` CALIFORNA STATE UNIVERSITY, NORTHRIDGE LIPID MEMBRANE-WATER PARTITIONING OF
advertisement

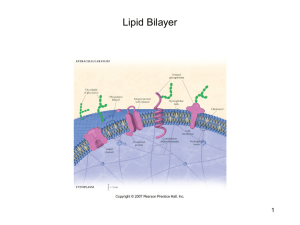
` CALIFORNA STATE UNIVERSITY, NORTHRIDGE LIPID MEMBRANE-WATER PARTITIONING OF LYSOPHOSPHOLIPIDS AND FATTY ACIDS A Thesis submitted in partial fulfillment of the requirements For the degree of Master of Science in Physics By Yasmin Alaee May 2014 ` The thesis of Yasmin Alaee is approved: Dr. Say-Peng Lim Date Dr. Yohannes Shiferaw Date Dr. Radha Ranganathan, Chair Date California State University, Northridge ii ` DEDICATION To my idols in life, Sadegh and Mariam Roohi Alaee Everything I have in my life, I owe it to you two. Thanks for everything! iii ` ACKNOWLEDGEMENT I am indebted to my thesis advisor Professor Radha Ranganathan, for her guidance on my work. It has been a great pleasure to work under her supervision who has always been kindly available to help me and spend time to advise me. I would like to express my appreciation to my committee members, Professor Say-Peng Lim and Professor Yohannes Shiferaw for their kind assistance and instruction which helped me in completion of this study. I would also like to thank Professor Miroslav Peric, my graduate advisor, for his advices throughout my graduate program. Special thanks to Dr. Jasmeet Singh who helped me to learn all the scientific techniques used in this project, without his instruction and supervision, completion of this work was impossible. I would also like to thank Ms. Carole Arciero, for her all time assistance through my Master’s program. Furthermore I would like to acknowledge the love of my life, Payman Pakzad, whose love and passion has always given me hope for brighter future. He always stood by me and gave me courage to move forward. In addition, I would like to thank Eli and Hadi Alaee, my little sister and brother, for being the greatest part of my life. You two are the source of love, joy and energy in my life and I can’t imagine my life without you. Finally, I would like to acknowledge the loving memory of Baba Joon, without you in my life, I wouldn’t be this person. You are greatly missed. iv ` TABLE OF CONTENTS Signature Page ii Dedication iii Acknowledgements iv Abstract vi CHAPTER 1: INTRODUCTION 1.1.Cell Membrane 1 1.2.Phospholipids 2 1.3. Phosphatidylcholines 4 1.4.This project 7 1.5.Brief Summary of Methodology 9 CHAPTER 2: THERMODYNAMICS OF PARTITIONING Theory 10 CHAPTER 3: MATERIALS AND EXPERIMENTAL METHODS 3.1. Materials 3.1.1. Lipids and Solutes 14 3.1.2. Fluorescence Probe 14 3.2. Experimental Method 3.2.1. Fluorescence Assay 14 3.2.2. Samples Preparations 17 v ` CHAPTER 4: EXPERIMENTAL RESULTS 19 CHAPTER 5: DISCUSSION AND CONCLUSION 5.1. Discussion 34 5.2. Conclusion 38 REFERENCES 40 vi ` ABSTRACT LIPID MEMBRANE-WATER PARTITIONING OF LYSOPHOSPHOLIPIDS AND FATTY ACIDS By Yasmin Alaee Master of Science in Physics The partition coefficients for the distribution of lysooleylphosphatdylcholine (LOPC) and oleic acid (OA) between water and dioleylphosphatidylcholine (DOPC) bilayer at temperatures of 5 C, 15 C, 37 C and 50 C were determined using a fluorescence assay. The enthalpy and entropy of the partitioning were determined from the temperature dependence of the partition coefficient. The Gibbs free energy decreases and entropy increases upon partitioning into the bilayer for both LOPC and OA, in the temperature region of study. The enthalpy changes of LOPC and OA partitioning are significantly different. LOPC partitioning into the bilayer is endothermic (increase in enthalpy) and OA partitioning is exothermic. This means that LOPC partitioning is entirely driven by entropy. Both enthalpy and entropy contribute favorably to OA partitioning into the lipid bilayer. Entropy contributions for both LOPC and OA are mainly due to the hydrophobic effect of the long hydrocarbon chains of these molecules. The difference between LOPC and OA in the enthalpies of partitioning is due to the difference in the polar characters of their head groups. vii ` CHAPTER 1: INTRODUCTION 1.1.Cell Membrane The Cell is the smallest unit of life that can replicate independently, which is why they are usually called ‘building blocks of life ’. It is the basic biological and functional unit of all known living organisms. A cell consists of various parts such as nucleus, ribosome, endoplasmic reticulum, lysosome, cell membrane, cytoplasm, microtubules, vacuoles, chloroplast and mitochondria. Structure and function of cells depend strongly on cell membranes. Cell membrane is a thin and semi-permeable membrane which surrounds the cytoplasm of the cell to protect the integrity of the cell’s interior by allowing certain substances to enter the cell environment and keeping others out through special protein channels. Cell membranes are involved in variety of cellular processes such as cell signaling, ion conductivity and cell adhesion, thus they have important roles in our body. The primary component of a cell membrane is a bilayer of lipids interspersed with proteins. Lipids can make up to 20 to 80 percent of the membrane, depending on membrane’s location and its role in our body. Lipids are responsible for the membrane’s flexibility. In this study we are mainly concerned with the lipid part of cell membranes. A schematic model of a section of the lipid membrane is shown in figure 1. Figure 1.Phospholipid Bilayer 1 ` In general, cell membranes consist of three kinds of lipids: phospholipids, glycolipids and sterols. They perform various physiological functions. Beyond their structural role, they have many functional roles such as serving as regulatory agents in cell growth and adhesion and increasing enzymatic activities of enzymes. Depending on the type of cell, the amount of the mentioned lipids is different but the most abundant types are phospholipids. 1.2.Phospholipids Phospholipids are major components (75 %) of cell membrane. The structure of phospholipid molecule consists of a hydrophilic head and hydrophobic tails as shown in figure 2. Figure 2.Chemical structure of a single phospholipid The hydrophilic part is called the head group and the hydrophobic part is a fatty acid hydrocarbon tail containing several methylene (CH2) groups and a terminal methyl (CH3) group. The head group contains a negatively charged phosphate group which is attracted to water due to ion-dipole or dipole-dipole interactions thus it interacts strongly with 2 ` water molecules. The hydrophobic tails consists of long fatty acid hydrocarbon chains. The chains have no charge so they will be repelled by water in an aqueous environment. Also, due to hydrogen bonding and dispersion forces, there are strong interactions between water molecules. This interaction along with the hydrophobic property of the fatty acid chain plays an important role in forcing the hydrocarbon chain out of the water (1). Phospholipids are thus surfactant molecules that exhibit dual properties of attraction and repulsion towards water. Due to this characteristic of phospholipids, placing them in an aqueous environment will cause the molecules to spontaneously arrange themselves such that the tails are shielded from the water. Resulting structures are typically vesicles (bilayers). A schematic diagram of hemisphere section of phospholipid vesicle is shown in figure 3. Diameter ≈ 49nm 4494949nm Figure 3.Hemisphere section of phospholipid vesicle In general there are five different types of phospholipid, which are phosphatidylserine, phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol and phosphatidylsphingomyelin. Their structure varies depending upon the element attached to the head and also which fatty acids make up the tails. Phosphatidylcholine is the major 3 ` component of cell membranes and it is very important for the liver and brain functions. Phosphatidylethanolamine is the second important phospholipids in animal and plant lipids. Phosphatidylinositol is present in all tissues and cell types, especially brain tissue. Although the amount of phosphatidylinositol compared to the other phospholipids is very low and in fact is a minor constituent of cell membrane. Phosphatidylserine is most concentrated in the cells of the brain and nerves. 1.3.Phosphatidylcholines Phosphatidylcholine (PC) is the most abundant phospholipid in animals and plants. Therefore it is the key building block of membrane bilayers. They are a class of phospholipids that incorporate a glycerol backbone connected to a phosphocholine head group with a variety of fatty acids (saturated or unsaturated) as the chains. The schematic diagram of the general structural formula of phosphatidylcholine is shown in figure 4. O C O CH2 C O CH O O Fatty Acid Chains H2C Ester Linkage Glycerol Backbone O P O O Phosphocholine headgroup Figure 4.General structure formula of Phosphatidylcholine 4 N ` In general the phosphatidylcholine molecule has a cylindrical shape. Due to its shape, being placed in aqueous environment it will spontaneously be forced to aggregate and form a bilayer, so it is ideally suited to serve as the main structural component of membranes. They are also the main phospholipid circulating in plasma as an integral component of lipoproteins, especially the high-density lipoproteins (HDL). Thus this kind of phospholipid is necessary for assembly and secretion of lipoproteins. Although they play an important role in HDL, they have strong influence on the level of all circulating lipoproteins especially very-low-density lipoproteins (VLDL). In addition to its function as an abundant component of membrane, it may also be responsible for signaling through diacylglycerols, especially in the nucleus. One of the most important physiological functions involving phosphatidylcholine is its catalytic hydrolysis by Phospholipase A2 (PLA2) (2,3). The result of this enzymatic hydrolysis is the breakdown of phosphatidylcholine into its two parts: Lysophosphatidylcholine (LPC) and Fatty acid (FA) both of which are involved in syntheses of lipid signaling molecules and several crucial physiological functions (3-5). LPCs are precursors for the platelet activating factor. The polyunsaturated fatty acid, arachidonic acid, liberated from hydrolysis of the corresponding phospholipid is the precursor for synthesis of the inflammation triggers, prostaglandins and leukotriene. A schematic diagram of the general chemical structure of LPC is shown in figure 5. 5 ` Figure 5.General chemical structure of LPC. R is the hydrocarbon chain. The removed fatty acid chain caused by the enzymatic hydrolysis consists of a chain of an even number of carbon atoms, with a carboxyl group (-COOH) at one end and hydrogen atoms at the other end. There are two types of fatty acids, saturated or unsaturated. In the saturated fatty acid, all the carbon-carbon bonds are single; they are saturated with hydrogen. Each carbon atom in a saturated fatty acid is bound to two hydrogen atoms since they have only single bonds. The schematic diagram of saturated fatty acid is shown in figure 6. Figure 6. Saturated fatty acid In the unsaturated fatty acids, one or more of the bonds are double or triple. There are two types of configurations for unsaturated fatty acids. If the adjacent hydrogen atoms are on the same side of the double bond, the cis configuration will occur. Due to the double bond rigidity in the cis isomer, the chain bends and restricts the conformational freedom of the fatty acid. If there is more double bonds in the chain in the cis configuration, the fatty acid will have less flexibility. This property of the cis bond, especially in a restricted 6 ` environment such as in a lipid bilayer, limits the ability of fatty acid to be closely packed, thus it will affect the melting temperature of the membrane. In contrast, if the two hydrogen atoms are bound to opposite sides of the double bond, the trans configuration occurs. In trans configuration, they do not cause the chain to bend much and they have a more similar shape to the saturated fatty acids. In general, trans configuration of fatty acids is not found in nature and they are a result of human processing such as hydrogenation. The schematic diagram of both cis and trans configurations of fatty acids are shown in figure 7. Figure 7.Unsaturated fatty acids; cis configuration vs. trans configuration fatty acids 1.4.This project This project concerns the distribution of LPC and FA between a phospatidylcholine model membrane bilayer and water. A schematic of the distribution is shown in fig. 8. Phospholipid LPC Figure 8b. Key Figure 8a.2-D cross sectional diagram of lipid vesicle Figure shows LPC and FA present in bilayer as well as in water 7 FA ` LPC and FA are formed upon hydrolysis of phospholipids by PLA2. After formation, they partition between water and the membrane. Within the membrane, they contribute to its structure and phase (6,7). As mentioned previously, they are responsible for several physiological functions, thus their concentration in water and in the membrane are of biological significance (8). The distribution of LPC and FA is given by the thermodynamics of partitioning of solutes between coexisting media: lipid bilayer and water. The solute distributions are also relevant to measuring Phospholipase A2 activity (8), because in such measurements the concentrations of the products expelled into the water are measured. Therefore the goal of this thesis research is to obtain the lipid bilayer/water partition coefficient and also the enthalpy and entropy of partitioning from the measured temperature dependence of the partition coefficient. The hydrolysis products are commercially available. For the purpose of measuring partition coefficients of these products, PLA2 is not needed to produce them. They can be added to prepared phospholipid vesicle solutions and experiments designed to measure the resulting distribution. The particular lipid chosen for study was the unsaturated dioleoylphosphatidylcholine (DOPC). The corresponding products of hydrolysis are LOPC and OA. Therefore the solutes in this study are LOPC and OA. Another important property of lipid bilayers relevant to this work is the gel (ordered) to liquid phase of the bilayers. In the gel phase the hydrocarbon tails are parallel to each other with little movement and in the liquid phase the chains are flexible and have greater movement. The gel to liquid transition 8 ` temperature of DOPC bilayer is -17 C. I DOPC bilayer is in the liquid phase is the temperature range of investigation (5 C-50 C). 1.5.Brief Summary of Methodology In this study, the solute concentration in water was measured using a fluorescence assay that was developed in the research laboratory at CSU Northridge(9). The fluorescence probe used is ADIFAB (Acrylodan-labeled rat-intestinal fatty acid binding protein), which selectively binds only the solutes present in water (10,11). The fluorescence response of AIDFAB is used to determine the solute concentration in water. Applying a model based on the thermodynamics of solute partitioning to the measured solute concentration in water as a function of the lipid concentration gives the partition coefficient. Temperature dependence of the partition coefficient gives the enthalpy and entropy of partitioning. 9 ` CHAPTER 2: THERMODYNAMICS OF PARTITIONING Theory The distribution of Lysooleylphosphatdylcholine (LOPC) and Oleic Acid (OA) between water and DOPC lipid bilayer may be treated as a thermodynamic problem of solute partitioning between two coexisting solvents: the lipid bilayer and water. The transfer and partitioning of solute molecules between coexisting, distinct media is governed by the chemical potential difference of the solute in the two media. The Gibbs free energy, GSW and GSB, in water and in lipid bilayer respectively, in the lattice model for dilute solutions, are given by, 𝐺𝑆𝑊 = 𝑁𝑆𝑊 µ0𝑆𝑊 + 𝑁𝑊 µ0𝑊 + 𝑘𝑇 [ 𝑁𝑊 𝑙𝑛 𝑁𝑊 𝑁𝑆𝑊 + 𝑁𝑆 𝑙𝑛 ] 𝑁𝑆𝑊 + 𝑁𝑊 𝑁𝑆𝑊 + 𝑁𝑊 = 𝑁𝑆𝑊 µ0𝑆𝑊 + 𝑁𝑊 µ0𝑊 + 𝑘𝑇 [ 𝑁𝑆𝑊 𝑙𝑛𝑁𝑆𝑊 + 𝑁𝑊 𝑙𝑛𝑁𝑊 − (𝑁𝑆𝑊 + 𝑁𝑊 )𝑙𝑛(𝑁𝑆𝑊 + 𝑁𝑊 ) ,(1) 𝐺𝑆𝐵 = 𝑁𝑆𝐵 µ0𝑆𝐵 + 𝑁𝐵 µ0𝐵 + 𝑘𝑇 [ 𝑁𝐵 𝑙𝑛 𝑁𝐵 𝑁𝑆𝐵 + 𝑁𝑆 𝑙𝑛 ] 𝑁𝑆𝐵 + 𝑁𝐵 𝑁𝑆𝐵 + 𝑁𝐵 = 𝑁𝑆𝐵 µ0𝑆𝐵 + 𝑁𝐵 µ0𝐵 + 𝑘𝑇 [𝑁𝑆𝐵 𝑙𝑛𝑁𝑆𝐵 + 𝑁𝐵 𝑙𝑛𝑁𝐵 − (𝑁𝑆𝐵 + 𝑁𝐵 )𝑙𝑛(𝑁𝑆𝐵 + 𝑁𝐵 ) , (2) Nsw and NSB are the number of solutes in water and lipid bilayer and NW and NB are the number of water molecules and lipid molecules (concentration of DOPC) in the mixture. (𝑁𝑆𝑊 µ0𝑆𝑊 + 𝑁𝑊 µ0𝑊 ) is the standard free energy of (solute in water + water) and likewise ( 𝑁𝑆𝐵 µ0𝑆𝐵 + 𝑁𝐵 µ0𝐵 ) is the standard free energy of (solute in lipid bilayer plus bilayer). Where µ0𝑆𝑊 and µ0𝑊 are standard chemical potential of solutes in water and standard 10 ` chemical potential of water respectively. µ0𝑆𝐵 and µ0𝐵 are similarly standard chemical potential of solutes in lipid bilayer and standard chemical potential of lipid bilayer. The terms 𝑘𝑇 [ 𝑁𝑊 𝑙𝑛 𝑁 𝑁𝑊 𝑆𝑊 +𝑁𝑊 + 𝑁𝑆 𝑙𝑛 𝑁 𝑁𝑆𝑊 𝑆𝑊 + 𝑁𝑊 ] and 𝑘𝑇 [ 𝑁𝐵 𝑙𝑛 𝑁 𝑁𝐵 𝑆𝐵 +𝑁𝐵 + 𝑁𝑆𝐵 𝑙𝑛 𝑁 𝑁𝑆𝐵 𝑆𝐵 + 𝑁𝐵 ] are the entropies of mixing of the solutes in each of the two solvents, water and lipid bilayer respectively. T is the temperature in Kelvin and 𝑘 = 𝑅 𝑁𝐴 , is the Boltzmann constant, where R is the gas constant and NA is the Avogadro number. Differentiating GSW and GSB with respect to NSW and NSB gives the chemical potentials; µ𝑆𝑊 = 𝜕𝐺𝑆𝑊 𝜕𝑁𝑆𝑊 (𝑁 𝑆𝑊 = µ0𝑆𝑊 + 𝑘𝑇 𝑙𝑛 𝑁 µ𝑆𝐵 = 𝜕𝐺𝑆𝐵 𝜕𝑁𝑆𝐵 +𝑁 ) = µ0SW + kT [lnNSW + 1 − ln(𝑁𝑆𝑊 + 𝑁𝑊 ) − (𝑁𝑆𝑊+𝑁𝑊)] 𝑁𝑆𝑊 𝑆𝑊 +𝑁𝑊 (3) , = µ0SB + kT [lnNSB + 1 − ln(𝑁𝑆𝐵 + 𝑁𝐵 ) − = µ0𝑆𝐵 + 𝑘𝑇 𝑙𝑛 𝑁 𝑁𝑆𝐵 𝑆𝐵 +𝑁𝐵 𝑊 (𝑁𝑆𝐵 +𝑁𝐵 ) ] (𝑁𝑆𝐵 +𝑁𝐵 ) (4) , At equilibrium, the chemical potentials of the solutes in the two solvents, water and lipid bilayer, must be equal to each other. µ𝑆𝑊 = µ𝑆𝐵 , (5) Using the expressions for the chemical potentials derived in eq. 3 and 4 in the equilibrium condition (eq. 5) gives, 11 ` µ0𝑆𝑊 + 𝑘𝑇 𝑙𝑛 𝑁 𝑁𝑆 𝑊 𝑆𝑊 +𝑁𝑊 = µ0𝑆𝐵 + 𝑘𝑇 𝑙𝑛 𝑁 𝑁𝑆𝐵 𝑆𝐵 +𝑁𝐵 , (6) Rearranging eq.6 together with the approximation for dilute solutions; NSB<<NB and NSW<<NW, gives 𝑁 µ0𝑆𝐵 −µ0𝑆𝑊 𝑁 𝑙𝑛 𝑁𝑆𝑊𝑁 𝐵 = 𝑘𝑇 𝑆𝐵 𝑊 , (7) The total number of solutes is, 𝑁𝑆 = 𝑁𝑆𝐵 + 𝑁𝑆𝑊 , (8) Eliminating NSB in eq. 7 using eq. 8 gives, 𝑙𝑛 𝑁 𝑁𝑆𝑊 𝑁𝐵 𝑊 (𝑁𝑆 −𝑁𝑆𝑊 𝑁𝑆𝑊 𝑁𝐵 𝑁𝑊 (𝑁𝑆 −𝑁𝑆𝑊 ) = ) = 𝑒 µ0𝑆𝐵 −µ0𝑆𝑊 𝑘𝑇 0 µ0 𝑆𝐵 −µ𝑆𝑊 𝑘𝑇 , (9) , (10) Solving eq.10 for the solute fraction NSW/NS denoted by XS, gives 𝑋𝑆 = 𝑁𝑆𝑊 𝑁𝑆 = 𝐵 𝐵+𝑁𝐵 , (11) where B is, 𝐵 = 𝑁𝑊 𝑒𝑥𝑝 0 0 (𝐺𝑆𝐵 −𝐺𝑆𝑊 ) 𝑅𝑇 , (12) 12 ` 0 0 0 Where 𝐺𝑆𝑊 and 𝐺𝑆𝐵 are the standard free energy per mole and 𝐺𝑆𝑊 = µ0SW NA and 0 𝐺𝑆𝐵 = µ0SB NA , where NA is the Avogadro number. In the next chapter it is shown that the experimental quantity measured in this work is directly proportional to XS. In the context of the present experiments NB is the concentration of lipids that form the bilayer (DOPC) and, NW is equal to CW where CW = 55.6 is the molar concentration of water. Eq.11, for solute (LOPC or OA) partitioning between water and DOPC bilayer may be rewritten as, 𝑋𝑆 = 𝐶𝑊 𝐾𝑊 [𝐷𝑂𝑃𝐶]+ 𝐶𝑊 , (13) where KW refers to the water-bilayer partition coefficient, 𝐾𝑊 = 𝑒𝑥𝑝 0 0 −(𝐺𝑆𝐵 −𝐺𝑆𝑊 ) 𝑅𝑇 , (14) In the experiments of this project XS is measured for various [DOPC]. The data of XS vs. [DOPC] is fit to eq. 13 and KW is extracted from the fit. 13 ` CHAPTER 3: MATERIALS AND EXPERIMENTAL METHOD 3.1. Materials 3.1.1. Lipids and Solutes Dioleylphosphatidylcholine (DOPC) and lysooleylphosphatdylcholine (LOPC) were obtained from Avanti Polar Lipids as lyophilized powders. Oleic acid (OA) was obtained from TCI America. 3.1.2. Fluorescence Probe The fluorescence probe used was Acrylodan-labeled rat-intestinal fatty acid binding protein (ADIFAB) obtained from FFA Sciences (San Diego, CA). 3.2. Experimental Methods 3.2.1. Fluorescence Assay ADIFAB fluorescence spectra were measured with Horiba Scientific Fluoromax-4 spectrometer. The sample was excited at wavelength of 386 nm and the fluorescence emission peak was at 432 nm. The concentration of the ADIFAB used in all samples was 0.4 µM. The fluorescence from ADIFAB is that of the label acrylodan. The wavelength of its peak emission depends on the polarity. When it is bound to the protein, the peak emission is at 432 nm (9,11). As explained previously, ADIFAB binds with only the LOPC or OA present in water. This binding displaces the acrylodan. The wavelength of the peak emission of the displaced acrylodan is at 505 nm. Thus, in the presence of binding solutes the fluorescence emission sifts. The peak intensity at 505 nm increases 14 ` and the intensity at 432 nm decreases with solute concentration. The fluorescence intensities at both peaks were obtained from the fluorescence spectra measured at various solute concentrations. The Generalized Polarization (GP) defined by (7,12,13) 𝐺𝑃 = 𝐼505 −𝐼432 𝐼505 +𝐼432 , (15) was used to represent the response of ADIFAB fluorescence emission to the presence of solutes. For solutes in water only (in the absence of lipids), GP vs. solute concentration was found to be linear. Therefore GP bears a linear relationship with solute concentration in water in the presence of the lipid bilayer as well. The relations between GP and total solute concentration is given by, 𝐺𝑃 − 𝐺𝑃0 = 𝑐1 [𝑠𝑜𝑙𝑢𝑡𝑒]𝑤𝑎𝑡𝑒𝑟 = 𝑐[𝑠𝑜𝑙𝑢𝑡𝑒], (16) where c and c1 are proportionality constants. c is the slope of GP vs. [solute], [solute] water is the concentration of the solute present in water (including solute bound to ADIFAB and free solute in water), and GP0 is the GP at zero solute concentration (8). The proportionality factor c between GP and [solute] from eq. 16 is, 𝑐= 𝑐1 [𝑠𝑜𝑙𝑢𝑡𝑒]𝑊 [𝑠𝑜𝑙𝑢𝑡𝑒] = 𝑐1 𝑋𝑠 = 𝑐1 𝐾 𝐶𝑊 𝑊 [𝐷𝑂𝑃𝐶]+ 𝐶𝑊 , (17) where Xs is the solute mole fraction in water and was defined in eq. 13, which also gives its dependence on [DOPC] as shown again in the equation above. For any given [DOPC], GP vs. [solute] would be linear if KW were constant for that range of solute concentration. 15 ` Expression for the temperature dependent partition coefficient was derived in Chapter 2 0 0 ); (eq. 14) and is given by the free energy of transfer from water to bilayer, (𝐺𝑆𝐵 − 𝐺𝑆𝑊 as shown again below, 𝐾𝑊 = [𝑆𝑜𝑙𝑢𝑡𝑒]𝐵 /[𝐷𝑂𝑃𝐶] [𝑆𝑜𝑙𝑢𝑡𝑒]𝑊 /𝐶𝑊 = 𝑒𝑥𝑝 0 0 −(𝐺𝑆𝐵 −𝐺𝑆𝑊 ) 𝑅𝑇 , (18) 0 where [solute]B is the solute concentration in the DOPC bilayer, and 𝐺𝑆𝐵 is the molar 0 Gibbs free energy for solute in the lipid bilayer and 𝐺𝑆𝑊 is the Gibbs free for solutes in water. R = 8.3144 J/mol /K is the universal gas constant, and T is the temperature. Fluorescence spectra measurements were conducted for solute concentration of 0 to 8 % of [DOPC] for several [DOPC]. The quantity c defined by eq. 17 was determined from the slope of GP vs. [solute] in the range of linear solute concentration dependence for each of the [DOPC]. This quantity is henceforth denoted by ΔGP/M. This slope is proportional to Xs (eq. 13). Its dependence on the lipid concentration given by eq. 13, can be written as, 𝛥𝐺𝑃⁄𝑀 = 𝐶1 𝐶𝑊 𝐶𝑊 +[𝐷𝑂𝑃𝐶]𝐾𝑊 , (19) The slope, ΔGP/M vs. [DOPC] was fit to the theoretical model given in eq. 19. The fit returns the partition coefficient. 16 ` 3.2.2. Sample preparations Hepes buffer (pH = 7.4, C = 20µM) was used as an aqueous medium for all samples. Small unilamellar vesicles of DOPC were prepared by hydrating a thin film of DOPC with the Hepes buffer, followed by vortexing and sonication (for about 5 minutes). Thin films were made by first making a solution of DOPC in ethanol and then evaporating the ethanol with a stream of nitrogen. Ultrasonic sonicator Model no. G112SP1G from Laboratory Supplies Inc., NY was used for sonication. The ultrasound power output was 80 W. The pH of the Hepes was measured and no change due to sonication was observed. DOPC was diluted, using Hepes buffer, to obtain concentrations of 20, 35, 50, 100, 200, 300 and 400 µM. Stock solution of ADIFAB was prepared by adding the storage buffer (supplied by FFA Sciences) to the vial of lyophilized ADIFAB powder to give a final concentration of about 100 µM. The approximate molecular weight of ADIFAB is 15000 g/mol; therefore for 1mg of ADIFAB, 667 µL of storage buffer was added. The ADIFAB stock solution was stored at 4°C after preparation (14). Quartz cuvettes were used to hold samples to minimize leeching. The cuvettes were cleaned thoroughly with an acid wash and dried before each experiment, to assure that they are free of traces of detergent. ADIFAB was added to the sample before performing the fluorescence assay. 17 ` The samples for the partitioning experiments were prepared as follows: LOPC and DOPC mixture: For each of the seven DOPC concentrations, a stock solution of LOPC (in Hepes buffer; pH = 7.4) at the same concentration was prepared. LOPC from the stock solution was added in steps to the DOPC solution in the fluorescence cuvette to achieve various solute concentrations of up to 8% of DOPC. The fluorescence emission spectra were measured at the end of each addition and stirring for 2 to 5 min. OA and DOPC mixture: Due to low aqueous solubility of OA, its stock solution at concentration 2mM was first prepared at pH = 10. This stock solution was then diluted with Hepes buffer to obtain OA stock solutions of the same concentrations as DOPC. No significant change in the pH of Hepes buffer due to addition of OA solution at pH 10 was observed. Measurements were conducted with OA from these latter stock solutions added to DOPC just as for LOPC and DOPC. 18 ` CHAPTER 4: EXPERIMENTAL RESULTS Fluorescence assays of solute concentration in water were performed at temperatures of 5°C, 15 C, 37 C and 50 °C. The fluorescence spectra of ADIFAB excited at 386 nm, in aqueous solutions of small unilamellar vesicles (SUV) of DOPC at 37 C for various LOPC and OA concentrations from 0% to 8% of [DOPC] are shown in fig. 9 and 10 respectively. These figures illustrate the response of ADIFAB to addition of LOPC or OA. Upon binding LOPC or OA, a new fluorescence peak appears at 505 nm which increases with solute concentration. 19 ` 4 10 6 0% Intensity (A.U.) 2% 3 10 6 2 10 6 1 10 6 4% 6% 8% 0 400 450 500 550 Wavelegth (nm) Figure 9.Fluorescence spectra of ADIFAB, excited at 386nm, in aqueous solution of SUVs of DOPC at 37 C for [DOPC] =100 µM at different LOPC concentrations. The appearance and increase of fluorescence at about 505 nm signals LOPC binding to ADIFAB. 20 ` 5 106 0% 6 Intensity (A.U.) 4 10 2% 3 106 4% 6% 6 2 10 8% 1 106 0 400 450 500 550 Wavelegth (nm) Figure 10.Fluorescence spectra of ADIFAB, excited at 386 nm, in aqueous solution of SUVs of DOPC at 37 ºC for [DOPC] =100 µM at different OA concentrations. The appearance and increase of fluorescence at about 505 nm signals OA binding to ADIFAB. Spectra like the ones in fig. 9 and 10 were obtain for several DOPC concentrations at each of the four different temperatures of study. The GP value using ADIFAB fluorescence intensities at wavelengths of 432 nm and 505 nm was calculated for each of the solute concentrations. Graph of GP variation with concentration of LOPC and OA for every DOPC concentration were then obtained. Representative graphs of GP vs. [solute] for the case of [DOPC] = 100 µM at different temperatures are shown in fig. 11 and 12. 21 ` -0.3 o 5 C 15 oC -0.35 o 37 C 50 oC GP -0.4 -0.45 -0.5 -0.55 0 -6 1 10 -6 2 10 3 10 -6 4 10 -6 [LOPC](M) Figure 11.Graph of GP vs. [LOPC] for [DOPC] =100µM 22 -6 5 10 ` 0.1 o 5 C 0 o 15 C o 37 C -0.1 o 50 C GP -0.2 -0.3 -0.4 -0.5 -0.6 0 1 10 -6 2 10 -6 3 10 -6 4 10 -6 [OA](M) Figure 12.Graph of GP vs. [OA] for [DOPC] = 100µM 23 5 10 -6 ` Graphs of GP vs. [LOPC] and GP vs. [OA] for different [DOPC] at 37 ºC are shown in fig. 13 and 14. -0.4 -0.42 GP -0.44 -0.46 [DOPC]=20M [DOPC]=35M [DOPC]=50M [DOPC]=100M [DOPC]=200M [DOPC]=300M [DOPC]=400M -0.48 -0.5 0 -6 5 10 -5 1 10 -5 1.5 10 -5 2 10 -5 2.5 10 [LOPC] Figure 13.GP vs. [LOPC] for different [DOPC] at 37 ºC 24 -5 3 10 ` -0.15 -0.2 -0.25 GP -0.3 -0.35 -0.4 [DOPC]=20M [DOPC]=35M [DOPC]=50M [DOPC]=100M [DOPC]=200M [DOPC]=300M [DOPC]=400M -0.45 -0.5 -0.55 0 2 10-6 4 10-6 6 10-6 8 10-6 1 10-5 1.2 10-5 1.4 10-5 1.6 10-5 [OA] Figure 14.GP vs. [OA] for different [DOPC] at 37 ºC As shown in fig. 11 and 12 the graph of GP variation with solute concentration is piecewise linear. At very low concentrations the precision of the data is low and at high concentrations, the dilute solution approximation may not be applicable. The change in slope with [solute] could signal a change in KW, because the presence of solutes can change the structure of the lipid bilayer. Therefore the linear part for 2-8% solute concentration was selected for further investigation. The linear portion was then fit to a straight line. The slope given by the fit is proportional to solute mole fraction in water, as explained in detail in Chapter 3 (eq. 16-17). 25 ` Graph of GP vs. [LOPC] and vs. [OA] for the case of [DOPC] = 100 µM at temperatures of 5 , 15, 37, and 50 C are shown respectively in fig. 15 and 16 for the 2 to 8 % solute concentration range. -0.3 5 oC o 15 C -0.35 37 oC o 50 C GP -0.4 -0.45 -0.5 -0.55 -7 5 10 1 10 -6 1.5 10 -6 -6 2 10 2.5 10 -6 -6 3 10 3.5 10 -6 4 10 -6 4.5 10 [LOPC](M) Figure 15.Graph of GP vs. [LOPC] for the case of [DOPC] = 100µM 26 -6 ` 0.1 0 5 oC o 15 C o 37 C GP -0.1 50 oC -0.2 -0.3 -0.4 -0.5 5 10-7 1 10-6 1.5 10-6 2 10-6 2.5 10-6 3 10-6 3.5 10-6 4 10-6 4.5 10-6 [OA](M) Figure 16.Graph of GP vs. [OA] for the case of [DOPC] = 100µM Graphs of GP versus concentrations of solute (either LOPC or OA) were plotted for each of the DOPC concentration, as in the examples shown in fig. 15 and 16. The data were fitted to a straight line and the slopes were determined from the fits. The data of GP slope versus concentration of DOPC for both LOPC and OA at all four temperatures were then fit to the thermodynamic model of eq. 19. 27 ` The graphs are shown in fig. 17 and 18. The fits yield the value of the partition coefficient at each of the temperatures. 10 o 5 C 8 o 15 C o 37 C GP / M x 10 -4 o 50 C 6 4 2 0 0 1 2 3 4 5 4 [DOPC] (M) x10 Figure 17.Graph of ΔGP/ M of LOPC vs. [DOPC] at temperatures of 5 ºC, 15 ºC 37 ºC and 50 ºC 28 ` 25 o 5 C 20 o 15 C o GP / M x 10 -4 37 C 15 o 50 C 10 5 0 0 1 2 3 [DOPC] (M) x10 4 5 4 Figure 18.Graph of ΔGP/ M of OA vs. [DOPC] at temperatures of 5 ºC, 15 ºC 37 ºC and 50 ºC 29 ` The values of KW for LOPC and OA were obtained from the fit and shown respectively in tables 1 and 2. Table 1.Values of KW for distribution of LOPC in lipid bilayer and water Temperature ( C ) 5 15 37 50 T-1 (K-1) 0.0036 0.0035 0.0032 0.0031 KWX10-6 4.43 5.9 6.79 2.88 Error in KW X10-6 0.854 0.24 0.390 0.972 Table 2.Values of KW for distribution of OA in lipid bilayer and water Temperature ( C ) 5 15 37 50 T-1 (K-1) 0.0036 0.0035 0.0032 0.0031 KW X10-6 7.57 5.32 2.97 5.30 Error in KW X10-6 0.992 0.197 0.336 0.28 The errors shown in the last column are uncertainties given by the fitting routine and not the standard deviations. The data show that the KW increases with temperature between 5 ºC and 37 ºC for LOPC and decreases for OA. The KW value at 50 ºC does not fall into this pattern. This could be because ADIFAB which is a protein begins to denature at 50 ºC. Previous experiments in our laboratory indicated that ADIFAB begins to denature at 50 ºC and it does not bind any solute at 60 ºC. Therefore the data point at 50 ºC was not included in further analysis. 30 ` The temperature dependence of KW for LOPC and OA are shown in fig.19 and 20. 6 6 10 6 5 10 6 K W 7 10 6 4 10 0.0031 0.0032 0.0033 0.0034 T -1 0.0035 -1 (K ) Figure 19.Graph of KW vs. T-1 for LOPC. 31 0.0036 0.0037 ` 7 9 10 6 8 10 6 7 10 6 6 10 6 5 10 6 4 10 6 3 10 6 K W 10 6 2 10 0.0032 0.0033 0.0034 T 0.0035 -1 0.0036 0.0037 -1 (K ) Figure 20.Graph of KW vs. T-1 for OA The temperature dependence of KW is given by eq. 14. The change in Gibbs free energy for one mole of solute transfer from water to bilayer is, 0 0 0 𝛥𝐺𝐵𝑊 = 𝐺𝑆𝐵 − 𝐺𝑆𝑊 = 𝛥𝐻𝐵𝑊 − 𝑇𝛥𝑆𝐵𝑊 , (20) where ΔHBW is the change in enthalpy of the system (both water and lipid bilayer) and ΔSBW is the change in entropy of the system. 32 ` Applying eq. 20 to eq. 14. gives, 𝐾𝑊 = 𝑒𝑥𝑝 −( 𝛥𝐻𝐵𝑊 𝑅𝑇 − 𝛥𝑆𝐵𝑊 𝑅 ) = 𝑒𝑥𝑝( 𝛥𝑆𝐵𝑊 𝑅 )𝑒𝑥𝑝 − ( 𝛥𝐻𝐵𝑊 𝑅𝑇 ), (21) The temperature dependence of KW is determined by ΔHBW. Fitting the data of fig. 19 and 20 to eq. 21 give ΔHBW and ΔSBW for the DOPC bilayer partitioning of LOPC and OA. The obtained values are shown in the tables below. Table 3.Values of ΔH for LOPC and OA ΔHBW = HSB – HSW (kJ mol-1) LOPC OA 8.9 ± 0.5 -20.76 ± 0.5 Table 4.Values of ΔS for LOPC and OA ΔSBW = SSB- SSW (kJ mol-1 K-1) LOPC OA 0.16± 0.002 0.057± 0.002 The errors in ΔHBW and ΔSBW were calculated as the propagated error due to a ± 20 % error in KW. The values of ΔGBW0 are obtained using eq. 20 and are shown in the table 5. Table 5.Value of ΔGBW0 for LOPC and OA at T = 37 C 𝜟𝑮𝟎𝑩𝑾 = ΔHBW – TΔSBW , (kJ mol-1) LOPC OA -40.7 ± 0.5 -38.4 ± 0.5 Discussion of the results follows in the next chapter. 33 ` CHAPTER 5: DISCUSSION AND CONCULSION 5.1. Discussion As the results show (Table. 5) the values of ΔGBW 0 for both LOPC and OA are negative. The ΔGBW0 value obtained for LOPC is -40.7 kJ mol-1 and -38.4 kJ mol-1for OA at 37 C. Negative change in Gibbs free energy means that it is lowered upon partitioning of solutes into the bilayer. In other words, the reaction is favorable towards partitioning of solutes, for both LOPC and OA, into DOPC at the temperatures of study. The enthalpy changes, however, are drastically different between LOPC and OA, being positive for LOPC and negative for OA. This difference in enthalpy change accounts for the difference in the behaviors of KW vs.1/T. Negative enthalpy change means that heat is released. Such a reaction is called exothermic. Positive enthalpy change means absorption of heat and partitioning is endothermic. The present results show that the change in enthalpy of the system is positive for LOPC, 8.9 kJ mol-1, and negative for OA, -20.8 kJ mol-1. This shows that change in enthalpy is favored towards partitioning of OA but not LOPC. Although the negative change in enthalpy makes the partitioning more spontaneous and favorable, it alone cannot be used to predict the overall case of partitioning. Similarly positive change in enthalpy does not mean partitioning is not favored. It’s the overall change in free energy that is the decisive factor. The entropy change for both LOPC and OA is positive. As shown in table. 4, change in entropy for LOPC is 0.16 KJmol-1 K-1 and for OA is 0.057 KJmol-1 K-1. . The increase in entropy causes the overall free energy to decrease. Entropy is a measure of disorder. The larger the number of states available for a system, larger is the entropy. When solutes are in water, the entropy is lowered because of the 34 ` ordering of water molecules around the hydrophobic chain of the solutes and loss of their orientational freedom. To gain back the entropy, the system prefers the transfer of the molecule into the bilayer. This is referred to as the hydrophobic effect(1). It is the origin of the increase in entropy. The negative change in free energy in spite of the positive change in enthalpy in the case of LOPC means that the change in entropy is positive enough to favor partitioning into the bilayer. This means that partitioning of LOPC into DOPC bilayers is entirely entropy driven. Partitioning of OA on the other hand is both enthalpy and entropy driven. Similar finding was reported for the partitioning of lysopalmitoylphosphatidylcholine (LPPC) and palmitic acid (PA) in the liquid phase (> 41 C) of dipalmitoylphosphatidylcholine (DPPC) where the enthalpy change was positive for LPPC and negative for PA (15). The structures of DOPC and LOPC together with OA are shown respectively in fig. 21 and 22. O C O CH2 C O CH O O DOPC H2C Figure 21.Chemical structure of DOPC 35 O P O O N ` O C O CH2 LOPC HO O CH H2C O P N O C OH O OA Figure 22.Chemical structures of LOPC and OA. Both head group interactions with water and tail interactions (Van der Waal) in the bilayer contribute to the enthalpy change. The head groups of LOPC and in general for all LPC are zwitterionic and therefore polar (fig. 22). Water molecules are also polar. The favorable dipole-dipole interaction between water and the polar LPC head group lowers the energy in the water, because of which the head group contribution to the change in enthalpy is positive. This could be the dominating reason for the overall enthalpy change to be positive for LOPC. This interaction is also the reason why LPC is more soluble in water than FA. Fatty acids have a small head group and are not completely ionized in water (fig. 22). They are minimally soluble in water. The head groups of fatty acids are much less hydrophilic than the head groups of LPC. In fact, fatty acids easily move between the two monolayers of the bilayer; a phenomenon referred to as flip-flop. In the flip-flop process the head group comes in contact with the non-polar tails. Fatty acid head groups being neutral are not repelled by this contact. That is, the fatty acid head groups do not prefer water to the bilayer. LPC exhibits an extremely slow flip-flop rate, because of the highly unfavorable contact with the tails that its polar head group must face in the process. Thus the favorable tail interaction in the bilayer mainly accounts for the negative change in enthalpy for OA partitioning. 36 ` A wide temperature range is needed for better precision in the values extracted by fits to temperature dependent properties. Practical considerations limit the temperature range over which the experiments could be performed. Since water freezes at 0 °C, the lowest temperature of measurements was 5 C. The fluorescence probe used in the measurements, ADIFAB, is a protein, and previous work in our laboratory showed that it begins to denature at temperatures higher than 50 C. Thus the temperature ranged employed in this study is 5 ºC – 50 C. The data at 50 C was excluded from the study of KW vs. 1/T citing ADIFAB denaturation as the reason. But water hydrogen bonding properties also change with temperature and this can contribute to the irregularity of the data at 50 C. In obtaining enthalpy and entropy of partitioning from temperature dependence of KW, it is assumed that the structure and properties of the solvents don’t change within the temperature range of investigation so that H and S are constants that can be determined from the fit to the dilute solution lattice thermodynamic model of K W vs. 1/T. DOPC was selected precisely for the reason that it might satisfy this required condition. The gel to liquid transition temperature of DOPC bilayers is - 17 C. For 5C T. 50 C, the DOPC bilayer was expected to remain in the liquid phase without structural changes. But there is not much information available on the phase and structural behavior of DOPC at these temperatures, particularly when LOPC or OA is present. 37 ` 5.2. Conclusions DOPC lipid bilayer partition coefficients of oleic acid (OA) and LOPC were measured. From the temperature dependence of the partition coefficient the enthalpy and entropy for partitioning were determined. The enthalpy change for LOPC partitioning is positive and negative for OA in the temperature 5 to 37 ºC. Such a result was also observed for LPPC and PA partitioning into DPPC bilayers (15). The partitioning of LOPC is entirely driven by the entropy term in the free energy, whereas both enthalpy and entropy contribute to OA partitioning. The origin of the entropy term is in the hydrophobic effect. This effect is the repulsion between the polar water molecules and the non-polar hydrocarbon tails, which causes ordering of water molecules around the hydrocarbon tails to prevent contact. Water experiences loss of entropy. Entropy is regained when the hydrocarbon is transferred into the bilayer away from water. Thus partitioning of both LOPC and OA is favored by entropy. The origin of the enthalpy change is due to both the head group interaction with water, bilayer, and the tail group interactions in the bilayer. The Van der Waal interactions between the tail groups in the bilayer favor partitioning for both LOPC and OA. But the polar head group of LOPC prefers the polar water region and repels the nonpolar region of the bilayer. This attraction of LOPC head group for water and its repulsion of bilayer lead to an unfavorable increase in enthalpy of partitioning. However the favorable entropy increase due to the hydrophobic effect wins over the unfavorable enthalpy effect with an overall reduction in the free energy upon partitioning into the bilayer. In the case of OA, the carboxylic head group interactions play a minimal role. 38 ` The enthalpy decrease is due mainly to favorable tail interactions in the bilayer. Both enthalpy and entropy contribute to the decrease in free energy of partitioning. 39 ` REFERENCES 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. Tanford, C. (1980) The Hydrophobic Effect: Formation of Micelles and Biological Membrane, 2nd ed., Wiley-Interscience, New York Dennis, E. A. (1997) The growing phospholipase A2 superfamily of signal transduction enzymes. Trends. Biochem. Sci. 22, 1-2 Dennis, E. A., Cao, J., Y-H., H., Magrioti, V., and Kokotos, G. (2011) Phospholipase A2 Enzymes: Physical Structure, Biological Function, Disease Implication, Chemical Inhibition, and Therapeutic Intervention. Chem. Rev. 111 (10), 6130-6185 Yan, J. J., Jung, J. S., Lee, J. E., Lee, J., So, H., Kim, H. S., Jung, K. C., Cho, J. Y., Nam, J. S., Suh, H. W., Kim, Y. H., and Song, D. K. (2004) Therapeutic effects of lysophosphatidylecholine in experimental sepsis. Nat. Med. 10(2) Kume, N., and Gimbrone, M. A. j. (1994) Lysophosphatidylcholine Transcriptionality Induces Growth Factor Gene Expression in Cultured Human endothelial cells. J. Clin. Invest. 93, 907-911 Henshaw, J. B., Olsen, C. A., Farnbach, A. R., Nielson, K. H., and John D. Bell, J. D. (1998) Definition of the Specific Roles of Lysolecithin and Palmitic Acid in Altering the Susceptibility of Dipalmitoylphosphatidylcholine Bilayers to Phospholipase A 2. Biochemistry 37, 10709-10721 Arouri, A., and Mouritsen, O. G. (2013) Membrane perturbing effects of fatty acids and lysolipids. Progress in Lipid Research 52, 130-140 Singh, J., Lai, A. J., Alaee, Y., and Ranganathan, R. (2014) Partitioning of lysolipids, fatty acids and their mixtures in aqueous lipid bilayers: Solute concentration/composition effects. Biochim. Biophys. Acta, Biomembr. 1838, 348-354 Singh, J., and Ranganathan, R. (2012) Quantitation of Lysolipids, Fattyacids, Phospholipase A2 Activity and Correlation with Membrane Polarity. J. Lipid Res. 53, 1993-2001 Richieri, G. V., and Kleinfeld, A. M. (1995) Continuous Measurement of Phospholipase A2 Activity Using the Fluorescent Probe ADIFAB. Analytical Biochemistry 229, 256-263 Richieri, G. V., Ogata, R. T., and Kleinfeld, A. M. (1999) The measurement of free fatty acid concentration with the fluorescent probe ADIFAB: A practical guide for the use of the ADIFAB probe. in Molecular and Cellular Biochemistry, Springer Netherlands Olson, E. D., Nelson, J., Griffith, K., Nguyen, T., Streeter, M., Wilson-Ashworth, H. A., Gelb, M. H., Judd, A. M., and Bell, J. D. (2010 ) Kinetic Evaluation of Cell Membrane Hydrolysis during Apoptosis by Human Isoforms of Secretory Phospholipase A2 J. Biol. Chem. 285 10993-11002 Harris, F. M., Smith, S. K., and Bell, J. D. Physical Properties of Erythrocyte Ghosts That Determine Susceptibility to Secretory Phospholipase A2. Richieri, G. V., Ogata, R. T., and Kleinfeld, A. M. (1992 ) A fluorescently labeled intestinal fatty acid binding protein. Interactions with fatty acids and its use in monitoring free fatty acids. . J. Biol. Chem. 267 23495-23501 Høyrup, P., Jesper Davidsen, J., and Jørgensen, K. (2001) Lipid Membrane Partitioning of Lysolipids and Fatty Acids: Effects of Membrane Phase Structure and Detergent Chain Length. J. Phys. Chem. B 105, 2649-2657 40