Electrochemical and Oxygen Reduction properties Graphene Nanoflakes (FLGs)
advertisement

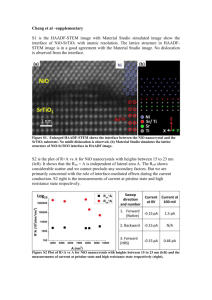
Supplementary Information Electrochemical and Oxygen Reduction properties of Pristine and Nitrogen Doped Few Layered Graphene Nanoflakes (FLGs) Navneet Soin†, §, *, Susanta Sinha Roy‡, Surbhi Sharma±, Thomas Thundat⊥ and James A. McLaughlin† † Nanotechnology and Integrated Bioengineering Centre (NIBEC), University of Ulster at Jordanstown, Shore Road, Newtownabbey BT37 0QB, United Kingdom ‡ Department of Physics, School of Natural Sciences, Shiv Nadar University, Gautam Budh Nagar, 203207, Uttar Pradesh, India ± School of Chemical Engineering, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom ⊥Chemical and Materials Engineering Department, University of Alberta, Edmonton, Alberta T6G 2V4, Canada § Present address: Institute of Renewable Energy and Environmental Technologies (IREET), Knowledge Centre for Materials Chemistry (KCMC), University of Bolton, Bolton BL3 5AB, United Kingdom Corresponding Author soin-n@email.ulster.ac.uk S1. SEM and TEM analysis of pristine and N-doped FLGs: Figure S1 shows the SEM images of pristine FLGs grown for various time durations. The TEM images of pristine and N-doped samples along with their corresponding line profiles are shown in Fig. S2, where the enhancement in the defects can be clearly observed. HRTEM analysis for N-doped samples confirms the structural and morphological changes occurring upon ECR treatment. Nitrogen doping in graphitic structures has been shown to produce pentagonal defects in the native hexagonal lattice [1-3]. These pentagonal defects cause graphene sheets to distort severely with the bending of graphene layers leading to structures with high curvatures and cross-linked structures. While, the pristine FLGs show highly parallel planes with a regular d spacing; the N doped samples exhibit large fluctuations in d spacing and appear severely distorted as shown in Figure S2. Fig. S1: SEM images of FLGs grown for (a) 30 sec., (b) 60 sec., (c) 600 sec. Fig. S2: HRTEM image of (a) pristine FLGs, showing regular and parallel basal planes, (b) 600 sec. N doped FLGs, showing distortion and buckling of planes. Inset shows line profiles of highlighted areas. S2. Raman spectroscopy of pristine and nitrogen doped FLGs: One of the easiest ways of identifying crystalline and disordered materials is through Raman spectroscopy. For amorphous materials, not only the FWHM of the bands is much larger than the crystalline material but also the 2D peak is completely absent [4]. Upon nitrogen doping, an increase in the disorder of the material can indeed be observed in the TEM images (Fig . S2) as well as the comparative Raman spectra of pristine and N-doped FLGs (Fig. S3). For pristine samples grown for 60 s, the FHWM of G band is nearly 23.8 cm -1, indicative that FLGs have a high degree of graphitisation. Also, the FWHM of G` (2D) band along with the absence of graphitic shoulder is one of the indicators of a FLG system [5-7] The calculated FWHM of G` band is around 43.3 cm -1, which lies within the range of 35-50 cm-1 observed for few layered graphene systems [5-7]. For pristine FLG nanoflakes grown at 60 s, the value of ID/IG obtained is around 0.55 while the I G/IG` ratio is nearly 1.1. These values of FWHM and ID/IG obtained are significantly smaller than those from previously reported structures such as carbon nanowalls; nanoflakes etc. which usually tend to show a very strong D band owing to their small crystallite size [8]. For N2 plasma treated samples, the ID/IG ratio increased monotonically from approx. 0.55 to about 0.65. This is accompanied by decrease in the I G`/IG ratio from approximately 1.1 to 0.6. In addition to the differences in the I D/IG and IG`/IG ratios, the spectra also exhibit a marked broadening of Raman D band for N doped samples. While for pristine FLGs, the D band shows a narrow FWHM of 30.7 cm -1, upon N doping this value increases to nearly 43 cm -1, implying a larger distribution of the nature of defects and an enhanced defect density. In the case of G band the change in the FWHM was not very prominent with a from 23.4 cm -1 to 24.5 cm-1. The increase in the FWHM of the bands is in agreement with the studies of Cuesta et al., that indicate first order band broadening correlates strongly with the degree of graphitic disorder [9]. Similar effects were observed by Gohel et al. in their study of N 2 plasma treated multiwalled carbon nanotubes [10]. Alongwith the change in the FWHM of the bands, a slight upshift in the D band can also be observed from approx. 1329 cm-1 to 1332 cm-1. This upshift in band positions has been observed previously as well and is attributed to appearance of new and enhanced disorder in the structure. The effects of N 2 plasma treatment can also be observed in the second order Raman spectra. In the second order Raman spectra; the change in the I G/IG` can also be observed implying a change in the crystallinity of the structure. While for pristine FLGs, the I G`/IG ratio is approx. 1.1, this value reduced monotonically to approx. 0.6 with the increase in the plasma treatement time. The increase in this ratio is indicative of the increase of disorder and a reduction in the overall crystallinity in N doped FLGs [9-11]. Hence, both the first and second-order Raman spectra suggest an increase in the disorder of N doped FLGs as observed in the HRTEM studies but do not lead to amorphisation, not at least at these levels of nitrogen doping. Fig. S3: Raman spectra of pristine N-doped FLGs. The dotted lines show the upshift in the peak positions of the Raman bands. S3. Electrochemical properties of nitrogen doped FLGs: The cyclic voltammetry response of pristine FLGs grown for 60 s and ECR treated FLGs (treated for 600 s) is shown in Fig. S3. A significant increase in the current response was observed in the case of N-doped FLGs as compared to the pristine FLGs while the value of ΔEp increased slightly from 63 mV to 70 mV. The increase in the ΔEp values can be attributed to the increase of the oxygen content [1]. However, the reaction on the electrodes are still semi-infinite diffusion controlled in nature as shown by the linear nature of peak current vs. the square root of the scan rate (Fig. 6.9 (b)) [2]. The increase in the current density can be ascribed to an increase in the electronic DOS as well as an increase in the disorder creating larger number of electrochemical sites. The increase in the disorder is also observed in the TEM images and Raman spectroscopy results shown above and can be profitably used for enhanced electrochemical sensitivity. Similar results were reported by Chen et al and Bandaru et al in their study of electrochemical behaviour of nitrogenated carbon nanotubes [2, 12]. Fig. S4: (a) CV curves of pristine and ECR treated FLGs in 5 mM K4Fe(CN)6 in 1M KCl, (b) peak current vs. square root of scan rate. S4. Oxidation of Hydrogen Peroxide In addition to ORR, the electrocatalytic reduction of H2O2 was also explored on pristine and N doped FLGs. Figure S5 (a-c) shows the electrochemical response of pristine and N-doped FLGs towards various concentrations of H2O2 ranging from 1 mM to 5 mM (in Phosphate Buffered Saline). Prior to running the scans, the solutions were purged with N2 for at least 30 minutes prior to running the scans and N2 blanket upon the solution was maintained throughout the scans. The peak potential for the reduction of H2O2 on pristine FLGs is approximately -0.55 V whereas the peak potential for N-doped graphene is only -0.28 V. Since the N-doped FLGs showed excellent ORR behaviour their high sensitivity towards the reduction of H2O2 is expected [13]. Fig. S5: CV scans of (a) pristine and (b) N-doped FLGs in N2 saturated PBS solution with different concentrations of H2O2, (c) comparison of pristine and N-doped FLGs towards addition of 5mM H2O2 at 10 mVs-1 scan rate. For pristine FLGs a weak response to 5 mM H2O2 was observed at -0.55 V, with the current density being -0.045 mAcm-2. However, at 600 s N-doped FLGs for the same 5 mM H2O2 the reduction current density was as high as -0.11 mAcm-2 at -0.28 V. Thus, on N-doped FLGs the reduction peak was shifted by almost 270 mV, which can be ascribed to the effects of N-doping on FLGs. In fact on pristine FLGs, no detection below 3 mM H2O2 was possible whereas Ndoped FLGs showed a strong response to the presence of even 1 mM H2O2. The literature reports that the for the electrocatalytic reduction of H2O2, the breaking of O-O bond is easier at the surface of N-doped graphene as compared to pristine graphene [13]. This is because of the enhanced charge delocalisation caused by the higher electronegativity of the N atoms, which cause the formation of a partial positive charge on the carbon atoms [13, 14]. An increase in the defect density and the localised electronic DOS is observed upon N-doping. It is believed that these very reasons that promote the high ORR activity on N-doped FLGs also provide enhanced sensitivity towards the detection of H2O2. References: [1] X. Ji, C. E. Banks, A. Crossley and R. G. Compton, ChemPhysChem, 2006, 7, 1337-1344. [2] Y. G. Lin, Y. K. Hsu, C. T. Wu, S. Y. Chen, K. H. Chen and L. C. Chen, Diamond Relat. Mater., 2009, 18, 433-437. [3] N. Soin, S. S. Roy, S. Roy, K. S. Hazra, D. S. Misra, T. H. Lim, C. J. D. Hetherington, J. A. D. McLaughlin, J. Phys. Chem. C, 2011, 115 (13), 5366-5372. [4] A .C. Ferrari, Solid State Commun., 2007, 143, 47-57. [5] A. Malesevic, R. Kemps, A. Vanhulsel, M.P. Chowdhury, A. Volodin, A.; C. Van Haesendonck, J. Appl. Phys., 2008, 104, 084301. [6] A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth, A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401. [7] N. Soin N, S. S. Roy, C. O’Kane, T. H. Lim, C. J. D. Hetherington, J. A. D. McLaughlin CrystEnggComm 2011, 13, 312-318 [8] Y. Wu , B. Yang , B. Zong , H. Sun , Z. Shen, Y. Feng, J. Mater. Chem., 2004, 14, 469. [9] A. Cuesta, P. Dhamelincourt, J. Laureyns, A. Martinez-Alonso, J. M. D. Tascon, Carbon, 1994, 32, 1523-1532. [10] A. Gohel, K. C. Chin, Y. W. Zhu, C. H. Sow, A. T. S. Wee, Carbon, 2005, 43, 2530-2535. [11] N. Soin, S. S. Roy, S. Roy, K. S. Hazra, D. S. Misra, T. H. Lim, C. J. D. Hetherington, J. A. McLaughlin, J Phys. Chem. C, 2011, 115, 5366-5372 [12] M. A. Hoefer, P. R. Bandaru, J. Appl. Phys. 2010, 108, 034308 [13] Y. Wang, Y. Shao, D. W. Matson, J. Li and Y. Lin, ACS Nano, 2010, 4, 1790-1798. [14] L. Qu, Y. Liu, J. B. Baek and L. Dai, ACS Nano, 2010, 4, 1321-1326. Acknowledgements: The authors would like to thank Mr. W. Hayes at the University of Ulster for his help in some of the electrochemistry experiments.