Chapter #9 Notes.doc
advertisement

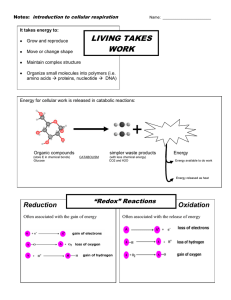
Chapter #9 Cellular Respiration Harvesting Chemical Energy - Notes Overview – Life Is Work I. Catabolic Pathways Yield Energy By Oxidizing Organic Fuels 1. Metabolism is the totality of an organism’s chemical reactions. 2. Two types of metabolic pathways are a) Catabolic pathways b) Anabolic pathways 3. Catabolic pathways – the breakdown of complex molecules to simpler compounds. a) One type of catabolic pathway is fermentation. b) Fermentation is a partial degradation of sugars that occurs without the use of oxygen. c) The most prevalent and efficient catabolic pathway is cellular respiration. d) Cellular respiration is the process in which oxygen is consumed as a reactant along with the organic fuel the exhaust is carbon dioxide and water plus energy and heat. 4. In eukaryotic cells, the mitochondria house most of the metabolic equipment for cellular respiration. The Integration of Chapter #8 5. Chemical reactions are classified as exergonic (energy outward) or endergonic (one that absorbs free energy from its surroundings). 6. Catabolism is linked to work by a chemical drive shaft-ATP (adenosine triphosphate). B. ATP Powers Cellular Work By Coupling Exergonic Reactions to Endergonic Reactions (Source – text: pp. 148-150) 1. A cell does three main kinds of worka) Mechanical work b) Transport work c) Chemical work 2. In most cases, the immediate source of energy that powers cellular work is ATP. C. The Structure & Hydrolysis of ATP 1. ATP is a nucleotide. 2. ATP contains a) the sugar ribose b) the nitrogenous base – adenine c) a chain of three phosphate groups 3. The bond between the phosphate groups of ATP’s tail can be broken by Hydrolysis. 4. Because their hydrolysis releases energy, the phosphate bonds of ATP are Sometimes referred to as high energy phosphate bonds. D. How ATP Performs Work 1. When ATP is hydrolyzed, it transfers a phosphate group to another molecule. 2. The recipient of the phosphate group is said to be phosphorylated. (This molecule undergoes a change that performs work). E. The Regeneration of ATP 1. ATP is a renewable resource that can be regenerated by the addition of phosphate to ADP. 2. This shuttling of inorganic phosphate and energy is called the ATP cycle, and it couples the cell’s energy yielding (exergonic) processes to the energy consuming (endergonic) ones. E. Redox Reactions: Oxidation and Reduction (Chapter #9 – p. 161) 1. Oxidation-reduction reactions – is the chemical reactions which involve partial or complete transfer of electrons from one reactant to another. a) Oxidation – is a redox reaction, the loss of electrons. b) Reduction – is the addition of electrons to another atom. 2. The electron donor is called the reducing agent. 3. The electron acceptor is the oxidizing agent. F. Oxidation of Organic Fuel Molecules During Cellular Respiration G. Stepwise Energy Harvest via NAD+ and the Electron Transport Chain 1. During cellular respiration, glucose is oxidized to carbon dioxide & oxygen is reduced to water. 2. Electrons lose potential energy during their transfer from organic compounds to oxygen. 3. Electrons from organic compounds are usually passed first to NAD+, reducing it to NADH. 4. NADH passes the electrons to an electron transport chain, which conducts them to oxygen in energy releasing steps. 5. The energy is used to make ATP. H. The Stages of Cellular Respiration: 1. Respiration is a cumulative function of three metabolic stages: a) Glycolysis b) The citric acid cycle c) Oxidative phosphorylation: electron transport and chemiosmosis 2. Cellular respiration is sometimes defined as including only the citric acid cycle and oxidative phosphorylation. II. Glycolysis Harvests Chemical Energy by Oxidizing Glucose to Pyruvate 1. The term glycolysis means “sugar splitting.” 2. Glycolysis can be divided into two phases: energy investment phase and energy payoff. 3. This process consist of ten steps – each catalyzed by a specific enzyme. 4. Glycolysis is a catabolic pathway that occurs in the cytosol. 5. This process oxidizes glucose into two pyruvate (3C) molecules. A. The Citric Acid Cycle Completes the Energy –Yielding Oxidation of Organic Molecules 1. Pyruvate is a charged molecule, so it must enter the mitochondrion via active transport, with the help of a transport protein. 2. Upon entering the mitochondrion pyruvate a 3C molecule is converted to a compound called acetyl coenzyme. 3. The conversion is accomplished by a multienzyme complex which – a carboxyl group is removed & given off as CO2. (The molecule is a 2C molecule which the mitochondrion will accept) 4. The remaining 2C fragment is oxidized to a compound – acetate. 5. An enzyme transfer the extracted electron to NADH. 6. Finally, coenzyme A, a sulfur containing compound is attached to the acetate by an unstable bond. 7. The product is ready to feed into the Krebs cycle (citric acid cycle tricarboxylic acid cycle). a) Hans Krebs is the scientist who was largely responsible for working out the pathway of the citric acid cycle. b) The Krebs cycle enzymes are located in the mitochondrial matrix. (Except one which resides in the inner mitochondrial membrane) 8. The Krebs cycle occurs in the matrix of the mitochondrion. 9. The end products of the Krebs cycle are the following: ATP, NADH, FADH2. IV. During Oxidative Phosphorylation, Chemiosmosis Couples Electron Transport to ATP Synthesis A. The Pathway of Electron Transport 1. In the electron transport chain, electrons from NADH and FADH2 lose energy in several energy-releasing steps. 2. At the end of the chain, electrons are passed to O2 reducing it to H2O. B. Chemiosmosis: The Energy-Coupling Mechanism 1. Chemiosmosis is the process in which energy stored in the form of hydrogen ion gradient across a membrane is used to drive cellular work such as the synthesis of ATP. 2 Chemiosmosis occurs in the inner membrane of the mitochondrion. 3. Populating the inner membrane of the mitochondrion are many copies of a protein complex called ATP synthase, the enzyme that actually makes ATP from ADP and inorganic phosphate. C. An Accounting of ATP Production by Cellular Respiration V. Fermentation and Anaerobic Respiration Enable Cells to Produce ATP Without the Use of Oxygen A. Types of Fermentation 1. There are many types of fermentation, differing in the end products formed from pyruvate. 2. Two common types of fermentation are – a) alcohol fermentation b) lactic acid fermentation 3. In alcohol fermentation, pyruvate is converted to ethanol in two steps. a) The first step releases carbon dioxide from the pyruvate, which is converted to the two-carbon compound acetaldehyde. b) In the second step, acetaldehyde is reduced by NADH to ethanol. 4. During lactic acid fermentation, pyruvate is reduced directly by NADH to form lactate as an end product, with no release of CO2.