Chapter 14: Introduction to Organic Chemistry
advertisement

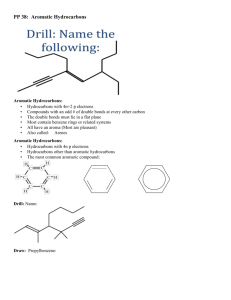
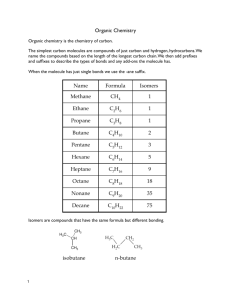
Organic Compounds (Chapter 14) Pencil Lead Diamond Student Learning Objectives • Identify substances which contain organic compounds • Sketch and describe isomer structures What is organic chemistry? Organic chemistry is the study of carbon compounds. 90% of compounds contain Carbon Carbon has a unique ability to bond with itself in complex covalent bonds. A single molecular formula may have several structures. Some Structures for C5H10 Practice How many structures can you draw for C7H16? Retinol (most common form of vitamin A) In what substances are hydrocarbons found? All hydrocarbons contain hydrogen and carbon. 1. Aromatic hydrocarbons contain the Benzene Ring (C6H6) Benzene Symbol Benzene Hydrocarbons Aromatics (benzene substances) have a strong aroma. Benzene is a carcinogen. Gasoline Adhesives Perfumes Paint Stripper Tobacco Household Cleaners Question What is a carcinogen? What does the word “carcinogen” mean? Aliphatic Hydrocarbons 2. Aliphatic hydrocarbons contain no Benzene Ring Methane Ethane Propane Alkane Aliphatic Hydorcarbons Alcane hydrocarbons have only single bonds. All Alcanes follow the same formula. Cyclo-Pentane CnH2n+2 Practice 1. What kinds of substances contain alkane hydrocarbons? a. Perfumes b. Fuels c. Food 2. What is the molecular formula for an alkane hydrocarbon that contains 16 carbons? Structural Isomers Alcane hydrocarbons may have structural isomers. Same molecular formula Different structure Different physical and chemical properties n-hydrocarbons iso-hydrocarbons neo-hydrocarbons Isomers of Pentane C5H12 Which Fuel? Octane ratings are based on the abundances of the different structural isomers of Pentane. neo-pentane highest octane rating iso-pentane n-pentane lowest octane rating Structural Symbols n-pentane Chain structure isopentane Branch structure Neopentane Cross structure cyclopentane Pentagon structure Questions 1) Which of the pentane structures do you expect to burn more slowly? Why? 2) In which fuel do you expect to have more neo-pentane molecules? a. Regular b. Super c. Supreme Alkyl Hydrocarbons (Alcohols) 3. Alkyl hydrocarbons are Alkanes with 1 hydrogen (H) replaced by hydroxide (OH) Alcohol Isomers Organic molecules have a base unit called a functional group. Alkene Ketone Carbonyl Amine Alkyl Benzyl http://sparkcharts.sparknotes.com/chemistry/organicchemistry1/section5.php Why are plastics and proteins difficult to break down? Polymers are very long molecules made up of repeating monomers. (chains of monomers) Naturally Occurring DNA Proteins Complex Carbohydrates Human Produced Carpet Plastics Chewing Gum DNA Practice Why do you think it is not a good idea to drink bottled water that has been in a hot car? many polymerization styrene polystyrene What are carbohydrates? Carbohydrates contain carbon and water. Glucose Fructose Sucrose Cellulose (Starch) C6(H2O)6 C6(H2O)6 C12(H2O)11 (C6H10O5)n Monosaccharide Monosaccharide Disaccharide Polysaccharides Practice Which breakfast cereal is highest in Fructose, Glucose, and Sucrose? ____________________ Fructose: 15,431 mg Glucose: 14,300 mg Sucrose: 170 mg http://nutritiondata.self.com/foods-008011010009000000000-1w.html

![LC Fuels and Thermochemistry [PDF Document]](http://s3.studylib.net/store/data/008241147_1-6ebc9d449a7896c353ddca434fe5df53-300x300.png)