Chico2005.ppt
advertisement
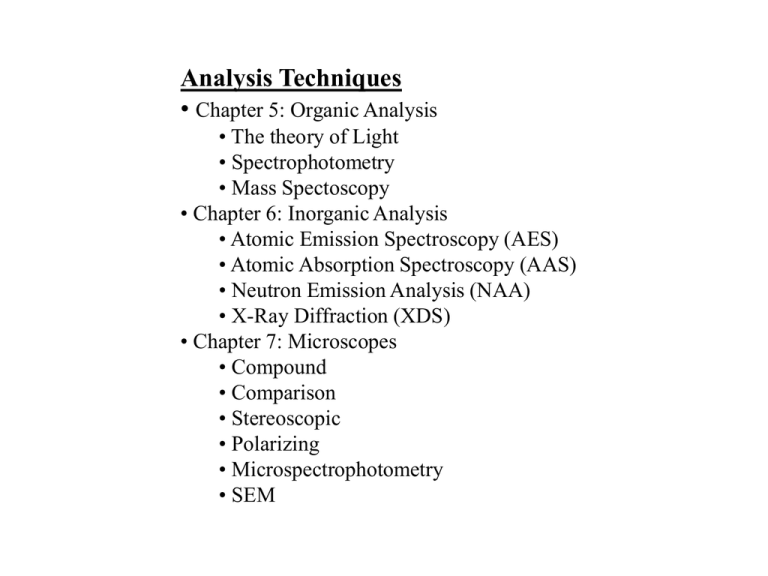
Analysis Techniques • Chapter 5: Organic Analysis • The theory of Light • Spectrophotometry • Mass Spectoscopy • Chapter 6: Inorganic Analysis • Atomic Emission Spectroscopy (AES) • Atomic Absorption Spectroscopy (AAS) • Neutron Emission Analysis (NAA) • X-Ray Diffraction (XDS) • Chapter 7: Microscopes • Compound • Comparison • Stereoscopic • Polarizing • Microspectrophotometry • SEM How Will I Approach These Topics? • The Theory of Light • The Structure of Matter • Analysis of Gross Features and Morphology • microscopes • Analysis of Compounds (Molecules and Crystals) • Photometry, GCMS, XDS • Analysis of Trace Elements • AES, AAS, NAA, SEM The Theory of Light Graphic representation of a Wave: • Crest Traveling waves • Trough • Amplitude (A) • Wave Length (l) l y(x) Crest A x(m) Trough Standing • Nodes waves • Anti-nodes • Period = T • frequency = f = 1/T Velocity = v = lf y(t) T A f Speed of Light = c = 3.00 x 108 m/s t The Wave Nature of Light: • 1801 Thomas Young performed the double slit experiment with light => demonstrated the Wave nature of light. • 1864 James Clerk Maxwell developed a unified theory of electromagnetism (includes theory of electromagnetic waves). • 1887 Heinrich Hertz experimentally confirms that light is electromagnetic waves. • Electomagnetic Spectrum: max frequency (Hz) wavelength • 103 • 107 • 107 • 1010 • 1012 • 3.9x1014 • 7.5x1014 • 1016 • 1019 RF AM TV,FM microwave infra-red visible ultra-violet x-rays gamma-rays 106 107 109 1012 3.9x1014 7.5x1014 1017 1020 … > 1 km 500 m - 50 m 10 m - 1 m 10 cm - 1mm 1 mm - 720 nm 720 nm - 390 nm 390 nm - 1 nm 10 nm - .5 pm < 10 pm Now, let’s look a little closer at a few regions of the Electromagnetic spectrum…. One can use specific absorption or transmission to identify a compound… Forensic investigation The Visible Spectrum: Why are there three colors of Light? Blue Green Red yellow Wavelength (l) Visible Analysis is easy. You just look at the sample.. IR Bands: Wavelength (l) IR light is not energetic enough to break molecular bounds, but it will excite specific vibrational modes in large complex molecules – can be used to identify specific organic molecules. UV Bands: UV is energetic enough to break covalent bonds. Causes sunburn. Wavelength (l) Quantization of Light: • Photo-electric effect is creation of an electric charge with light. • 1905 Einstein explains the photo-electric effect (Won the Nobel prize for this in 1921). Ephoton = hf When photons eject electrons from a metallic surface: hf = KEmax + W0 photon energy of the electron Binding Energy of the outer electrons “Work Function” of the metal g Main Concept => Light is quantized! => photon, particle nature of light Intensity of the light does not effect KEmax or W0. Quantization of Energy: Visible Range 6000 K “White Hot” 1900 Max Planck explained the black-body curves assuming atomic oscillations with discrete Energy values 4000 K “Red Hot” E = 0, hf, 2hf, 3hf, …. 500 En = nhf h = Planck’s Constant 1000 l (nm) 1500 h = 6.626 x10-34 Js Main Concept: => Energy is Quantized! Introduce the Photon 2000 Absorption and Emission of Light • Gamma rays Nuclear Levels • x-rays inner atomic levels • UV Molecular Bonds • Visible • IR vibrational modes • microwaves rotational modes Covalent Ionic van der Waals Scattering - Probe wavelength and Size of Subject Visible - 500 nm x-rays - 10-8 to 10-12 m Atoms - 10-10 m The Structure of Matter What is matter? It has mass and volume The Basis Building Blocks of Matter: Particle Mass Proton 1.67x10-27 kg neutron 1.67x10-27 kg electron 9.11x10-31 kg Charge +1 0 -1 The Fundamental unit of Matter – the Atom: Atom Proton Neutron Electron Size 1 fm 1 fm 0 (1 fm = 10-15m) Nucleus The Nucleus: Discovered by James Chadwick, 1932 Made up of neutrons and protons, collectively called ‘nucleons’ A X Z X = Element name A = Atomic mass number Z = Atomic number Same Z, different A => ‘Isotopes’ Atomic wt (12C) = 12.0 amu Example: 12C and 14C Atomic Number (Z) = number of protons Determines which chemical element Atomic Mass Number (A) = # of n’s + # of p’s Determines which isotope How is the nucleus held together? => Strong Force! PE • Strong • Short range attraction • Hard-core Repulsion r The Valley of Stability: Light Elements N=Z Example: 16O (N = 8, Z = 8) Strong -DPE ~ A Electric +DPE ~ Z2 (other corrections from surface area, and from neutron+ proton ratio) Heavy Elements N>Z Example: 197Au (N = 118, Z = 79) Most light nuclei have the same number of neutrons as protons. Binding Energy per Nucleon: (In the valley of stability) 56 A DPE/A One of the Most Important Plots in the Universe! Too Much Surface Too Much Coulomb PE 8 Best Bound Nuclei: DPE / A aV A aS A 2/3 56Fe, 62Ni Z2 (Z N )2 aC 1/ 3 a A A3 / 4 A A Light Elements Fusion aV=15.68 aS=18.56 aC=0.717 aA=28.1 = 34 Fusion: combining light nuclei to build up toward 56 DPE/A A 56 The universe is 94% H, 6%He, traces of 7Li Fe Energy release in the fusion process is 8.70 MeV/nucleon. (It does, however, take several steps to release all that energy) Thermonuclear weapons are 103 more powerful than A-bombs 8 1 “Abridged” Stellar Burning: H 1H 2H e 2 H 2H 4He energy 4 He 4He 8Be 8 Be 4He 12C C 4He 16O 12 O 12C 28Si 16 28 Si 28Si 56Fe Hydrogen burning Helium burning C+O burning Silicon burning Where do the Heavy Elements Come from? => SuperNovae! Consider a main sequence star approachng the end of it’s life…. Gravity is trying to collapse the star. Thermal pressure and electrostatic repulsion of the iron nucleii support the core. When enough inert iron builds up in the core, the core cools, the iron nuclei are forced together, … they touch => BOOM! • The strong force is ‘turned on’ • The core becomes one huge nucleus => neutron star • Recoil => mantle of the star is blown off • R-process of nucleosnythesis => forms the heavy nuclei, rapid neutron capture and b decay Formation of the Planets: and their elemental compositions • Start with heavy elements coalescing… coffee talk… gravitationally capturing atoms • Mass of the planet determines the mass of the molecules which can be captured • lighter molcules • Earth captures AMU~18 O2=32, N2=28, Si=28, Al=27, H2O=18, Ne=20 (Most terrestial rock is composed of Si and Al) • Mars captures CO2 (mass 44), rocks are mostly iron ores. • Jupiter captures Hydrogen => protostar… No fusion. • Heavy Elements in the core • Radioactive decays heat the core => this is where ALL He gas comes from • Moon… We know the moon did not form independently => lunar rocks are SiO and aluminates. Relative Abundances of the Elements in the Earth’s Crust: Trace Elements Trace Element Analysis The Wave Nature of Matter: 1923 Arthur Compton describes photon scattering (Compton Scattering) Must conserve momentum: g’ g Relativistically e- pe gmv Ee gmc2 P v 2 E c P 1 Pg hf h Pg For a photon: E c hf c l • 1923 Louis deBroglie proposed that le = h/p • 1927 Davisson and Germer demonstrated electron diffraction on a crystal of nickel. Main Concept => Wave nature of Matter Quantum Mechanics (Basics): Consider a particle in a 1D “box” Like a standing wave! Wavefunction MUST go to zero at the boudaries. Etot = KE = (1/2)mv2 = n2h2/(8mL2) E1 = h2/(8mL2) E2 = 4 E1 l = h/p = h/mv v = h/ml = 2nh/mL E3 E3 = 9 E1 • Quantized energy levels. • Need energy to change levels. nl/2 = L g E2 E1 ground state The Nature of the Atom: • 1911 Ernest Rutherford observed backscattered alphas, which indicted that there was a concentrated positively charged nucleus. • 1913 Niels Bohr --- Model of the Atom r +Z e Fcent Felec mv 2 kZe2 2 r r Assume the states are quantized => Standing waves around the orbit circumference = nle h h 2r n n p me v Quantization leads to discrete energy levels. The exact energies of the transitions are unique to each given element. Energy Levels for hydrogen The Periodic table is defined by the electronic structure of the atoms. Alakine Metals Alkai Earths Group determines the chemistry Transistion Metals Rare Earth Metals Noble Gases Halogens Classification of elements •periodic table compactly shows relationships between elements features of the periodic table •Periods are horizontal rows on the table. •Groups (or families) are columns on the table. All have similar chemical properties. •Blocks are regions on the table. •important groups: •alkali metals (Group IA, first column ) •soft, extremely reactive metals react with cold water to form hydrogen gas form +1 ions •alkaline earth metals (Group IIA, second column): •soft, reactive metals, compounds are a major component of earth's crust, form +2 ions •halogens (Group VIIA, next-to-last column): •poisonous and extremely reactive nonmetals, all form -1 ions •fluorine and chlorine are yellow-green gases •bromine is a volatile red-brown liquid •iodine is a volatile blue black solid •noble gases (Group 0, last column) •all are monatomic gases, a. k. a. inert gases; almost completely unreactive •Important blocks: •transition metals are the elements in the region from the third to twelfth columns. •hard, dense metals, less reactive than Group IA and IIA •rare earth metals are the elements in the annex at the bottom of the table. •lanthanides (annex, top row), actinides (annex, bottom row) •main group elements are all elements except the transition and rare earth metals. •group numbers end with "A" •metals, nonmetals, and metalloids (semimetals) Bonds: Molecules and Crystals • Ionic versus Covalent Bonds • Molecule - simple vs. complex • Crystals • Solids and Liquids • Organic vs. Inorganic Microscopes Lens: Converging (+f) and Diverging (-f) “Projector” o>f => Real Image, Inverted M = -i/o “Magnifying Glass” o<f => Virtual Image, upright M = -i/o Condenser Concave Lens (diverging, -f) Always Virtual Image, upright M = -i/o Thin Lens Equation: Lens are used to bend light. The refractive index of glass is 1.3. A single lens can give you a magnification of 10 to 40 without distortion. A compound scope uses two lens to get much higher magnification. You can multiple M1 by M2 to get Mtot. 1 1 1 o i f Compound Microscope Parts: Eyepiece Objectives Fine Adjustment Knob Power Switch Stage Diaphragm Base Body Tube Stage Clips Stage Stop Coarse Adjustment Knob Aperture Arm Light Source Schematic view of A compound scope. Use transmitted light for a transparent sample. The light illuminates from below. The condenser concentrates the light. It takes light that is dispersing and concentrates in on the sample. The aperature controls the total amount of light. The objective lens creates a magnified real image. The Ocular lens creates a magnified virtual image that your eye can see and interpret. The typical illumination of specimens in which light passes through the specimen and travels to you eye is called Bright Field microscopy. Elodea Comparison Microscope Has a split image. It consists of two coupled compound scopes. You are looking at two separate objects that you want to compare side-by-side. There is a bridge that connects the two scopes. You need to have the same magnification and lighting on both sides. Primarily used to compare bullets, fibers, or even to match up jagged pieces of glass. Stereomicroscope Two Compound scopes – no bridge to form a single image. Two independent images The goals is to create depth perception. The two scopes view the sample from slightly different angles. Best for viewing samples that have some shape to them – i.e. not just looking at a flat surface. Stereomicroscopes have characteristics that are valuable in situations where three-dimensional observation and perception of depth and contrast is critical to the interpretation of specimen structure. Polarizing Microscope You put polarizers where the condenser would be on a normal compound scope. The polarizers select a specific electromagnetic orientation. You adjust the polarizers to see how the colors of the field changes. Best for identifying certain types of polymers and glasses. Scanning Electron Microscope • Totally different than a light microscope. • It focuses a high energy scan of electrons at a sample. One observes how the electrons scatter back. • One can probe extremely small things with the electron beam. • The beam scans across the surface and recreates the surface with extremely good depth of field. • One can do trace element analysis with the SEM. • Can be coupled with an x-ray detector for elemental analysis. Trace Element Analysis We want to determine the distribution of trace elements. These are elements heavier that iron. The “trace element finger print” is unique to materials that come from a single source. These trace elements are on the ppb level. If you can identify the trace elements, you can identify the source of material destructive • Atomic Emission Spectroscopy • Atomic Absorption Spectroscopy • Neutron Activation Analysis • SEM Atomic Emission Spectroscopy • AES is a destructive technique. One vaporizes the sample by using an electrical arc. This ionizes the sample causing it to emit light a frequencies characteristic of the elements in the sample. You can determine the elemental distribution in the sample, but you do not know anything about the molecular structure. • The technique will tell us what the trace elements used to be in the sample. Atomic Absorption Spectroscopy • AAS is more sensitive than AES as the elements will not be obscured. As above, the sample is vaporized. You shine a light source with a known and calibrated frequency into the vaporized sample. When you want to look for a specific trace element, expose the vapor to a specific wavelength and determine the amount of absorption. Neutron Activation Analysis • NAA is a non-destructive technique. The sample is placed inside a region of high neutron flux (like the core of a nuclear reactor). The bath the sample in neutrons. The nuclei in the sample will absorb some of the neutron, leaving unstable radioactive elements which decay with characteristic gamma ray emissions. Use of a high precision gamma detector to acquire the spectrum will allow one to determine the distribution of all trace elements in a single shot Scanning Electron Microscope • SEM’s are becoming quite common. You can do a lot with an SEM. SEM uses x-ray emission spectrocopy. You bombard the sample with an energetic electron beam (cheaper than a nuclear reactor). The electrons excite the atoms in the material. The atoms emit their characteristic xrays. A detector picks up the x-rays from which we can determine the elemental composition. • One can both ID the trace elements, and determine their physical location on the surface of the sample. Analysis of Compounds • UV Spectrophotometry • IR Spectrophotometry • GC Mass Spectroscopy • X-ray Diffraction UV Spectrophotometry • Spectrophotometry the absorption of specific frequencies of light. You start with a bright, broad frequency light source, then pass it through a prism; this breaks the light into its component frequencies. You pass this dispersed light through a narrow slit to get a particular frequency. You can then rotate the prism to take a measurement at a different frequency and thereby determine the absorption as a function of frequency. • UV spectrophotometry gives a gross absorption curve; you are not exciting specific modes; you are degrading the material which you are looking at, because your are breaking covalent bonds. The complex molecules of heroin, sugar and cocaine are all white powders. You can do a gross ID with UV spectrophometry, you should do a final ID using IR spectrophotometry. • UV spectra and Visible spectra can be used to identify an unknown compound by a comparative analysis. One can compare the UV or Visible spectra of the unknown with the spectra of known suspects. Those that match are evidence that they could be one and the same. However using a match on UV or Visible is not conclusive. Usually IR and Proton NMR spectra must nail down the exact identification. IR Spectrophometry • The IR spectrophotometer uses IR light to excite vibrational bands in complex molecule. These absorption spectra provide quick positive ID for complex organic molecules whose “IR fingerprints” are stored in its memory. GC Mass Spectrometry • Mass spectrometer is usually used in conjunction with gas chromatography. The result of the GC goes through an ionizer where it is bombarded by a high energy electron beam. This beam breaks the complex molecules like heroin into a standard set of chunks (fragments). The ionized samples then go through a magnet that separates the fragments based upon their mass. A detector picks up the fragments of a certain mass. By adjusting the magnetic field, one can determine the fragment mass spectrum. This is usually done with complex organic molecules. Inorganic molecules do not create complicated bonding structures. X-ray Diffraction • ID’s specific inorganic crystals. Can also be used on organic molecules that one can crystalize (i.e. you can crystalize DNA etc.) • Use an x-ray in the nm range to illuminate the sample. Reflections off the different layers will yield bright spots at specific angles. The pattern of spot from an x-ray diffraction measurement allows one to ID specific substances the pattern is unique to each crystal. END OF LECTURE Radioactivity: the quest for a more stable state 1896 Discovered by Henri Bequerel => Researched by the Marie and Pierre Curie a => 4He nuclei b+/- => e+/g => high energy photons bbb aaaa N(t) = N0e-lt T1/2 = ln(2)/l a and b decay change the nuclear species. g’s are emitted during internal restructuring of the nucleus, NOT changing A or Z. Fission: when a and b emission just isn’t fast enough! 235U + n => two neutron rich fragments and several neutrons U Kr Ba Average energy release ~ 1 MeV/nucleon Chain Reaction… 235U is pretty stable. 236U is NOT. Add a neutron to 235U and it immediately fissions, creating several extra neutrons... Hiroshima: 104 Joules or 20 kilotonnes of TNT from 3 moles of 235U fissioning rewrite Go back to nh v me 2r mv 2 kZe2 2 r r 2 2 2 2 nh kZe me r me 2r rn n 2 h 2 4 2 kZe2 me Etot KE PE 1 2 kZe2 1 2 1 2 2 Etot mv mv mv mv 2 r 2 2 2 2 1 me Z Etot 2 2kZe2 h 2 13.6eV n 2 n Main Concept: Demonstrates quantization of energy, wave nature of matter, and structure of the atom. Law of Reflection: qi=qr Reflection: “Specular Reflection” from a smooth surface Incident ray qi qr DEMO: Optics Board Reflected ray Surface “Diffuse Reflection” off a rough surface Often, not all light is reflected from a surface => Reflectivity - Dependent on frequency of light - Dependent on angle of incidence - Dependent on polarization What happens to the Light that is NOT Reflected? 1) Absorbed 2) Refracted opaque transparent Neutral filters translucent Varies w/ frequency q1 Wavefronts, l apart Incident rays n1 n2 l = v/f v1=c/n1 v2=c/n2 q2 Snell’s Law: n1sinq1 = n2sinq2 Nvacuum nwater nglass ndiamond = 1.000 = 1.329 = 1.500 = 2.417 Total Internal Reflection q2 qi qr Low n to high n => Bent towards normal High n to low n => Bent away from normal The Critical angle (qc): n1sinqc = n2sin90 sinqc = n2/n1 qc = arcsin(n2/n1) DEMO: Optics Board Optical Systems: (Mirrors and Lens) Rays diverge • Object Converges diverges, bends reflects light • Optical system A point from which rays diverge or appear to diverge • Image You • Observer object Your eye expects to see diverging rays from an object. No optical system. No “image” rays Observer (focuses diverging rays only) Now Add an Optical System: Focus Real Image Virtual Image Reflect and De-Focus Optical system Defocus Virtual Image Reflect and Focus Real Image Images: Our eyes focus diverging rays…. Our eyes can not distinguish: an object, a real image, or a virtual image. • Eye can see after the image • Can Focus on screen • Eye can see • Can NOT Focus on screen The Simplest Optical System -- The plane mirror o i Reflected virtual image Spherical (or Parabolic) Mirrors: A parabolic mirror will focus parallel light to a single point = the “focal point” f focus The mirror is characterized with a given “focal length”, f. From Pre-Calculus: ( x f ) 2 y 2 x f y 2 4 fx y directorix Incident para-axial rays (-f,0) focus (f,0) x Ray Tracing: 1) 3) 2) i o f With Mirror or Lens: Typically draw 3 Rays: 1) Parallel Ray reflected thru focus 2) Ray thru focus reflected parallel 3) Ray to center qi=qr for reflection Thin Lens Equation: In this case, o>f => real inverted image Magnification = -i/o 1 1 1 o i f Positives and Negatives: 1) In this case, o<f, i must be negative => virtual upright image Object distance (o): always positive Image distance (i): positive if same side as viewer 3) f Magnification = -i/o o i Focal length (f): positive if converging lens/mirror negative if diverging lens/mirror






