Aromatic compound
advertisement
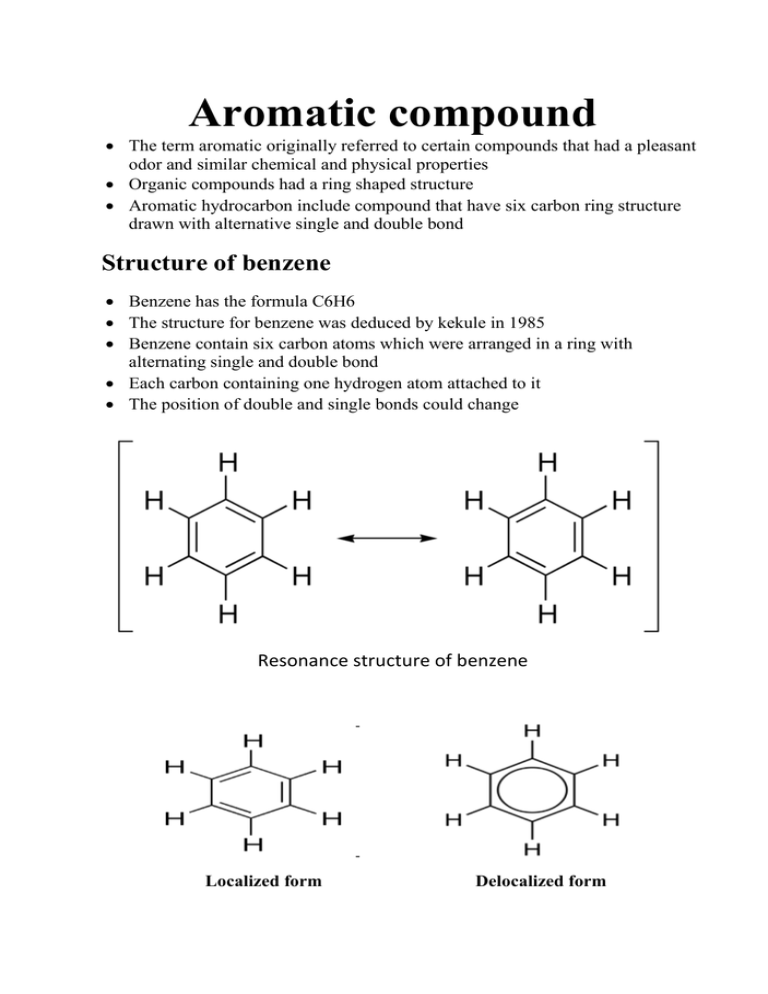
Aromatic compound The term aromatic originally referred to certain compounds that had a pleasant odor and similar chemical and physical properties Organic compounds had a ring shaped structure Aromatic hydrocarbon include compound that have six carbon ring structure drawn with alternative single and double bond Structure of benzene Benzene has the formula C6H6 The structure for benzene was deduced by kekule in 1985 Benzene contain six carbon atoms which were arranged in a ring with alternating single and double bond Each carbon containing one hydrogen atom attached to it The position of double and single bonds could change Resonance structure of benzene Localized form Delocalized form Properties of benzene 1. Benzene is a colorless liquid with a distinct gasoline like odor 2. It is insoluble in water 3. Benzene is toxic taken internally 4. Contact with skin is harmful 5. Continued inhalation of benzene vapors decreases red and white blood cell counts 6. it undergoes electrophilic substitution reactions and nucleophilic aromatic substitutions. 7. Benzene is a mild carcinogenic (causing cancer). Phenol Aspirin Analine Nitrobenzene Paracetamol Benzoic acid Picric acid Aromaticity An aromatic compound contains a set of covalently bound atoms with specific characteristics: 1. A delocalized conjugated π system, most commonly an arrangement of alternating single and double bonds 2. Coplanar structure, with all the contributing atoms in the same plane 3. Contributing atoms arranged in one or more rings 4. A number of π delocalized electrons that is even, but not a multiple of 4. That is, 4n + 2 number of π electrons, where n=0, 1, 2, 3, and so on. This is known as Hückel's Rule. Naming of aromatic compounds 1) Monosubstitution compounds Monosubstitution of benzenes are named as derivative of benzene. Phenol analine Chlorobenzene Nitrobenzene Toluene 2) Di substitution compounds When benzene ring bears two side chains or groups, indicate their positions by numbering them, or as is more common, by using prefixes Ortho indicates a 1,2-disubstituted benzene Meta indicates a 1,3-disubstiuted benzene Para indicates a 1,4-disubstituted benzene 1,2-dimethylbenzene ortho-dimethylbenzene 1,3-dimethylbenzene meta-dimethylbenzene 1,4-dimethylbenzene para-dimethylbenzene Importance of aromatic compounds 1. Aromatic compounds play key roles in the biochemistry of all living things. 2. The four aromatic amino acids histidine, phenylalanine, tryptophan, and tyrosine each serve as one of the 20 basic building blocks of proteins. 3. All 5 nucleotides (adenine, thymine, cytosine, guanine, and uracil) that make up the sequence of the genetic code in DNA and RNA are aromatic purines or pyrimidines. 4. The molecule of heme contains an aromatic system with 22 π electrons. 5. Chlorophyll also has a similar aromatic system. 6. Aromatic compounds are important in industry. Key aromatic hydrocarbons of commercial interest are benzene, toluene, ortho-xylene and para-xylene. 7. Preservations of urine specimens, preparation of drugs and power explosives. 8. Phenol is poison if taken internally and it make deep burns and blisters. Also phenol may be used as antiseptic and disinfectant for surgical instruments Uses of aromatic compounds: Toluene: 1) Preservation for urine specimens 2) Preparation of dyes 3) Air plane glue 4) Powerful explosive (TNT) 5) Toluene can be used to break open red blood cells in order to extract hemoglobin in biochemistry experiments. Phenol: 1) White crystal turn reddish if exposure to light 2) Phenol is poisonous if taken internally and make deep burns and blisters 3) Phenol is used to as antiseptic and disinfectant for surgical instruments. Naphthalene: 1) Is white crystalline solid obtained from coal tar, 2) Naphthaline was used in home under the name mothball Phenantherene: It is an important component of male and female sex hormone, vitamin D, Cholesterol, bile etc. Reaction of benzene (substitution reaction) 1) Nitration: Nitric acid (HNO3 = HO-NO2) reacts with benzene in the presence of sulphuric acid and provides a nitro group to it finally making nitrobenzene. Benzene 2) Sulphonation: Nitrobenzene When sulphuric acid (H2SO4 = HO – SO3H) is heated with benzene, it gives a sulphonic group which attaches with benzene displacing hydrogen to make benzene sulphonic acid. Benzene Benzene sulphonic acid 3) Halogenations: Benzene reacts with chlorine (Cl2 = Cl – Cl) in the presence of FeCl3 to give chlorobenzene and hydrochloric acid. Benzene Chlorobenzene 4) Alkylation: When an alkyl halide is reacted with benzene in the presence of aluminium chloride, the alkyl group gets attached with benzene to form toluene displacing one hydrogen which reacts with the chloro group of alkyl halide to form hydrochloric acid. Benzene Toluene