CH08/chapter8.ppt
advertisement

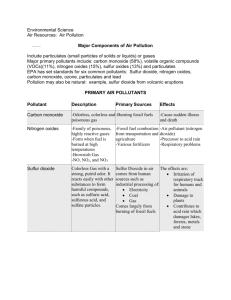
Pollution from Energy Generation and Energy Use Air Pollution in Eastern Europe Atmospheric Physics Sources of Air Pollution Air Quality Standards Energy in the News OPEC Price Changes Lackner Lecture on Friday Sustainable Energy Roundtable (Thursday) Air Pollution in Eastern Europe Trabant: East German car Radioactive waste dumps in Moscow Air pollution in Moscow No information on health under communism Copsa Mica, Romania; most polluted city in the world Composition of Normal Air Pollutants: parts of normal Air that are added by humans And are toxic to animals, Vegetation or property Two main components of Air are….. Air Pressure We live at the bottom of an ocean Of air. Pressure = force per unit area Pressure =mgh/area Units of pressure=psi=lb per square Inch OR newtons per square meter 1 Newton per square meter = 1 Pascal (MKS system) Air pressure changes as a function Of elevation- How? A Simple Barometer Atmospheric Pressure Although you can’t see it, the atmosphere exerts Pressure on you Examples: 1) suction cup holding a tile 2) glass of water upside down with paper on top 3) sucking water through a straw Other examples? A Barometer Temperature Decrease with Elevation PV=nRT (ideal gas law) Pressure * Volume = number of moles * R * Temperature If decrease pressure, what happens to temperature? Adiabatic lapse rate: 7 degrees C per kilometer, Or about 3.5 degrees F per thousand feet Buoyant Force A solid object will float if its density is equal to or less than the density of the medium it is in. So: light air rises and heavy air sinks. What determines if air is light or heavy? Normal Air Temperature Profile Y axis:elevation Elevation increases Towards the top X axis: temperature Temperature increases Towards the right What do all profiles Have in common? Unusual Air Temperature Profile X:Temperature increasing To right Y: Elevation Increasing Towards the top What is the common feature of All of these Temperature Profiles? Temperature Inversion What you get when there is a hot layer Of air on top of colder air. Hot layer: lid that traps pollutants Why? Air Pressure Systems Why does warm rising Air produce lower Pressures Why does cold sinking air Produce higher pressures? Wind Systems What are the pressure differences at ground level Between a high pressure and low pressure system? Global Wind Systems Winds move pollutants Which direction do they Move pollutants in the United States (on average)? Form of Air Pollutants Aerosols: Small droplets of liquid suspended in a gas What are common aerosols that you encounter? (3 or more) Particulates: Small particles of solid material? Examples of common particulates? (3 or more) Does size of particulates matter? Why or why not? Particulates can be charged: they are then more likely to Adsorb other materials on their surfaces Types of Pollutants PM10- Particulate matter under 10 microns in size PM2.5-Particulate matter under 2.5 microns in size Carbon Monoxide-produced by incomplete combustion Sulfur Oxides-from burning of fossil fuels and oxidation of sulfur. Produce acid rain Pb (plumbum or lead)-heavy metal, neurotoxin Hg-(mercury)-also a heavy metal and neurotoxin Nitrogen Oxides-from high temperature combustion,also source Of acid rain VOC-volatile organic compounds, also O3-Ozone Biggest < 10 micron Particulate Sources Power Co. 2-6 8-9? 18 20-22 25 Coal Mining 11 Non Attainment Areas PM10 Do Industrial Sources Dominate The Emission Of PM10 Why or Why not? Particulates:Why Size Matters Smaller particulates can enter the lungs and remain there. All particulate matter less than 10 microns in diameter can do this. Separation of matter less than 10 microns and less than 2.5 microns Is relatively new. Why? Difficult to measure size. What diseases are caused directly and indirectly by small particulates? (at least three) Black Lung Diseases Produced by high Concentrations Of small particulate matter: Black lung (emphysema) Asthma Lung cancer (Indirectly: congestive Heart failure) Trace Elements in Coal Which trace elements are most dangerous? Why? Urban Particulates and Dust Concentrations normalized to Al (that is Aluminum Concentration is set to be 1.) This is because most common weathering product of crustal weathering is clay. All clay minerals are rich in Al. What dangerous elements are Enriched by over 100 times? Relative Contributions to City Dust Minor constituents Major constituents Which are most surprising? Sootprints All carbon is not alike !!! Gray:Carbon char Dark aqua: Carbon ash Purple:Carbon from gas turbine Light Blue:Carbon from high pressure Diesel engine Red:Carbon from medium pressure Diesel engine Green: carbon from idling diesel engine Dark Blue: amorphous carbon Energy loss in electron volts: product Of thermal history of carbon Why is this work important? Largest Pb Emission Sources Power Co Nos: 5-7 10 15 17 Others: Steel GE Navy Pb non attainment areas Do Industrial Sources Dominate Pb Emission? Why or Why Not? Largest VOC Emissions Sources Oil Company Nos: Including Refineries 2-4.6, 15,16,23 Electricity 9,18 Natural Gas 10 VOC Sources Oil Refineries Cars Methane from decay (also termites) Aromatic compounds from trees= terpenes e.g. pine, eucalyptus Largest SO2 Emissions Sources Power Plant Nos: 1-25 What Fossil Fuel Do These Plants Use? Non Attainment Areas Sulfur Dioxide Do industrial Sources Dominate SO2? What Else is controlling SO2 Pollution Levels? Sources of Sulfur Dioxide Coal (sulfur content variable) Volcanoes Hydrogen sulfide gas from decay of organic matter Oil (sulfur content variable) Effects of Sulphur Dioxide on Health At high exposure levels and/or longer durations of exposure To sulphur dioxide: 1) airway resistance increases (harder to breathe) 2) health of bronchitis patients declines 3) number of hospital admissions increases 4) number of deaths from heart attacks increases 5) incidence of heart diseases increases How Sulfur Oxides Produce Acid Rain 2SO2+O2= 2SO3 SO3+H2O=H2SO4 Sulfate Deposition pH 2000 What areas have the highest pH in rain? Why? pH 1994 Has there been improvement since 1994 in acid rain? Where? Fish Populations in Adirondacks What changes Have occurred In fish stocks? Why? (also geology) (projections for Future) Largest NO2 Emission Sources Power Plant Nos: 1-25 Are these Old or Modern Plants? Why? How Nox and VOC Produce Smog Photodissociation of NO2: Light energy +NO2 = NO + O O + O2 = O3 O3+NO = NO2 + O2 Ordinarily an equilibrium Volatile organic compounds disturb this equilibrium By producing organic radicals that are strong oxidizing Agents. Result is SMOG. Strong oxidizing agents:prevent the destruction of ozone Therefore more ozone accumulates. What would a strong reducer do? Effect of Time of Day on Pollutants (LA basin) Why does ozone Peak later in the day than Nitric oxide and Nitrogen dioxide? Largest Carbon Monoxide Stationary Sources Chemical Plant Nos: 5,10 16-18 22 (not all Organic) Others: Steel Paper Aluminum Non Attainment Areas: CO Are Industrial Sources Dominating CO? Why or Why not? Why do western States Dominate? Total Precipitation: 2000 Does rain Wash CO Out of the Air? Or it is Legislative? (gasoline Additives MTBE) Mercury: Sources of Pollution What is the best way to Reduce the overall amount Of Mercury pollution? What does mercury do? (Alice in Wonderland) (methyl mercury) Sources of Major Air Pollutants What pollutants Are dominated By stationary Sources? By moving Sources? Indoor Air Pollutants US National Air Quality Standards Particulate Levels in Different Cities Moscow (personal Experience) Truck drivers in Moscow Russian truck and car factories Personally Imposed Pollution Toxicity Definitions: Are they relevant To these products? More Personal Choices New Silent Spring: Our Stolen Future by Theo Colbourn et al