1412-PracticeExam4.doc
advertisement

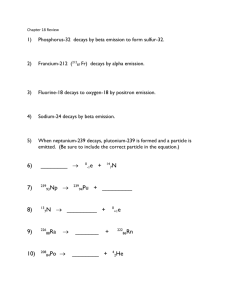
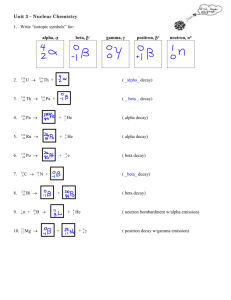
CHEM 1412 Practice Exam 4 (Chapters 21, & 23) F = 96485 J/V mol = 96485 C/mol of e- G = –n F E°cell Amp = C/sec E = E° – (0.0592/n) log Q G = –RTlnKeq R = 8.314 J/mol K mass of proton = 1.007276470 amu lnNt = –kt + lnNo c = 2.998 X 108 m/s mass of neutron = 1.008664904 amu A periodic table is also attached. 1. 2. Alpha particles are identical to A. protons. B. helium atoms. C. hydrogen atoms. D. helium nuclei. E. electrons. Predict the other product of the following nuclear transformation. A. B. C. D. E. 3. When atoms of beryllium-9 are bombarded with alpha particles, neutrons are produced. What new isotope is also formed? A. B. C. D. E. 4. In the decay series there are six radioisotopes that decay by alpha emission, including Th-232 itself, and four radioisotopes that decay by beta emission. The final product of this series is a stable isotope. The symbol for this product is A. B. C. D. E. 5. Sulfur-35 decays by beta emission. The decay product is A. B. C. D. E. 6. The only stable isotope of iodine is iodine-127. Predict the mode of decay of A. alpha emission B. beta emission . 7. 8. 9. C. positron emission D. electron capture Carbon-11 is a radioactive isotope of carbon. Its half-life is 20.3 minutes. What fraction of the initial number of carbon-11 atoms in a sample will remain after 81 minutes? A. 1/16 B. 1/4 C. 1/2 D. 1/32 E. 1/8 Cobalt-60 is a beta emitter with a half-life of 5.3 years. Approximately what fraction of cobalt-60 atoms will remain in a particular sample after 26.5 years? A. 1/5 B. 1/16 C. 1/26 D. 1/32 E. 1/64 Which isotope, when bombarded with nitrogen-15, yields the artificial isotope dubnium-260 plus 4 neutrons? A. californium-245 B. thorium-257 C. nobelium-245 D. californium-249 E. dubnium-249 10. In the following reaction, identify X. A. B. C. D. E. 11. 12. 13. Decay of tin-110 by electron capture yields A. tin-109. B. cadmium-106. C. indium-110. D. antimony-110. E. tellurium-114. Decay of ruthenium-93 by positron emission yields A. technetium-93. B. molybdenum-89. C. palladium-97. D. rhenium-93. E. ruthenium-92. When atoms of aluminum-27 are bombarded with alpha particles, a neutron and an element are produced. The particular isotope formed is A. B. C. D. E. 14. 15. The general formula for alkenes is A. CnH2n+2 B. C2nH2n C. CnHn+2 D. CnH2n E. CnH2n-2 Which one of these formulas is that of an unsaturated hydrocarbon? A. B. C. D. E. 16. Which one of these hydrocarbons does not have isomers? A. C7H16 B. C6H14 C. C5H10 D. C4H8 E. C3H8 17. 18. 19. 20. Which of these species are structural isomers of C6H14? A. I and II B. I and III C. II and III D. II and IV E. III and IV Which of these species is an aromatic compound? A. C2H2 B. C6H12 C. C6H4Br2 D. C5H10 E. C2H4Br2 The name for the compound with the formula CH3CH2CH2CH2OH is A. propanol. B. propane. C. butanol. D. pentane. E. pentanol. Which type of organic compound does not contain a carbonyl group? A. ethers 21. B. carboxylic acids C. ketones D. aldehydes E. esters Which one of these compounds will result from the addition of HCl to A. B. C. D. E. 22. 23. none of these Which of these is the systematic name for the compound represented below? A. 2-ethylbutane B. 3-methylpentene C. 3-methyl-1-pentene D. 3-methyl-1-hexene E. 2-methylhexane The correct structure for 2,3,3-trimethylpentane is A. ? B. C. D. 24. 40 25. The gamma rays from cobalt-60 are used in cancer radiation treatment. What is the nuclide produced in this decay process? Ar can be prepared by electron capture from 36 a) Cl. 40 b) K. 41 c) Ar. 40 d) Ca. 33 e) S a) 60 27 Co b) 59 27 Co c) 60 28 Ni d) 59 26 Fe e) 58 26 Fe Part II – Show Your Work. 1. Name the following compound: CH3 | CH3CHCH2CH2CHCH2CHCH2CH3 | | CH3 CH2CH3 b. trans-5-methyl-2-hexene c. Draw the following molecule: 1-ethyl-1-methylcyclopentane 2. Draw and name the structural isomers which have the molecular formula C4H9Br. 3. Carbon-11 has a half-life of 20.4 minutes. Assuming you start with a 500 g sample of carbon-11,how much will remain after 24 hours? (Show calculation) 4. i. Write the nuclear equation for the beta decay of iodine-131. ii. By what process does oxygen-15 decay to nitrogen-15? iii. The alpha decay of what isotope of what element produces lead-206? iv. In the nuclear transmutation, 168O (p, ) X, what is the product nucleus X?