Chapter 1 Problems.doc
advertisement

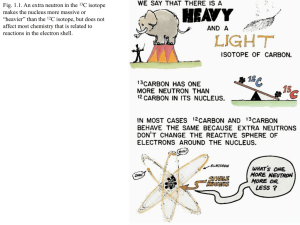
Problems for Chapter 1 1. True or false: Isotopes are forms of the same element with the same number of neutrons and electrons, but different numbers of protons. 2. True or false: Working with stable isotopes of the HCNOS elements needs special permits and licensing due to radioactivity hazards. 3. True or false: The heavy isotopes of an element are always rarer than their light isotope counterparts. 4. True or false: Most modern instrumentation is not equipped to detect isotopes, with the exception of mass spectrometers and some laser systems. 5. True or false: If the human in Fig. 1.3 were composed exclusively of light isotopes, that human would be 10% lighter than the human depicted. 6. True or false: The element fluorine has only one stable isotope form, while the element chlorine has two stable isotopes. 7. True or false: Sulfur has more stable isotopes than does hydrogen or nitrogen. 8. True or false: Early scientists who discovered stable isotopes in the late 1880s were awarded Nobel prizes. 9. True or false: Stable isotopes are similar forms of the same element, differing in the number of neurons and spin orientation. 10. True or false: Ecologists can freely add stable isotopes to field experiments with little cost or effort, but interpretation of the isotope addition experiments is not always simple.