SECTION 23 ² AMINES 23-1 -- Nomenclature of Amines
advertisement

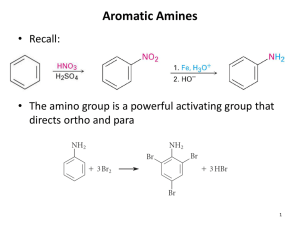
SECTION 23 ² AMINES 23-1 -- Primary (1°), Secondary (2°), and Tertiary (3°) Amines 23-1 -- Nomenclature of Amines x Common Names ² ´Alkyl Aminesµ x IUPAC Names ² ´Alkanaminesµ x Naming Amines With ²NH2 as a Substituent (Branch) x Use of ´N-´ as a Locator x Ammonium and Iminium Ions 23-3 -- Structures of Amines x Simple Amines are Pyramidal x Complicated Amines (i.e. Aniline) Can ´Flatten Outµ 23-4 -- Spectroscopy of Amines x IR Spectroscopy of Amines x 1H NMR Spectroscopy of Amines 23-5 -- Basicity of Amines x Amine Basicity Relative to H2O and OHx Alkylamines and Their Conjugate Acids (pKa ~ 10-11) x Resonance Effects and Electron-Withdrawing Substituents 23-7 -- Acidity of Amines x Alkyl Amines (pKa ~ 35-38) Have Very Strong Conj. Bases - Amide Ions, R2N23-8 -- Reactions of Amines ² pp.23-8 through pp.23-19 ² BIG TOPIC 23-8 -- Alkylation of Amines x Direct Alkylation of Amines (SN2 with Amines as Nucleophiles) x Exhaustive Alkylation of Amines x Reductive Alkylation of Amines (Use of NaBH4 or H2/catalyst) 23-10 -- Gabriel Synthesis of 1° Amines x Use of Pthalimide in an SN2 Reaction x Gabriel Synthesis Adds ²NH2 to the R-group of R-Br 23-12 -- Preparation of Amines via Reduction Reactions x Reduction of Azides (R-N3) x Reduction of Nitriles (R-CƱN:) x Reduction of Amides Using Lithium Aluminum Hydride (LiAlH4, or ´LAHµ) 23-13 -- The Hofmann Elimination x Step 1 - Overalkylation of the Amino Group x Step 2 ² ´Switchingµ I- with OHx Step 3 - E2 Elimination (via Heat, ¨) to Form Less-Substituted Alkene x ´Hofmann Regiochemistryµ is Observed 23-14 -- The Hofmann Rearrangement www.OChemNotes.com x Involves Transformation of 1° Amide in Basic Solution to an Amine 23-15 -- The Curtius Rearrangement x Converts an Acyl Azide to an Amine x Mechanism Involves an Isocyanate Intermediate (O=C=N-R) x Curtius Rearrangements Performed in H2O vs. in Alcohol (R·OH) 23-16 -- Diazonium Ion Chemistry x [ R-NƱN ÅÆ R-N=N ]+1 , an Alkyl Diazonium Ion (Add lone pairs on N·s!!) x N2 is an Excellent Leaving Group (l.g.) x Diazonium Ions are Very Unstable x Aryl Diazonium Ions (Ar-NƱN:)+1 are More Stable x Aryl Diazonium Ions in Electrophilic Aromatic Substitution (E.A.S) Reactions x Azobenzenes and UV-VIS Spectroscopy 23-18 -- Rearrangement of Alkyl Azides x Analogous to Curtius Rearrangement x Rearrangement Occurs Thermally (via Heat), or Photochemically (via UV light) www.OChemNotes.com SECTION 23 – AMINES Were the FREE Section 23 Notes Useful? Want the FULL VERSION of the Section 23 Notes? Download Them Instantly for Only $6.99 at: OChemNotes.com www.OChemNotes.com