Unit 27 Applications of Acids and Bases (Chapter 7) •Acid Rain (7.8)
advertisement

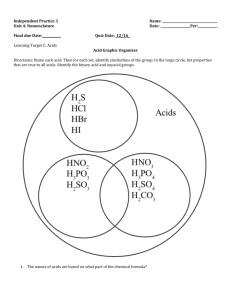
Unit 27 Applications of Acids and Bases (Chapter 7) •Acid Rain (7.8) •Antacids (7.9) •Acids/Bases in Industry (7.10) •Acids/Bases in Health (7.10) Still needs interesting stuff – acid rain pictures of forest, statues, maps, sinkholes, etc. Acid Strengths (7.4) •Recall that an acid reaction with water may be written generally as: HA (aq) + H2O (ℓ) → A- (aq) + H3O+ (aq) •Any acid reacting with water will undergo the same sort of reaction but possibly to a different degree. Acids may be classified as strong or weak depending on the extent to which they break apart. •Strong acids: A strong acid will undergo the reaction above to about 100% to form the A- and the H3O+. The only seven strong acids are HCl, HBr, HI, HNO3, H2SO4, HClO3, and HClO4. •Weak acids: A weak acid will only undergo the reaction above to small extent – maybe 5%. Acids that are not one of the seven above will be classified as weak acids. Examples include HF, HNO2, HC2H3O2, and others Image from aidhyl.wordpress.com Statue over entrance to castle in Westphalia, Germany built in 1702. The left picture was taken in 1908 and the right one in 1968. Image from http://www.epa.gov http://www.howstuffworks.com Image from http://pubs.usgs.gov