Lab 4 pH
advertisement

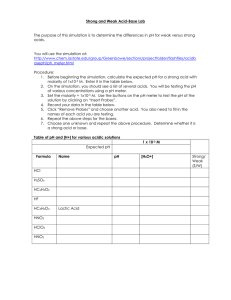
pH By Dr. Mohammed Golam Rasul 2 3 Overview of Today’s lecture 1. Introduction to pH 2. pH scale 3. Importance of pH in Everyday Life 4. Testing the pH of Solutions 6.pH demonstration Introduction Living things have certain pH ranges for normal biochemical processes to occur. pH is a logarithmic scale for measuring acids and bases. Measured through indicators or pH meter. What is pH? The term "pH" is defined as the negative logarithm of H+ ion concentration of a given solution. pH = -log10 [H+] pH value The pH value of a substance is directly related to the ratio of the hydrogen ion and hydroxyl ion concentrations. If the H+ concentration is higher than OHthe material is acidic. If the OH- concentration is higher than H+ the material is basic. 7 is neutral, < is acidic, >7 is basic pH Scale The pH of a solution is used to indicate the acidity of a solution has values that usually range from 0 to 14 is acidic when the values are less than 7 is neutral with a pH of 7 is basic when the values are greater than 7 8 Strongest acid Weak acid Neutral Weak Base Strongest base Importance of pH in Everyday Life • pH plays a very significant role in biochemical reactions. For example, the blood in our bodies is maintained at a pH value of 7.35 - 7.45. • The stomach produces hydrochloric acid that helps in the digestion of food. • The bacteria present in the mouth act on sugar and food particles remaining in the mouth after eating to produce acids. The best way to prevent this is to clean the mouth after eating food or use toothpastes , which are generally basic, for cleaning the teeth so as to prevent tooth decay. Learning Check Identify each solution as A) acidic, B) basic, or N) neutral ___ 1) HCl with a pH = 1.5 ___ 2) pancreatic fluid [H3O+] = 1 x 10−8 M ___ 3) Sprite soft drink, pH = 3.0 ___ 4) pH = 7.0 ___ 5) [OH−] = 3 x 10−10 M ___ 6) [H3O+ ] = 5 x 10−12 M 11 Solution Identify each solution as A) acidic, B) basic, or N) neutral A 1) HCl with a pH = 1.5 B 2) Pancreatic fluid [H3O+] = 1 x 10−8 M A 3) Sprite soft drink pH = 3.0 N 4) pH = 7.0 A 5) [OH-] = 3 x 10−10 M B 6) [H3O+] = 5 x 10−12 12 Testing the pH of Solutions The pH of solutions can be determined using a pH meter pH paper indicators that have specific colors at different pH values 13 pH meter pH paper Measuring pH of various solutions using a pH paper The pH paper is orange in color (before use) and changes color depending on the pH being tested. Look at the pH key to determine the pH of the solution ranging from 1-12. A pH strip has been dipped into the solution and is displayed sitting outside the beaker pH paper pH paper Indicators SAFETY NO tasting, smelling, touching of liquids NO tasting, smelling of papers NO wandering – be aware while cleaning up. First Aid – wash affected area with plenty of water. Calculating pH Mathematically, pH is the negative log of the hydronium ion concentration pH = −log [H3O+] For a solution with [H3O+] = 1 x 10−4, pH = −log [1 x 10−4 ] pH =[4.0] pH = 4.0 20 Example of Calculating pH Find the pH of a solution with a [H3O+] of 1.0 x 10−3. STEP 1 Enter the [H3O+] value: STEP 2 Press log key and change the sign: log (1 x 10−3) = [3] STEP 3 Make the number of digits after the decimal point (2) equal to the number of significant figures in the coefficient (2): [H3O+] = 1.0 x 10−3 pH is 3.00 21 Learning Check What is the pH of coffee if the [H3O+] is 1 x 10−5 M? 1) pH = 9.0 2) pH = 7.0 3) pH = 5.0 22 Solution What is the pH of coffee if the [H3O+] is 1 x 10−5 M? STEP 1 Enter the [H3O+] value: STEP 2 Press log key and change the sign: log (1 x 10−5) = [5] STEP 3 Make the number of digits after the decimal point (1) equal to the number of significant figures in the coefficient (1): [H3O+]= 1 x 10−5, pH is 5.0 (3) 23