Unit 26 Applications of Acids and Bases (Chapter 7) •Acid Rain (7.8)
advertisement

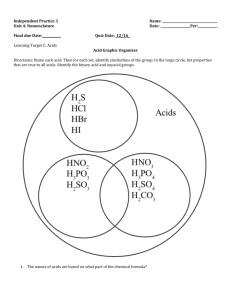
Unit 26 Applications of Acids and Bases (Chapter 7) •Acid Rain (7.8) •Antacids (7.9) •Acids/Bases in Industry (7.10) •Acids/Bases in Health (7.10) Still needs interesting stuff – acid rain pictures of forest, statues, maps, etc. Acid Strengths (7.4) •Recall that an acid reaction with water may be written generally as: HA (aq) + H2O (ℓ) → A- (aq) + H3O+ (aq) •Any acid reacting with water will undergo the same sort of reaction but possibly to a different degree. Acids may be classified as strong or weak depending on the extent to which they break apart. •Strong acids: A strong acid will undergo the reaction above to about 100% to form the A- and the H3O+. The only seven strong acids are HCl, HBr, HI, HNO3, H2SO4, HClO3, and HClO4. •Weak acids: A weak acid will only undergo the reaction above to small extent – maybe 5%. Acids that are not one of the seven above will be classified as weak acids. Examples include HF, HNO2, HC2H3O2, and others