AbstractID: 7947 Title: Considerations and Caveats for Implementing a Gliasite...
advertisement

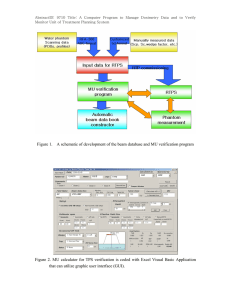
AbstractID: 7947 Title: Considerations and Caveats for Implementing a Gliasite Brachytherapy Program The Gliasite1 is a novel brachtherapy device used in the treatment of brain tumors. It is implanted at the time of surgical resection in the cavity created by removal of the gross tumor volume. The device consists of a double layer, inflatable, spherically shaped, balloon attached to a flexible fill/withdraw line catheter connected to an access port. At time of treatment the device is filled with a liquid solution of I-125 ( IOTREX cr) with a typical activity of several hundred mCi resulting in a typical dwell time of several days. Considerations and caveats for implementing this type of program will be reviewed including: instrumentation for assay and survey; patient simulation for confirming placement, size, and device sphericity; dosimetry; and radiation safety considerations for patients, visitors, and staff. Based on our clinical experience of 20 patients, parameters of clinical interest will be presented including a comparison of expected vs. measured source activities; dilution efficacy to reduce contrast media remaining in applicator from simulation, and transfer efficiency from filling syringe to applicator. Lastly, important dosimetry considerations to be noted for recently available, “unit doses” will be reviewed. 1 Ref Dempsey et al Int Jrnl Rad Onc Biol Phys, Vol. 42, No. 2. pp 421-429, 1998