Chapter- 4.doc
advertisement

Chapter 4: Aqueous Reactions & Solution Stoichiometry
Aqueous Solution- Solution in which water is the solvent. Solvent is the substance doing
the dissolving; solute is the substance(s) being dissolved.
Concentration Units:
(molarity i.e. moles solute per liter of solution)
(molality i.e. moles solute per kilogram of solvent).
Percent composition (mass of solute per total mass of solution x 102 ).
Parts per million (mass of solute per total mass of solution x 106 ).
Normality (equivalents of H+ or OH- per liter soln) = n x M = N
One equivalent is 1 mole of H+ ion or 1 mole of OH- ion
One mole of Ca(OH)2 will dissociate in water to give two equivalents (OR 2 moles) of
OHEx. Ca(OH)2(aq) → Ca2+ + 2OHMole Fraction (moles of solute per sum of moles of substances).
Use mole fractions ( X1 = n1/ nt); (nt = n1 + n2 + n3)
Molarity: M = n/V; n is moles; V is volume of solution in liters(L); Symbol = [ ]
n = M x L (Remember!!!!!)
Volume of solvent = n/molarity
Example: Molarity of soln if 20 grams of NaOH (mwt. = 40g) is dissolved in solution
volume of 250mL?
M = n/V = (20g/40g) x (1/0.25L) = 2M
Strong Electrolytes- are compounds that dissociate completely or almost completely into
ions when dissolved in water. Their solutions are good electric current conductors. They
dissociate essentially completely in water. Examples: (NaCl, HCl….)
NaCl(aq) Na+(aq) + Cl-(aq)
1mole
1mole
1mole
K2SO4(aq) 2K+(aq) + SO42-(aq)
1mole
2moles
1mole
(NH4)3PO4(aq) → 3NH4+ (aq) + PO43- (aq) .
1 mole
3 moles
1 mole
Weak Electrolytes- are compounds in which a very small amount dissociates when
dissolved in water. Example:
HC2H3O2(aq) H+(aq) + C2H3O2-(aq)
1mole
10-3mole
10-3mole Extent of dissociation is small.
We do not write weak electrolytes in dissociated form in equations!!!!!!
Nonelectrolytes- are compounds that do not dissociate at all when dissolved in water.
Example: Common sugar, methanol, ethanol
Molecular Equation (Metathesis or Double Displacement Reactions)
Pb(NO3)2(aq) + 2KI(aq) PbI2(s) + 2KNO3(aq)
Complete Ionic Equation
Pb+2(aq) + 2NO3-(aq) + 2K+(aq) + 2I-(aq) 2NO3-(aq) + 2K+(aq) + PbI2(s)
Net Ionic Equation
Pb+2(aq) + 2I-(aq) PbI2(s)
Spectator Ions
NO3-(aq), K+(aq)
2HC2H3O2 + Ca(OH)2 → Ca(C2H3O2)2 + 2H2O
2HC2H3O2 + Ca+2 + 2OH- → Ca+2 + 2C2H3O2 - + 2H2O
2HC2H3O2 + 2OH- → 2C2H3O2 - + 2H2O
The driving force in a metathesis reaction is that a precipitate is formed, or a weak or
non-electrolyte is formed, or a gas is produced. If all reactants and products are strong
electrolytes, no reaction takes place. Ex.:
Molecular Equation: KNO3 + NaCl → KCl + NaNO3
Complete Ionic Equation: K+ + NO3- + Na+ + Cl- → K+ + Cl- + Na+ + NO3No Net Ionic Equation: All ions are spectator ions.
Use solubility guidelines for common ionic compounds to determine what compounds
are strong, weak, or nonelectrolytes.
------------------------------------------------------------------------------------------------------All ionic compounds of alkali metals, nitrates (NO3-), and most acetates (C2H3O2-) are
strong electrolytes (except for acetates of Pb+2 & Pb+4 , Ag+ Hg2+2, Hg+2).
All compounds of chloride, bromide, and iodide are strong electrolytes (except with Ag+,
Hg2+2, Hg+2 and Pb+2).
All sulfates (SO42-) are strong electrolytes (except with Sr2+, Ba2+, Hg2+2, and Pb+2).
All compounds of sulfides (S2-) and hydroxides (OH-) are weak electrolytes (except alkali
metals, Ca2+, Sr2+, Ba2+ and NH4+ (except ammonium hydroxide).
All phosphates (PO43-) and carbonates (CO32-) are weak electrolytes (except alkali metal
and NH4+ phosphates).
All strong acids and bases are strong electrolytes. Weak acids and bases are weak
electrolytes.
------------------------------------------------------------------------------------------------------
Acids and Bases
Acids produce H+ ions in water; bases produce OH- ions in water.
Strong acids and bases completely dissociate into their respective cations and anions.
Strong acids: HCl, HBr, HI, HClO3, HClO4, HNO3, H2SO4
Strong bases: Group1 metal hydroxides (LiOH, NaOH, KOH, RbOH, CsOH) and 3
Group 2 metal hydroxides {Ca(OH)2, Sr(OH)2, Ba(OH)2}
ACIDS & BASES (3 DEFINITIONS)
Definition
Arrhenius:
Bronsted-Lowry:
Lewis:
ACIDS
increase [H+ ] in water
H+ donor
electron-pair acceptor
BASES
increase [ OH ] in water
H+ acceptor
electron-pair donor
In Acid-Base reactions (also called Neutralization or titration reactions), an acid and base
react to form a salt and water:
HCl + NaOH → NaCl + H2O
ACID/ BASE NEUTRALIZATION
In an acid/ base neutralization reaction( titration), one brings equal moles of H+ and OHto react, reaching what is called the endpoint of the titration.
Neutralization equation: naMaVa = nbMbVb
The a means acid, M is molarity of solutions and V means volume of solutions.
For H3PO4: na = 3
For H2SO4: na = 2
For HCl : na = 1
For Al(OH)3 : nb = 3
For Ca(OH)2 : nb = 2
For NaOH : nb = 1
If one knows any three of Ma, Mb, Va, & Vb, one can calculate the fourth.
Neutralization equation: naMaVa = nbMbVb
Ma & Mb are molarity of acid and base, respectively. Va & Vb are volumes of acid and
base, respectively.
And na is the number of hydrogen atoms per acid molecule. Ex. For H3PO4, phosphoric
acid, na is 3. For base, nb is the number of hydroxide ions per molecule of base. Ex for
Ca(OH)2, nb is 2.
Ex What volume of a 0.5M Ca(OH)2 solution is required to neutralize 25ml of a 1.0M
solution of H3PO4?
Va = 25ml, Ma = 1.0M, na = 3, Mb = 0.5M, nb = 2, Vb = ?
naMaVa = nbMbVb => Vb = naMaVa
nbMb
= (3)(1.0M)(25ml)
(2)(0.5M)
= 75ml Ca(OH)2
Mixing Different Concentration of same type of strong electrolyte solutions
Molarity = M1V1 + M2V2 +…
V1 + V2…
Ex What is the new concentration when 10ml 0.5M HCl, 30ml of 1.0M HCl and 50ml of
1.5M HCl solutions are mixed together?
Molarity = (10ml)(0.5M) + (30ml)(1.0M) + (50ml)(1.5M) = 110 mmoles = 1.2M
10ml + 30ml + 50ml
90ml
Dilution Equation = MiVi = MfVf , i means initial; f means final.
How much water must one add to a 30ml of a 1.5M HCl solution to dilute it to a
concentration of 0.55M? Mi = 1.5M, Vi = 30ml, Mf = 0.55M, Vf is
Vf = MiVi = (1.5M)(30ml) = 81.8ml , so one must add 51.8ml of water
Mf
0.55M
OR
MiVi = MfVf => (1.5M) (30 mL) = (0.55M)(30mL + x) ; x = 51.8mL
Remember: Molarity can be used as a conversion factor for relating moles in a solution to
volumes in the same solution. Ex. How many milliliters will contain 0.9 mole in a 2.5M
solution? Invert the molarity value and use it as a conversion factor.
1 liter
│0.9 mole = 0.36 liter = 360 ml
2.5 moles│
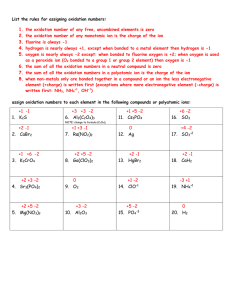
Oxidation Number
Oxidation number is the charge an atom (either alone as a neutral atom or ion, or as part
of a polyatomic ion or compound) has according to certain rules.
Rule 1. An atom in its elemental form has an oxidation number of 0(ie. Each H in H2 has
an oxidation number of 0, same for Na, He, P4, S8).
Rule 2. The oxidation number of monoatomic ions is the charge of the ion(ie. K+ has
oxidation number of +1, S-2 has -2 oxidation number).
Rule 3. Oxygen usually has -2 oxidation number and takes precedence unless it is bound
to fluorine, or is a peroxide ion(O2-2) or a superoxide ion(O2-1).
Rule 4. Hydrogen as an ion has an oxidation number of +1 when bound to a nonmetal ie.
H2O; or is -1 when bound to a metal ie. NaH.
Rule 5. As monoatomic ions, usually the oxidation number of oxygen, hydrogen, group 1
& 2 metals and the halogens is the charge on the atom when it tries to reach the electronic
noble gas stability.
The sum of the oxidation number of the individual atoms of an ion or compound is
equal to the net charge on the ion or compound!!!!!
Ex. What is the oxidation number of sulfur in the bisulfate ion, HSO4- ? The net charge
on the ion is -1.
-1 = 1(+1) + 4(-2) + S
-1 + (-1) + 8 = S
S = +6. So the oxidation # of S in the bisulfate ion is +6.
What is the oxidation number of Cr in Cr2O7-2?
-2 = 7(-2) + 2Cr
-2 + 14 = 2Cr,
Cr = +6. So the oxidation # of Cr in the dichromate ion is +6.
Oxidation-Reduction Reactions
In this type of reaction, one reactant loses electron(s) and is therefore oxidized; the other
reactant gains the electron(s) and is reduced.
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
0
+1 -1
+2 -1
0
Reaction
Oxidation Number
Mg(s) + 2H+ + 2Cl-(aq) → Mg+2 + 2Cl-(aq) + H2(g)
Net Ionic Equation
Mg(s)
+
2H+(aq)
→ Mg+2(aq) + H2(g)
Mg loses 2 e- and is oxidized. Therefore, Mg is the reducing agent (reactant).
H in HCl gains e-‘s and is reduced. Therefore, HCl is the oxidizing agent (reactant).
Reaction
Oxidation Number
Ni + 2HCl → NiCl2 + H2
0
+1 -1
+2 -1
0
Al(s) + H+(aq) →
Al(s) + Ca+2(aq) →
First reaction: Al metal will go to Al cation & H cation will go to Hydrogen gas.
Separate into two half reactions; balance them by atoms and charges; add them.
2(Al
→ Al+3 + 3e- ) = 2Al → 2Al+3 + 6e+
3(2H + 2e- → H2 ) = 6H+ + 6e- → 3H2
.
+
+3
2Al + 6H → 2Al + 3H2 .
Balanced in terms of atoms and charge!!!
Balance the following redox reactions
What about second reaction?
According to the activity series list, will these reactions proceed?
Activity Series List
Li, K, Ba, Ca, Na, Mg, Al, Mn, Zn, Cr, Fe, Co, Ni, Sn, Pb, H, Cu
Reaction
2Mg(s) + O2(g) → 2MgO(s)
Oxidation #
0
0
+2 -2
Mg is oxidized (reducing agent) O is reduced (O2 is oxidizing agent)
Ex.
Reaction
V2O5(s) + 5Ca(l) 2V(l) + 5CaO(s)
Oxidation#
+5
0
0
+2
Ca is oxidized (reducing agent) V is reduced (V2O5 is oxidizing agent)
Ex.
2H2O2(aq) → 2H2O(l) + O2(g) (Disproportionation)
-1
-2
0
↑
In this type of reaction, an element on the reactant side is both reduced and oxidized.
Ex.
Reaction
Oxidation#
Activity Series
Active metals tend to lose their electron(s) in a chemical reaction. The more active the
metal, the easier it loses its electron(s). In order of decreasing activity, the activity series
is:
Li, K, Ba, Ca, Na, Mg, Al, Mn, Zn, Cr, Fe, Co, Ni, Sn, Pb, H, Cu
The metal on left side will displace any metal to its right in a single displacement
reaction.
For the halogens, the displacement order is:
F2 > Cl2 > Br2 > I2 .