ARDS
advertisement

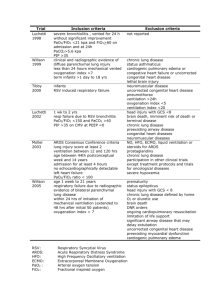
ARDS ABDULLAH M. AL-OLAYAN MBBS, SBP, ABP. ASSISTANT PROFESSOR OF PEDIATRICS. PEDIATRIC PULMONOLOGIST. OUTLINES Definition AND Diagnosis. Pathophysiology. Pulmonary (Direct) Versus Non-pulmonary (Indirect) ALI/ARDS. Differences Between Children and Adult. Treatment. Definition AND Diagnosis (ARDS) is a diffuse, progressive inflammatory lung disease. Bilateral pulmonary infiltrate on CXR. 2. Pulmonary capillary wedge pressure of 18 mmHg (for pediatric purpose excluding heart failure due to congenital heart disease or cardiomyopathy). 3. PaO2 / FIO2 ratio , >200 or >300 for ARDS and ALI respectively. 1. Pathophysiology ALI/ARDS arises as a consequence of an inflammatory process at the alveolar-capillary interface in the lung. The resultant endothelial and epithelial disruption leads to increased alveolar-capillary permeability and flooding of alveoli with protein rich edema fluid. Alveolar gas exchange is impaired and surfactant function is disrupted. Pathophysiology Acute Exudative Phase characterized by the production of pulmonary edema, cytokine release, and activated neutrophils. The acute exudative stage is associated with increased intrapulmonary shunting, reduced functional residual capacity (FRC), and a decrease in lung and chest wall compliance. Pathophysiology The acute exudative phase may resolve within hours or days, and not all patients progress to the subsequent fibroproliferative—early repair—phase. “Repair” may not result in disease resolution in all patients but may presage the development of fibrosing alveolitis. Pathophysiology The recovery stage, occurs within 10 to 14 days, with gradual improvement in lung compliance and oxygenation. The mechanism of resolution of the acute inflammatory process and fibrosis is not well established. Etiology Direct Injury : Common Causes : Pneumonia 28%. Aspiration 14%. Less Common Causes : Pulmonary contusion. Fat emboli. Near drowning. Inhalational injury. Davis et al., J Peds1993;123:35 Etiology Indirect Injury : Common Causes : Sepsis 32%. Shock after severe trauma 5%. Less Common Causes : Cardiopulm. Bypass. Drug overdose. Acute pancreatitis. Massive blood transfusions. Davis et al., J Peds1993;123:35 Pulmonary Versus Non-pulmonary ALI/ARDS In 1994, the NAECC made a distinction between : (ARDSp) such as aspiration pneumonia. (ARDSexp) such as sepsis. Data provided by Flori and colleagues suggest that ARDSp constitutes 60% of pediatric ARDS. The same authors also reported sepsis as the major cause (13% to 21%) of ARDSexp in children. Pulmonary Versus Non-pulmonary ALI/ARDS Differences between ARDSp and ARDSexp may be most apparent in the acute exudative phase of the disease. These differences may impact the response to treatment and outcome. Recent study in children reported that ARDSexp had a greater mortality than ARDSp. Pulmonary Versus Non-pulmonary ALI/ARDS ARDSp is characterized by a primary injury to the alveolar epithelium resulting in intra-alveolar edema, and reduced lung compliance with preservation of the chest wall compliance. In ARDSexp, the primary insult is systemic and the major injury is to the capillary endothelium;here the edema is predominantly interstitial, and the greater reduction of compliance occurs in the chest wall. Pulmonary Versus Non-pulmonary ALI/ARDS Gross pathology of the lung in ARDSp suggests predominant consolidation, and in ARDSexp the striking finding is atelectasis. Pulmonary Versus Non-pulmonary ALI/ARDS Epidemiology and Outcome The incidence of ALI in children is 3 to 5/100,000/Y. 5 to 10 times less than that in adults. 2% to 10% of all PICU admissions. 80% of children with ALI will progress to ARDS and 2/3 require early mechanical ventilation. 60% with ALI are less than 4 years of age and have an underlying chronic disease. Epidemiology and Outcome Mortality among children with ARDS decreased from between 65%-80% in the 1980s, to less than 20% now. In clinical trials, the reported mortality among children with ALI ranges from : 7% to 20% (degree of hypoxemia) Country (28% to 44% in developed countries, 61% in a developing country). Etiology (up to 40% to 60% in immunocompromised children, minimal in bronchiolitis). Comorbidities (e.g., nonpulmonary organ dysfunction, CNS dysfunction). Severity Score Blood gas analysis, specifically oxygenation, is regarded as the standard for assessment of severity of ARDS. (A − a) O2 difference can express the degree of hypoxemia. (OI) = [(MAP × Fio2)×100]/PaO2. Severity Score In contrast to oxygenation , VI [Paco2 × peak airway pressure × respiratory rate]/1,000 has been employed to reflect the difficulty involved in clearing CO2. Severity Score Genetic Modifiers of ALI/ARDS In 2002, Marshall and colleagues were the first investigators to describe a preliminary association between a gene variant and ALI mortality. This group described the increased incidence of a high producer polymorphism of the ACE gene in patients with ARDS. Pulmonary surfactant- associated protein B (SFTPB), interleukin-6 (IL-6), and coagulation factor V (F5). Differences Between Children and Adult The mechanical properties of the lungs of children and infants are different from those of adults. Chest wall compliance is inversely related to age, and with pressure preset ventilation, higher chest wall compliance may increase delivered tidal volume and thereby increase the risk for ventilator-associated lung injury (VALI) in young children. Differences Between Children and Adult The infant lung has low inherent elastic recoil, which may protect against lung collapse, so lower PEEP levels may be required to maintain lung recruitment. Finally, there are important outcome differences: Mortality for children with ARDS is less than in adults and high Fio2 is associated with worse outcome in children but not in adults. Treatment Conventional Mechanical Ventilation : 28% of patients with ALI do not require mechanical ventilation at the onset of the lung injury and almost all children with ARDS require mechanical ventilation. The ventilation parameters associated with this iatrogenic lung injury are high levels of end-inspiratory airway pressure, large tidal volumes, low levels of endexpiratory airway pressure, and possibly high Fio2. Treatment Conventional Mechanical Ventilation : lower VT, up to 10 mL/kg; lower inflation pressure (<30 cm H2O); higher PEEP (~5 to 12 cm H2O); and lower Fio2. If the achievement of normal pH, Paco2, and Pao2 levels require respiratory support strategies that may injure the lungs, then lower pH, Pao2, and higher Paco2 (permissive hypercapnia) are tolerated. Treatment Conventional Mechanical Ventilation : Because the likelihood of lung injury is greater if the airway pressure, VT, and concentration of inspired oxygen are elevated, many clinicians will reduce the target SaO2 (85% to 88%) if necessary. Permissive hypercapnia (Paco2 60 to 80 mm Hg) with a pH > 7.2 . Treatment Conventional Mechanical Ventilation : Few studies regarding mechanical ventilation have been performed in children, and as a result, no specific approaches have been proved superior to others. Currently there are no data to support the superiority of one mode of ventilation over another (e.g., pressure control versus volume control). Treatment Noninvasive Ventilation : A trial of NIPPV may be attempted in any child with early respiratory failure; however, one should not persist with its use if there is no clinical benefit within 2 to 3 hours. Treatment High Frequency Oscillatory Ventilation : HFOV achieves effective gas exchange while avoiding high peak airway pressures and the inflationdeflation cycles. Lung volume is maintained by the application of a high continuous mean airway pressure. CO2 removal is achieved despite small tidal volumes (2 to 4 mL/kg) by imposing a breath frequency of 300 to 900 (5 to 15 Hz) per minute, resulting in large minute volumes. Treatment Airway Pressure Release Ventilation (APRV): APRV is a ventilator modality characterized by cyclical alternation between two levels (high and low) of positive airway pressure, while permitting spontaneous breathing activity at both levels of pressure support. APRV, facilitates an open lung ventilatory approach, avoids cyclical recruitment and derecruitment of alveolar units, permits homogenous gas distribution during inspiration, minimizes (volutrauma), and reduces the risk of (atelectrauma). Treatment Neurally Adjusted Ventilatory Assist (NAVA): The NAVA system utilizes the electrical activity of diaphragmatic muscle to signal the initiation of patient inspiratory effort. Although there are few reports of NAVA use in either adults or children, it has been demonstrated that NAVA prevents excessive lung distension, efficiently unloads respiratory muscles, and improves but does not abolish patient-ventilator dyssynchrony Adjuvants to Mechanical Ventilation : Prone Positioning : Prone positioning is safe and has been reported to produce a rapid and sustained improvement in oxygenation in 90% of children with ALI/ARDS. NO reduction in days of ventilation or mortality. Prone positioning is best reserved for patients with persistent refractory hypoxemia. If it is not improve oxygenation, it should be discontinued. Adjuvants to Mechanical Ventilation : Inhaled Nitric Oxide (iNO): It has been shown to result in short-term improvements in oxygenation in some patients with ARDS/ALI, but it has no substantial impact on the duration of ventilator support or mortality when used as a routine part of care. Inhaled NO is best reserved for patients with refractory hypoxemia,with Fio2 > 0.6. Adjuvants to Mechanical Ventilation : Surfactant: Despite several studies in adults and children with ALI/ARDS the role of surfactant administration has not been established. Walmrath and colleagues reported improvement in oxygenation when a high dose of bovine surfactant was administered to patients with ARDS and sepsis. In children, a relatively small randomized controlled trial (RCT) reported a reduction in mortality following administration of natural calf surfactant,however, no effect on ventilator-free days or length of hospitalization was reported. Adjuvants to Mechanical Ventilation : Corticosteroids: The anti-inflammatory properties of corticosteroids and the potential inhibition of both fibroblast proliferation and collagen deposition make corticosteroids an attractive option. High doses (≥30 mg/kg/day) of corticosteroids for a short period of time (≤24 hours) either had no impact on mortality or, in one case, increased mortality. Adjuvants to Mechanical Ventilation : Corticosteroids: A more recent study of early low-dose corticosteroids (1 mg/kg/day) for 25 days demonstrated a treatment benefit with improvements in lung injury score, days of ventilation, and mortality. In summary, current evidence from clinical trials does not support the use of corticosteroids in any phase of ARDS in adults. In children, no data are available. Adjuvants to Mechanical Ventilation : Neuromuscular Blocking Agents: No data are available regarding the use of neuromuscular blocking agents (NMBA) among ventilated children. A recent RCT in adults has suggested that continuous neuromuscular blockade (with cisatracurium besylate) in the first 48 hours of ventilation in ARDS significantly reduced mortality. Optimization of patient/ventilator synchronization. Adjuvants to Mechanical Ventilation : Beta-Adrenergic Agonists: The alveolar edema in ALI/ARDS is mainly due to increased permeability and increased capillary hydrostatic pressure. Alveolar fluid clearance is enhanced through upregulation of Na+ transport in the alveolar epithelial cells. In addition, pulmonary vasodilatation and resulting reduction of pulmonary vascular pressure results in lowered capillary hydrostatic pressures, and β agonism may independently decrease endothelial permeability. An observational review of children with ARDS suggested that inhaled bronchodilators were associated with a lower mortality. Adjuvants to Mechanical Ventilation : Tracheostomy: There is no literature describing the preemptive use of tracheostomy in children in whom prolonged ventilation is anticipated, nor is it frequently employed in the pediatric population. A recently published study revealed that among ventilated children in Canada, the prevalence of tracheostomy was less than 1.5%. Reference Thank You