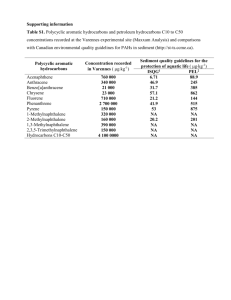
Document 14681202
advertisement

International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 211 Petrochemical Industrial Waste: Bioremediation Techniques An Overview Sheetal Koul and MH Fulekar* Department of Life Sciences, University of Mumbai, Santacruz (E), Mumbai- 400 098, India * School of Environment and Sustainable development, Central University of Gujarat, Gandhinagar – 482030, India * Email: mhfulekar@yahoo.com ABSTRACT The petrochemical industry is one such major source of hazardous waste, produced during IJOART manufacture of Petrochemical products. These wastes are often released in the environment. Petrochemical Solid waste is generally associated with more hazardous constituents and accordingly carries a higher level of public health and environmental risk potential. In the present paper the petrochemical waste compound in particular Polycyclic Aromatic Hyrocarbons (PAH) were described as persistent pollutant in soil-water environment. The microbial sources which have been found and reported for PAH compound degradation have been cited with examples viz: potential species of Bacteria, species of Fungal and actinomycetes have been described for microbial degradation. The factors influencing microbial degradation including the influence of GMO’s on bioremediation have been cited in the paper. This would serve as a bioremediation technique for microbial degradation of petrochemical waste. Keywords: Bioremediation, Polycyclic Aromatic Hydrocarbons’ (PAH), Petrochemical Solid waste, GMO’s Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 1. 212 Introduction: The persistence of a pollutant in the environment is influenced by the nature of the contaminant, the amount of the contaminant present and the interplay between chemical, geological, physical and biological characteristics of the contaminated site. In the present era, petroleum hydrocarbon contamination is considered a major widespread environmental problem distributed in atmosphere, terrestrial soil, marine waters and sediments [1]. Anthropogenic activities which include improper management and disposal of oil sludge waste results in leaks and accidental spills during the exploration, production, refining, transport and storage of petroleum products. Polycyclic aromatic hydrocarbons (PAHs) are one class of toxic environmental pollutants and perhaps the first recognized environmental carcinogens that have accumulated in the environment whether accidentally or due to human activities [2]. Polycyclic aromatic Hydrocarbons (PAHs) are fused ring hydrocarbon compounds IJOART that are highly recalcitrant under normal conditions due to their structural complexity, strong molecular bonds, low volatility and aqueous solubility and high affinity for soil material and particulate matter [3]. Overtime, they accumulate in the surrounding soil sediments and ground water causing extensive damage to animal tissues due to their carcinogenic, mutagenic and potentially immune toxicant properties. [4], [5]. Although in the natural environments they are readily degraded by indigenous microbial communities, these processes are very time consuming. Various physical and chemical applications like mechanical burying, evaporation, dispersion and washing are currently employed to remediate the problems caused by PAHs pollution. However, these forms of treatments are either expensive or can lead to incomplete decomposition of contaminants [6]. The process of bioremediation, defined as the use of microorganisms to detoxify or remove pollutants owing to their diverse metabolic capabilities is an evolving method for the removal and degradation of many environmental pollutants including the products of petroleum industry [7]. In addition, bioremediation technology is believed to be non-invasive and relatively cost effective [8]. Bioremediation by natural populations of microorganisms represents one of the primary mechanisms by which petroleum and other hydrocarbon pollutants can be removed from the environment [9] and is cheaper than other remediation technologies [10].The success of PAH degradation and bioremediation depends on one’s ability to establish and maintain conditions that favour enhanced oil biodegradation rates in Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 213 the contaminated environment which include the employment of correct population of microorganism [3] with the appropriate metabolic capabilities [6] and ensuring adequate concentration of nutrients, oxygen and optimal pH and temperature [11],[12],[13],[14],[15].The characterization of novel catalytic mechanisms, physiological adaptations of microbes, biochemical mechanisms involved in hydrocarbons accession, uptake and the application of genetically engineered and enhanced microbes for bioremediation are the recent advances employed in the removal of persistent organic pollutants like PAHs. Therefore, the intent of this review is to update information on microbial degradation of Polycyclic Aromatic Hydrocarbons towards the better understanding in bioremediation challenges. 2. Polycyclic Aromatic Hydrocarbons (PAHs) 2.1 Physical and chemical properties: IJOART Polycyclic Aromatic Hydrocarbons or PAHs as they are fondly called are chemical compounds made up of two or more fused benzene rings [3] and some “Penta-cyclic Moieties” in linear, angular, and/or cluster arrangements [16]. Many PAHs contain a “bay-region” and a “K-region”. The bays-and K-region epoxides, which can be formed metabolically, are highly reactive both chemically and biologically. Phenanthrene is the simplest aromatic hydrocarbon which contains these regions. The bay-region of phenanthrene is a sterically hindered area between carbon atom 4 and 5 and the K-region is the 9, 10 double bond [17] According to the Schmidt-Pullman, electronic theory K-region epoxides should be more Carcinogenic than the parent hydrocarbon.[17]. PAHs generally accumulate in the environment because they are thermodynamically stable compounds, due to their large negative resonance energies; they have low aqueous solubility’s and they absorb to soil particles [16].Generally solubility decreases and hydrophobicity increases with an increase in number of fused benzene rings also volatility decreases with an increasing number of fused rings [18] High molecular weight [HMW] PAHs (four or more rings) sorb strongly to soils and sediments and are more resistant to microbial degradation. Because of solid state, high molecular weight and hydrophobicity expressed as its log P value between 3 and 5, PAHs are very toxic to whole cells [19]. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 214 Figure1. PAHs representatives and their chemical structures [17] Table 1. Structure and physical-chemical properties of some three-, four-, five- and six-ring polycyclic aromatic hydrocarbons . [20] IJOART No. of rings mpa (oC) bpb(oC) Solc(mg l-1) log K p d Vapour pressure (torr at 20(oC) 3 101 340 1.29 4.46 6.8 x10-4 3 216 340 4.45 0.07 2.0 x 10-4 Fluoranthene 4 111 250 0.26 5.33 6.0 x 10-6 Benz[a ]anthracene 4 158 400 0.014 5.61 5.0 x 10-9 Pyrene 4 149 360 0.14 5.32 6.8 x 10-7 Chrysene 4 255 488 0.002 5.61 6.3 x 10-7 Benzo[a ]pyrene 5 179 496 0.0038 6.04 5.0 x 10-7 Dibenz[a,h]anthracene 5 262 524 0.0005 5.97 1.0 x 10-10 Benzo[g,h,i]perylene 6 222 - 0.0003 7.23 1.0 x 10-10 Indeno[1,2,3-c,d ]pyrene 6 6163 0.062 7.66 1.0 x 10-10 PAH Phenanthrene Anthracene 536 a mp: melting point;bbp: boiling point; c Sol: aqueous solubility. d log K p : logarithm of theoctanol:water partitioning coefficient. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 215 2.2 Production, Occurrence and Toxicity of PAHs The major source of PAHs is from the incomplete combustion of organic material (Guerin & Jones., 1988). PAHs are formed naturally during thermal geologic production and during burning of vegetation in forest and bush fires [21].In Industrial countries, anthrogenic combustion activities are a principal source of PAHs in soils where they arise from atmospheric deposition [22]. PAHs have been detected in a wide variety of environmental samples including air [23],soil [22], sediments [24],water, oils, tars and foodstuff [25], [26].Oil leakage from storage tank bottoms, oil-water separators, and drilling operations etc. has led to an increase in soil PAH concentration over the last few decades [17] PAHs are also a major constituent of Creosote (approximately 85% PAH by weight) and anthracene oil, which are commonly used pesticides for wood treatment. [27].These contaminated soils vary in hydrocarbon composition. Table2. Characteristics of a typical PAH-contaminated soil [28]. IJOART PAH-Contaminated areas pose a health risk to humans [29]. One-, two and three-ring compounds are acutely toxic [20]. Low Molecular weight PAH pollutants exert toxic, Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 216 mutagenic, carcinogenic and potential endocrine-disrupting properties [29],while higher molecular weight PAHs are considered to be Genotoxic [30]. Table 3.standard limiting PAH content (µg/kg) in the soil surface layer [31] Total PAH Content Pollution Class < 200 Soil Assessment 0 Unpolluted (natural content) 200-600 I Unpolluted (natural content) 600-1000 II slightly polluted 1000-5000 III Polluted 5000-10000 IV Heavily Polluted >10000 V Very heavily polluted 3. Biodegradation of PAHs- Bioremediation Strategies IJOART Biodegradation of petroleum hydrocarbons particularly PAHs is a complex process. Petroleum hydrocarbon compound bind to soil components, and they are difficult to degrade [32]. The natural presence of PAHs in the environment allows many microorganisms to adapt to the use and exploitation of these naturally occurring potential growth substrates. Thus many bacterial, fungal and algal strains have been shown to degrade a wide variety of PAHs containing from three to five aromatic rings [27]. 3.1General Aspects of PAH-Degradation: The fate of PAHs in the soil very much depends on the physical characteristics of the PAH constituents, like molecular size and the topology of the compound [33] For low molecular weight (LMW) PAHs, removal through evaporation is the first line of elimination [3]. Generally the increase in molecular size and angularity of the PAH compound results in a concomitant increase in hydrophobicity and electrochemical stability and due to their lipophilic nature, PAH have potential for bio-magnification through tropic transfers [33].However, with prolonged exposure to soil particles, bioavailability is greatly reduced and biodegradation rate become slower, therefore in order to enhance the biodegradation process, bioavailability of PAHs in soil needs to be increased [34].The indigenous organisms have only limited capacity to degrade all the fractions of hydrocarbons present, hence, a Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 217 consortia consisting of outside microbes isolated from the contaminated sites and the indigenous microorganisms can degrade the constituents and have a higher tolerance to toxicity [35]. The efficiency range for biodegradation of hydrocarbons by bacteria, yeast and fungi is reported to be 6%-82% [36] for soil fungi, 0.13%-50% [37],[38] for soil bacteria and 0.003%-100% [39] [40] for marine bacteria. 3.2Bacterial Degradation Bacteria are the most active agents in petroleum degradation and they work as primary degraders of spilled oil in environment [40],[39],Bacteria initially oxidise aromatic hydrocarbons to cis-dihydrodiols [41],[42], [43] by incorporating both atoms of molecular oxygen, catalysed by a dioxygenase into the aromatic ring to produce a cis-dihydrodiol [44]. The dioxygenase that catalyses these initial reactions is a multicomponent enzyme system. The initial ring oxidation is usually the rate-limiting step in the biodegradation reaction of PAH [45] Cis-dihydrodiols are re-aromatised through a cis-dihydrodiol IJOART dehydrogenase to yield a dihydroxylated derivative [41].Further oxidation of the cisdihydrodiols leads to the formation of catechols [46] via a NAD+ dependent dehydrogenation reaction. The important step in catabolism of PAHs is ring fission by dioxygenase enzymes that cleave the aromatic ring to give aliphatic intermediates [47] Catechol can be oxidised via two pathways, the Ortho-pathway involves cleavage of the bond between carbon atoms with a hydroxyl group. Ring cleavage results in the production of succinic, fumaric, pyruvic and acetic acids and aldehydes, all of which are utilised by micro-organisms for the synthesis of cellular constituents and energy [48] A by-product of these reactions is the production of carbon dioxide and water. Once the initial hydroxylated aromatic ring of the PAH is degraded to pyruvic acid and carbon dioxide, the second ring is then attached in the same manner. High molecular weight PAH’s such as benzo(a)pyrene (BaP) are only degraded with difficulty. However degradation has been observed via a co-oxidation or co-metabolism mechanism through less recalcitrant compound. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 218 IJOART Figure 2: Microbial metabolism of the aromatic ring by ortho or meta cleavage [45] A great diversity of bacterial genera have been repeatedly isolated mainly from the soil that are capable of degrading the low molecular weight PAHs like naphthalene, acenapthanes and phenantherne, these are usually gram-negative bacteria, most of them belong to the genus Pseudomonas. The biodegradative pathways have also been reported in bacteria from the genera Mycobacterium ,Corynebacterium, Acromonas, Rhodococcusand Bacillus [49], [50]. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 219 Table 4: Polycyclic aromatic hydrocarbons oxidised by different species of bacteria [45] PAH Naphthalene Organisms References Acinetobacter calcoaceticus, Alcaligenes Ryu et al. (1989), Weissenfels et al. (1990, 1991), Kelly et al. (1991), Dunn denitrificans, Mycobacterium sp., Pseudomonas and Gunsalus (1973), Davies and Evans (1964), Foght and Westlake (1988), sp., P. putida, P. fluorescens, Sp. Jeerey et al. (1975), Mueller et al. (1990a), Kuhm et al. (1991), Walter et al. paucimobilis,Brevundimonasvesicularis, (1991), Dua and Meera (1981), Tagger et al. (1990), Garcia-Valdes et al. Burkholderiacepacia, Comamonastestosteroni, (1988), Trower et al. (1988), Grund et al. (1992), Barnsley (1975a); Rhodococcus sp., Corynebacteriumrenale, Barnsley (1983a), Yang et al. (1994), Burd and Ward (1996), Allen et al. Moraxella sp.,Streptomyces sp., B. cereus, P. (1997), Stringfellow and Aitken (1995), Filonov et al. (1999), Hedlund et al. marginalis, P. stutzeri, P. saccharophila, (1999), Geiselbrecht et al. (1998), Foght and Westlake (1996), Goyal and Neptunomonasnaphthovorans, Cycloclasticus sp. Beijernickia sp., fluorescens,Bu.cepacia, Acenaphthene P. putida, P. Pseudomonas sp., Cycloclasticus sp., Neptunomonasnaphthovorans, Zylstra (1996) Chapman (1979), Schocken and Gibson (1984), Ellis et al. (1991), Geiselbrecht et al. (1998), Hedlund et al. (1999), Selifonov et al. (1993) Alcaligeneseutrophus, Alcaligenesparadoxus Phenanthrene Aeromonas sp., A. faecalis, A. denitrificans, Kiyohara et al. (1976, 1982, 1990), Weissenfels et al. (1990, 1991), Keuth Arthrobacter polychromogenes, Beijernickia sp., and Rehm (1991), Jerina et al. (1976), Colla et al. (1959), West et al. (1984), Micrococcus sp., Mycobacterium sp., P. putida, Kiyohara and Nagao (1978), Heitkamp and Cerniglia (1988), Guerin and Sp. paucimobilis, Rhodococcus sp., Vibrio sp., Jones (1988a, 1989), Treccani et al. (1954), Evans et al. (1965), Foght and Nocardia sp., Flavobacterium sp., Streptomyces Westlake (1988), Mueller et al. (1990b), Sutherland et al. (1990), Ghosh and sp., S. griseus, Acinetobacter sp., P. aeruginosa, Mishra (1983), Savino and Lollini (1977), Trower et al. (1988), Barnsley P. stutzeri, P. saccharophila, Stenotrophomonas (1983b), Yang et al. (1994), Kohler et al. (1994), Stringfellow and Aitken maltophilia, Cycloclasticus sp., P. uorescens, (1995), Boonchan (1998), Juhasz (1998), Geiselbrecht et al. (1998), Foght Acinetobacter calcoaceticus, and Westlake (1996), Acidovorax dela®eldii, Gordona sp., Kastner et al. (1998), Lal and Khanna (1996), Shuttleworth and Cerniglia Sphingomonas sp., Comamonas (1996), Mahro et al. (1995), Goyal and Zylstra (1996), Dyksterhouse et al. testosteroni, Cycloclasticus pugetii, Sp. (1995), Allen et al. (1999), Aitken et al. (1998), Romero et al. (1998), yanoikuyae, Agrobacterium sp., Iwabuchi et al. (1998), Churchill et al. (1999), Bacillus sp., Burkholderia sp., Sphingomonas sp., Juhasz (1991) IJOART Pseudomonas sp., Rhodotorula glutinis, Nocardioides sp., Flavobacterium gondwanense, Halomonas meridiana Anthracene Beijernickia sp., Mycobacterium sp., P. putida, Colla et al. (1959), Akhtar et al. (1975), Jerina et al. (1976), Evans et al. Sp. paucimobilis, Bu. (1965), Ellis et al. (1991), Weissenfels et al. (1991), Foght and Westlake cepacia, Rhodococcus sp., Flavobacterium sp., (1988), Walter et al. (1991), Mueller et al. (1990a), Savino and Lollini Arthrobacter sp., P. marginalis, Cycloclasticus (1977), Tongpim and Pickard (1996), Burd and Ward (1996), Geiselbrecht sp., P. fluorescens, Sp. yanoikuyae, et al. (1998), Foght and Westlake (1996), Kim et Acinetobactercalcoaceticus, Gordona sp., al. (1997), Lal and Khanna (1996), Mahro et al. (1995), Goyal and Zylstra Sphingomonas sp., (1996), Dyksterhouse et al. (1995), Allen et al. (1999) Comamonastestosteroni,Cycloclasticuspugetii Fluoranthene A. denitrificans, Mycobacterium sp., P. putida, Sp. Kelly and Cerniglia (1991), Walter et al. (1991), Weissenfels et al. paucimobilis, Bu. (1991), Foght and Westlake (1988), Barnsley (1975b), Mueller et al. cepacia, Rhodococcus sp., Pseudomonas sp., (1990a, 1990b), Ye et al. (1996), Kelly et al. (1993), Boonchan (1998), Stenotrophomonas Juhasz (1998), Lal and Khanna (1996), Shuttleworth and Cerniglia (1996), maltophilia, Acinetobactercalcoaceticus, Mahro et al. (1995), Willumsen and Karlson (1998), Sepic et al. (1998), Acidovoraxdelafieldii, Gordona Willumsen et al. (1998), Churchill et al. (1999), Chen and Aitken (1999) sp., Sphingomonas sp., P. saccharophilia, Pasteurella sp. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 Pyrene 220 A. denitrificans, Mycobacterium sp., Rhodococcus Heitkamp et al. (1988a), Walter et al. (1991), Weissenfels et al. (1991), sp., Sp. paucimobilis, Grosser et al. (1991), Schneider et al. (1996), Ye et al. (1996), Stenotrophomonasmaltophilia, Boonchan (1998), Juhasz (1998), Kastner et al. (1998), Lal and Khanna Acinetobactercalcoaceticus, Gordona sp., (1996), Mahro et al. (1995), McNally et al. (1999), Jimenez and Bartha Sphingomonas sp., P. putida, Bu cepacia, P. (1996), Churchill et al. (1999), Juhasz et al. (1997), Chen and Aitken (1999) saccharophilia Chrysene Rhodococcus sp., P. marginalis, Sp. paucimobilis, Walter et al. (1991), Burd and Ward (1996), Ye et al. (1996), Boonchan Stenotrophomonas (1998), Lal and Khanna (1996), Aitken et al. (1998), Chen and Aitken maltophilia, Acinetobacter calcoaceticus, (1999) Agrobacterium sp., Bacillus sp., Burkholderia sp., Sphingomonas sp., Pseudomonas sp., P. saccharophilia Benz[a] A. denitrificans, Beijernickia sp., P. putida, Sp. Gibson et al. (1975), Maha€ey et al. (1988), Weissenfels et al. (1991), anthracene paucimobilis, Schneider et al. (1996), Ye et al. (1996), Boonchan (1998), Juhasz (1998), Stenotrophomonasmaltophilia, Agrobacterium Aitken et al. (1998), Chen and Aitken (1999) sp., Bacillus sp., Burkholderia sp., Sphingomonas sp., Pseudomonas sp., P. saccharophilia Dibenz[a,h] Sp. paucimobilis, Stenotrophomonasmaltophilia Ye et al. (1996), Boonchan (1998), Juhasz (1998) anthracene IJOART 3.3 Anaerobic PAH Degradation: Rapid depletion of dissolved oxygen during PAH degradation results in decrease of redox potential which eventually favours the growth of denitrifying, sulphate reducing and even methanogenic microbial population [51], [52] proposed links of bacterial genera like Bacillus, Rhodococcus and Herbaspirillum with anaerobic anthracene degradation using 16srRNA analysis under methanogenic conditions in an aquifer.[53] suggested that the archaeal members most closely affiliated with Methanosaeta and Methanocellues and bacterial members most closely realted to “Clostridiaceae” play an important role in methanogenic metabolism of substituted. There has been tremendous interest in understanding the fate of PAHs in ground water subsurface environments, that are largely micro-aerobic or anaerobic [51]. In anaerobic conditions like that of aquifers the degradation of PAH compounds depend on the availability of suitable nutrients and soil microorganisms which can degrade the compounds through utilization of electron acceptors other than oxygen.[54]. Microbial transformation of aromatic compounds under denitrifying, sulphate-reducing and methanogenic conditions, however is fundamentally different from degradation under aerobic condition [51] Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 221 Kartikeyan A & Bandari A [51] summarized the suggested pathways of anaerobic bacterial degradation involving enzymes that include CoA ligase, oxido-reductases and decarboxylases. The possible peripheral metabolic reactions occurring during the anaerobic transformation process are carboxylation, reductive dehydroxylation, reductive deamination, reductive dehydroxylation, oxidation of carboxymethyl groups, methyl oxidation,odemethylation, trans- hydroxylation and decarboxylation. [55] identified carboxylation reaction as a prototype for the initial activation of anaerobic degradation of naphthalene. Meckenstock., etal [56], studied the degradation of naphthalene by sulphate-reducing culture isolated from a freshwater source. The authors suggested a stepwise reduction of the aromatic ring system before ring cleavage. The first step is the carboxylation of the aromatic ring to 2naphthoic acid, which may activate the aromatic ring prior to hydrolysis. Stepwise reduction of 2-naphthoic acid via a series of hydrogenation reactions results in decaclin-2-carboxylic acid which is subsequently converted to decahydro-2-naphthoic acid [57] In a similar study by [58], anaerobic degradation of naphthalene, 2- IJOART methylnaphthalene, and tetralin (1,2,3,4-tetrahydronaphthalene) was investigated with a sulfate-reducing enrichment culture obtained from a contaminated aquifer (fig) .It was proposed that the naphthalene (compound A) or2-methylnaphthalene (B) is activated in peripheral, upper degradationpathways to generate 2-naphthoic acid (I), naphthoic acid isthen reduced to 5,6,7,8-tetrahydro-2-naphthoic acid (II), whichis also the entry of anaerobic tetralin (C) degradation into thepathway. A further hydrogenation may lead to octahydro-2napthoic acid (III), which could be hydrated to generate hydroxydecahydro-2-naphthoic acid (V) and subsequently oxidizedto oxodecahydro-2-naphthoic acid (VI). Decahydro-2naphthoic acid (IV) is probably a dead-end metabolite. Athiolytic ring cleavage and a subsequent oxidation can generatethe tentatively identified C 11 H 16 O 4 -diacid (VII). Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 222 IJOART Fig3: Proposed reductive 2-naphthoic acid pathway of anaerobic PAH degradation. (I) 2-naphthoic acid, (II) 5,6,7,8-tetrahydro-2-naphthoic acid, (III) hydroxydecahydro-2-naphthoic acid, (IV) b-oxo-decahydro-2 naphthoic acid, (V) C 11 H16 O 4 -diacid, (VI) 2- carboxycyclohexylacetic acid. Compounds III and IV are putative intermediates. [58] 3.4 Alkyl Substituted Poly Aromatic Hydrocarbons: The ring structure of PAH compounds are generally substituted with alkyl groups having one to four saturated carbon atoms, producing different structural isomers and homologs for each poly aromatic hydrocarbon family. Abundance of alkyl substituted PAHs such as alkyl naphthalenes, alkyl phenanthrenes and alkyl dibenzothiophenes are found in fossil fuels, crude oil, petroleum and petroleum derived products [59].Alkylated PAH’s are found to be more abundant, less water soluble and persistent than their respective “Parent” Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 PAH compound [60]. Alkyl PAHs like 1-Methylanthracene 223 and 7, 12- Diethylbenzo[a]anthracene are among the 16 Priority pollutant PAHs designated by US-EPA. Within an aromatic series, acute toxicity increases with increasing alkyl substitution on the aromatic nucleus [60]. Since the presence of alkyl branch inhibits the proper orientation and accessibility of PAHs to dioxygenase enzyme resulting the involvement of more diverse enzymes. These include oxidation of methyl group to alcohol, aldehyde, or carboxylic acid, decarboxylation, demethylation, and dioxygenation [59]. Mahajan et al [61], suggested the possibility of occurrence of multiple pathways in the degradation of 1 and 2 methyl naphthalene. Enzyme activity study of Pseudomonas putida CSV86, growing on 1&2 methyl naphthalene in one of the proposed pathway suggests the oxidation of the aromatic ring adjacent to the one bearing the methyl moiety ,leading to the formation of methyl-salicylates and methyl catechols. In the other pathway the methyl side chain is hydroxylated to –CH 2 OH which is further converted to –CHO and –COOH, resulting IJOART in the formation of napthoic acid as the end product. Annweiler et al, [60] investigated the anaerobic degradation of 2-methyl naphthalene by a sulphate reducing enrichment culture. The findings proposed the addition of fumarate to the methyl group of 2-methylnapthalene as the first activation step by naphthyl-2-methyl succinate synthase, Napthyl-2-methyl-succinic acid is activated by a succinyl CoA dependent CoA transferase and subsequent oxidation to yield napthyl-2 methylene succinyl CoA. A sequence of reactions proceeds via beta oxidation and leads to the 2-napthoic acid CoA ester intermediate.(Fig 4). Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 224 IJOART FIG 4.. Proposed scheme of the upper pathway of anaerobic 2-methylnaphthalene (1) degradation to the central intermediate 2-naphthoic acid ( 2), fumaric acid; (3), naphthyl-2-methyl-succinic acid;(4), naphthyl-2-methylsuccinyl- CoA; (5), naphthyl-2-methylene-succinyl-CoA;(6), naphthyl-2-hydroxymethyl-succinylCoA; (7), naphthyl-2-oxomethyl-succinyl-CoA. Asterisk marked compounds were identified as free acids. 3.5Fungal Degradation Some fungi have the enzymatic apparatus to degrade a wide variety of structurally diverse PAHs via pathways that are generally similar to those used by mammalian enzyme systems [16]. Many fungi oxidize PAHs via a cytochrome P-450 monooxygenase by incorporating one atom of the oxygen molecule into the PAH to form an arene oxide and the other atom into water. Most arene oxides are unstable and can undergo either enzymatic hydration via epoxide hydrolase to form trans-dihydrodiols or nonenzymatic rearrangement to form phenols, which can be conjugated with sulfate, glucose, xylose, or glucuronic acid. Whereas a diverse group of non ligninolytic fungi is able to oxidize PAHs to transCopyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 225 dihydrodiols, phenols, tetralones, quinones, dihydrodiol epoxides, and various conjugate of the hydroxylated intermediates, only a few have the ability to degrade PAHs to CO 2 [16]. Fungal genera, namely, Amorphoteca, Neosartorya, Talaromyces, and Graphiumwere isolated from petroleum contaminated soil and proved to be the potential organisms for hydrocarbon degradation [62] .A group of terrestrial fungi, namely, Aspergillus, Cephalosporium, and Pencillium which were also found to be the potential degrader of crude oil hydrocarbons [63]. The yeast species, namely, Candida lipolytica, Rhodotorula mucilaginosa, Geotrichum sp, and Trichosporon mucoides isolated from contaminated water were noted to degrade petroleum compounds [64]. White-rot fungi have remarkable potential to degrade PAHs. Degradation of substrate is carried out through the action of extracellular enzymes, the best characterized of which are laccase, lignin peroxidases and manganese peroxidases. The specificity of these enzymes is low, so the substrate spectrum is broad. Other advantage of this non-specific mode of enzyme IJOART action is that it does not require preconditioning to individual pollutants and avoids the uptake of potentially toxic substances [65]. Furthermore, because the induction of the degradative enzymes is independent of the presence of the pollutants, the fungi can degrade pollutants at extremely low concentrations. White-rot fungus, Phanerochaete chrysosporium, has been reported to mineralise phenanthrene, fluorene, fluoranthene, anthracene and pyrene in nutrient-limited cultures [66]. Degradation of BaP to carbon dioxide and water has also been reported [67]. However, since a huge amount of fungal inoculum is required for bioremediation, it makes this technique costly also achieving homologous distribution of mycelium into the soil remains a setback for employment of fungal strains for bioremediation [16]. . Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 226 Table 5: Polycyclic aromatic hydrocarbons oxidised by different species of fungi PAH Organisms References Absidaglauca, Aspergillusniger, Basidiobolusranarum, Candida utilis, Choanephoracampincta, Circinella sp., Clavicepspaspali, Cokeromyces poitrassi, Conidiobolusgonimodes, C. bainieri, C. elegans, C. japonica, Naphthalene Emericellopsis sp., Epicoccumnigrum, Gilbertellapersicaria, Gliocladium sp., Helicostylumpiriforme, Hyphochytriumcatenoides, Linderinapennispora, Mucorhiemalis, Neurosporacrassa, Cerniglia and Gibson (1977), Cerniglia Panaeoluscambodginensis, Panaeolus subbalteatus, et al. (1978, 1982a), Penicilliumchrysogenum, Pestalotia sp., Phlyctochytrium Smith and Rosazza (1974), Cerniglia reinboldtae, Phycomyesblakesleeanus, Phytophthoracinnamomi, and Crow (1981), Ferris etal. (1973) Psilocybe cubensis, Psilocybestrictipes, Psilocybestuntzii, Psilocybesubaeruginascens, Rhizophlyctisharderi, Rhizophlyctisrosea, Rhizopusoryzae, Rhizopus stolonifer, S. cervisiae, Saprolegniaparasitica, Smittiumculicis, Smittium culisetae, Smittiumsimulii, Sordaria ®micola, Syncephalastrumracemosum, Thamnidiumanomalum, Zygorhynchusmoelleri IJOART Pothuluri et al. (1992a), Johannes et al. Acenaphthene C. elegans, T, versicolor (1998) Cerniglia and Yang (1984), Cerniglia et Phenanthrene C. elegans, P. chrysosporium, P. laevis, Pleurotusostreatus, T. versicolor, al. (1989), Morgan et al. (1991), Bjerkanderaadjusta, Pleurotusostreatus, Cylindrocladium simplex, Sutherland et al. (1991), Bumpus Monosporiumolivaceum, Curvularialunata, Curvulariatuberculata, (1989), Hammel et al. (1992), Bezalel et Laetiporus al. (1996), Brodkorb and Legge (1992), sulphureus, Daedaelaquercina, Flamulinavelutipes, marasmiellus sp., Schutzendubel et al. (1999), Lisowska Penicullium sp., Kuehneromycesmutabilis, Laetiporussulphureus, and Dlugonski (1999), Bogan and Agrocybe aegerita, Aspergillusniger, Syncephalastrumracemosum Lamar (1996), Sack & Gunther (1993), Sack et al. (1997a), Collins and Dobson (1996), Casillas et al. (1996), Sutherland et al. (1993) Anthracene Bjerkandera sp., Bjerkanderaadjusta, C. elegans, P. chrysosporium, P. Cerniglia (1982), Cerniglia and Yang laevis, Ramaria sp., R. solani, Trametesversicolor, Pleurotusostreatus, (1984), Hammel et al. Cylindrocladium simplex, Monosporiumolivaceum, Curvularialunata, (1991), Sutherland et al. (1992), Field et Curvulariatuberculata, Cryphonectriap arasitica, al. (1992), Collins et al. Ceriporiopsissubvermispora, Oxysporus sp., Cladosporiumherbarum, (1996), Schutzendubel et al. (1999), Drechsleraspicifera, Verticillium Lisowska and Dlugonski (1999), lecanii, Fusariummoniliforme, Rhizopusarrizus, Coriolopsispolyzona, Krivobok et al. (1998), Andersson and Laetiporussulphureus, Daedaelaquercina, Flamulinavelutipes, Henrysson (1996), Johannes et al. marasmiellus sp., Penicullium sp. (1996), Bogan et al. (1996), Bogan and Lamar (1996), Vyas et al. (1994), Sack and Gunther (1993) C. elegans, C. blackesleeana, C. echinulata, Bjerkanderaadjusta, Fluoranthene Pleurotus ostreatus, Sporormiellaaustralis, Cryptococcus albidus, Pothuluri et al. (1990, 1992b), Cicinoboluscesatii, Pestalotiapalmarum, Beauveria alba, Schutzendubel et al. (1999), Salicis Aspergillusterreus, Mortierella ramanniana, Rhizopusarrhizus, et al. (1999), Sack and Gunther (1993) Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 227 Laetiporussulphureus, Daedaelaquercina, Flamulinavelutipes, marasmiellus sp., Penicullium sp. Pyrene C. elegans, P. chrysosporium, Penicillium sp., P. janthinellum, P. Cerniglia et al. (1986), Hammel et al. glabrum, P.ostreatus, Syncephalastrumracemosum, Bjerkanderaadjusta, (1986), Launen et al.(1995), Bezelel et Pleurotus sp., Dichomitussqualens, Flammulinavelutipe, al. (1996), Schutzendubel et al. (1999), Trammetesversicolor, Kuehneromycesmutabilis, Laetiporussulphureus, Martens & Zadrazil (1998), Sack et al. Agrocybeaegerita (1997b), Wunder et al. (1997), Sack and Fritsche (1997), Lang et al. (1996), Stanley et al.(1999), Boonchan (1998) Cerniglia et al. (1980a), Andersson and Chrysene P. janthinellum, Syncephalastrumracemosus, Penicillium sp. Henrysson (1996), Bogan and Lamar (1996), Stanley et al. (1999), Boonchan (1998) Kiehlmann et al. (1996), Stanley et al. Benz[a] anthracene C. elegans, Trametesversicolor, P. laevis, P. janthinellum (1999), Boonchan (1998) Dibenz[a,h]anth Trametesversicolor, P. janthinellum Andersson and Henrysson (1996), Stanley et al. (1999), Boonchan (1998) racene IJOART 3.6` Degradation of PAH compounds by Algae and Cyanobacteria. Algae and cyanobacteria have also been shown to oxidise PAH [2]. Eukaryotic algae and cyanobacteria (blue-green algae) oxidize PAHs under photoautotrophic conditions to form hydroxylated intermediates. Cyanobacteria oxidize naphthalene and phenanthrene to metabolites that are similar to those formed by mammals and fungi. In contrast, the green alga Selenastrum capricornutum oxidizes benzo[a]pyrene to isomeric cis-dihydrodiols similar to bacterial metabolites [68]. Walker et al [69] isolated an alga, Protothecazopfi which was capable of utilizing crude oil and a mixed hydrocarbon substrate and exhibited extensive degradation of n-alkanes and iso-alkanes as well as aromatic hydrocarbons. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 228 Table 6:Polycyclic aromatic hydrocarbons oxidised by different species of cyanobacteria and algae [45] PAH Organism Reference Oscillatoria sp., Microcoleuschthonoplastes, Nostoc sp., Anabaena sp., Agmenellumquadruplicatum, Coccochloriselabens, Cerniglia et al. (1979, 1980d, 1980b, 1982b), Narro et al. Naphthalene Aphanocapsa sp., Chlorella sorokiniana, Chlorella autotrophica, (1992a) Dunaliellatertiolecta, Chlamydomonasangulosa, Ulvafasciata, Cylindrotheca sp., Amphora sp., Nitzschia sp., Navicula sp., Porphyridiumcruentum Phenanthrene Oscillatoria sp., Agmenellumquadruplicatum Narro et al. (1992b) 3.7Biodegradation by Yeast IJOART Studies on yeast able to use various petroleum components as sole carbon source, showed that their biodegradability decreases from n-alkanes >branched alkanes>low molecular weight aromatic hydrocarbons >cycloalkanes>high molecular weight aromatic and polar compounds. Yeasts, however cannot grow on polycyclic aromatic hydrocarbons (PAH) but are able to cooxidizebiphenyl, naphtalene and benzopyrene using the mono-oxigenase cytochrome P450 pathway induced by the presence of n-alkanes. Studies on fungi and yeast (Candida,Rhodotorula, Trichosporon) communities from aquatic environments polluted with PAH, especially phenantherene, revealed high degradation rates for Trichosporon penicillatum [70]. 3.8 Field Scale Application of Biodegradation of PAHs The bioremediation of PAH contaminated sites include a wide range of remediation strategies like land farming, slurry-phase bioreactors, land-treatment systems, enhanced washing of PAHs from soil using surfactants, combination of advanced chemical oxidation with bioremediation by thermal pre-treatment etc. An engineered land treatment system was operated for the treatment of soils containing wood-preserving chemicals at a creosote wood-treatment site. The landtreatment unit contained polluted soil which was nutrient amended, tilling weekly and Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 229 moisture was applied periodically; pH was maintained at 6.5-7.5. The decrease in total PAH concentration was seen during active bioremediation period from 21%-82%.The treatment period s ranged from 2months to 12 months. The end point was observed at 1000mg/Kg [16]. Pilot study of slurry phase removal of 10,000 tons of creosote-contaminated soil from the southern wood preserving superfund site in Canton, Missippi [71] was performed by the American Company OHM remediation services Corporation. Four slurry phase bioreactors were employed each with volume of 680m3. The soil was sieved to less than 200 mesh and Aeration of the slurry was provided by diffusers and blower. The initial total PAH concentration ranged from 8000 to 1500 mg/kg, the concentration of carcinogenic PAH was from 1000 to 2500 mg/kg. The majority of the PAH biodegradation occurring in the initial 5-10 days of treatment. The treatment goal was easily achieved after 10days residence time in a bioreactor [71]. IJOART 4. FACTORS AFFECTING PAH DEGRADATION A number of physical, chemical, biological or environmental factors may influence the rate and extent of PAH degradation [72] 4.1Bioavailability (Use of Biosurfactant): The composition and inherent biodegradability of the petroleum hydrocarbon pollutant is the first and foremost important consideration when the suitability of a remediation approach is to be assessed [73]. Bioavailability could be the rate determining factor for the degradation of PAH. Decrease in the mineralisation of high molecular weight PAHs attributed to the association of PAHs to soil organic matter [74],[75] which results in the reduction of the rate and extent of PAH degradation due to the slowing of PAH desorption from soil organic matter into the soil aqueous phase [76]. According to Leahy & Colwell [10], biosurfactants are important agents in the effective uptake of PAHs by bacteria and fungi. The formation of emulsions in the presence of biosurfactants is reported to be in 96% of hydrocarbon metabolizing freshwater bacteria [77]. Additives and bulking agents enhance the overall hydrocarbon catabolism [78] .The use Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 230 of surfactants like SDS, TritonX-102, Brij 35, Marlipal 013/90 and Genapol X150 increases the concentration of hydrophobic compounds in the water phase by solubilisation or emulsifycation [79]. The increase of biodegradation rate has been observed in studies in which organic solvents were used to improve PAH-bioavailability to bacteria [80]. Soils pre-treated with solvents like acetone and ethanol showed faster biodegradation rate than soils without pretreatment. Chemotactic migration of mobile bacterial cells towards or away from the elevated level of attractant chemical compounds has also been found to play an important role in the biodegradation of organic compounds. Calvo ortega et al [81] Chemotaxis of PAH degrading microorganisms present in polluted rhizosphere soils inferred that the chemotactic attraction towards PAHs increase their bioavailability and consequently the biodegradation rate of PAH. 4.2 pH IJOART pH is an important factor for the degradation activity of introduced microorganisms in the soil or water systems. PAH mineralization is favoured by near neutral pH values [82]. Small pH shifts have dramatic effects on the degradation of low concentrations of PAH’s in oligotrophic aquatic environments [83]. However fungi are known to be more tolerant to acidic conditions. Biodegradation study of PAH’s in extremely acidic environments in the presence of acidophilic microorganisms by [84] reported that the indigenous microorganisms oxidized about 50% of the supplied naphthalene to CO 2 and water within 24 weeks, while the extent of mineralization of phenenthrene and anthracene was only 10-20%. It was suggested that initial fungal attacks on the hydrocarbons may have produced intermediates that were available for further degradation by bacteria [82]. 4.3Temperature Temperature plays a significant role in controlling the natural and the extent of microbial hydrocarbon metabolism, which is of special significance for in-situ bioremediation. [82]. Temperature also affects the bioavailability and solubility of hydrocarbons [85] possessing direct effect on the physical nature and chemical composition Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 231 of the PAHs constituents [46]. At low temperature PAHs tend to be more viscous with less microbial growth and propagation [86]. Studies show increased temperature made PAH’s more bioavailable to microorganisms because the increased temperature made the PAH’s more soluble [57]. In a field study done by [87].on the biodegradation of dispersed crude oil in cold and icy seawater (-1.8 to5.50 C), half-life times of PAHs ranged from 1.5-1.7 days (naphthalene) to 2.4-7.5 days(phenanthrene) under favourable conditions, i.e. at temperature above 0ºC and with effective chemical dispersion. The highest degradation rates that generally occur are in the range of 30-40 ºC in soil environments and 15-20oC in marine environment [88],[89], [90] isolated microorganisms which are able to convert naphthalene, phenanthrene and antheracene under thermophilic conditions. Bacillus thermoleovorans thermophilic bacteria degraded naphthalene at 60oC showing significantly different metabolites and different metabolic pathway known for mesophilic bacteria. For mesophilic bacteria at temperature above optimal, enzymatic activities are inhibited as protein denatures [10]. 4.4Nutrients IJOART Apart from degradable carbon source in the form of PAH compounds, microorganisms require mineral nutrients such as nitrogen (N), phosphate (P) and potassium (K) for cellular metabolism and growth. In contaminated sites, where organic carbon levels are often high due to the nature of the pollutant, available nutrients like Nitrogen, phosphorous and in some cases Iron (Fe)[89] rapidly deplete during microbial metabolism hence become limiting factor and greatly affect the microbial degradation of hydrocarbons [72] e.g in marine environment where low levels of Nitrogen and Phosphorous are found [86]. Therefore it is common practice to supplement contaminated land with nutrients, generally nitrogen and phosphates to stimulate the in situ microbial community and therefore enhance bioremediation. Fungi are able to effectively recycle nutrients (specifically nitrogen). In fact, the high molecular weight PAH-oxidising ligninolytic enzymes of the white-rot fungi are produced under nutrient deficient (often low nitrogen) conditions. Excessive nutrient concentrations can also inhibit the biodegradation activity [91] negative effects of high N, P, K levels on aromatic hydrocarbon degradation have also been reported [92],[93], [94]. 4.5Salinity: There is an inverse relationship between salinity and solubility of PAHs, with the increase in salinity there was an increase in the sorption of aromatic hydrocarbon as seen in Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 232 pyrene in various sediment types, was due to the salting-out effects occurring in both the solution and solid (sediment organic matter) phases. However hyper salinity results in the decreased microbial growth but an unidentified halophytic archaeon was found to degrade PAH’s (acenaphtene, phenanthrene, anthracene; 500mg/l. Four bacterial strains, belonging to the genera Micrococcus, Pseudomonas and Alcaligenes and tolerating 7.5% w/v NaCl, could grow on 0.1% naphthalene and anthracene. [95] Rhykerd et al [96] Showed inhibitory effect of artificial salinity on mineralization of Petroleum oil (50g/ Kg soil),soils fertilized with inorganic N and P and emended with NaCl ( 0.4,1.2 and 2% w/w) . Highest salt concentration after 80 days at 25º C had considerably inhibited the mineralization of petrochemical compounds. Thus the removal of salt from PAH contaminated soils may reduce the time required for bioremediation, however some indigenous microorganisms are expected to be salt adapted. 4.6 Oxygen IJOART The amount of available oxygen will determine whether the system is aerobic or anaerobic. PAHs degradation occurs primarily under aerobic condition, however in anaerobic environment such as aquifers and marine sediments anaerobic biodegradation has also been reported [97] with negligible rate and were initially limited to halogenated aromatic compounds like halobenzoates chlorophenols etc only [98], [99].Though it is now well established that bioremediation of organic contaminants such as PAHs can proceed under both aerobic and anaerobic conditions. During aerobic PAH metabolism, oxygen is integral to the action of mono- and dioxygenase enzymes in the initial oxidation of the aromatic ring. In the absence of molecular oxygen, alternative electron acceptors such as nitrate, ferrous iron and sulphate are necessary to oxidise these aromatic compounds, with recent research clearly demonstrating that PAH degradation will occur under both denitrifying [100] and sulfatereducing [97] anaerobic conditions. Promotion of anaerobic bioremediation however has several drawbacks, for not all environments contain an active population of anaerobic PAH degraders. Also, under anaerobic conditions when electron acceptors like nitrate, ferric iron and sulphate are reduced, this results in the release of excess of phosphorous and ferrous iron, both of which are toxic to the environment. In addition, release of greenhouse gases (CH 4 , NO 2 etc) and increase in pH has also been observed during anaerobic degradation of PAH. [57]. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 233 To increase the oxygen amount in the soil it is possible to till or sparge air. In some cases, hydrogen peroxide or magnesium peroxide can be introduced in the environment. 4.7Genetic Enhancement: A bacterium needs the appropriate catabolic gene in order to be a degrader of a compound these catabolic genes can be either chromosomal or plasmid borne. The metabolic pathways for compounds such as naphthalene, salicylate, camphor, octane, Xylene and toluene have been shown to be encoded on plasmid in Pseudomonas spp.by [101] Applications for genetically engineered microorganisms (GEM) in bioremediation have received a great deal of attention, but have largely been confined to the laboratory environment. This has been due to regulatory risk assessment concerns and to a large extent the uncertainty of their practical impact and delivery under field conditions. There are at least four principal approaches to GEM development for bioremediation application. These include: IJOART (1) modification of enzyme specificity and affinity, (2) pathway construction and regulation, 3) bioprocess development, monitoring, and control, and (4) bioaffinity bioreporter sensor applications for chemical sensing, toxicity reduction, and end point analysis. [102] Collective metabolism by mixed culture of microorganism may result in enhanced PAH utilization as compared to single bacterial strain degradation by indigenous community of bacteria. 5. Current Approaches to Improve PAHs Degradation 5.1 Bioaugementation Bioaugementation is an in situ treatment method involving the addition of microorganisms indigenous or exogenous to the contaminated sites. The addition of exogenous microorganisms with PAH degrading capabilities overcome the catabolic limitations of the indigenous microflora towards PAH [2], [90] and [103] proposed that Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 234 bioaugementation is especially important for sites containing high PAH concentrations, site which contain a significant proportion of high molecular weight PAHs and for recently polluted soils which do not have an adapted microbial population. Bioaugementation study demonstrated by [2] showed significant decrease in the concentration of all PAH compounds including Benzo(a) pyrene at bench scale trials for the clean-up of petroleum contaminated soil using a mixed bacterial culture isolated from MGP site [104] and had previously shown the ability to degrade three-,four-,five- and seven-ring PAH compounds. Some factors however limit the use of added microbial cultures in land which include die-off of the laboratory strains, and the inability of the inocula to compete with the indigenous microflora to develop and sustain useful population levels. 5.2 Bacterial-Fungal Co-cultures Degradation of PAH is by bacteria is often limited by the incapability of the organisms to hydroxylate the compound, and the inability of high molecular weight PAH compounds like BaP to pass through bacterial cell wall [105]. Since fungi have the ability to IJOART produce extracellular enzymes (lignin-degrading enzymes)the initial transformation of PAH by fungi followed by bacterial degradation of polar metabolites could lead to an effective strategy for PAH degradation[2]. Gomez et.al [106]performed a study consisting of sixteen co-cultures composed of four bacteria and four fungi grown on sugarcane bagasse pith were tested for phenanthrene degradation in soil. The four bacteria were identified as Pseudomonas aeruginose, Ralstonia pickettii, Pseudomonas sp. and Pseudomonas cepacea. The four fungi were identified as: Penicillium sp., Trichoderma viride, Alternaria tenuis and Aspergillus terrus that were previously isolated from different hydrocarbon-contaminated soils. Fungi had a statistically significant positive (0.0001<p) effect on phenanthrene removal, that ranged from 35% to 50% and bacteria removed the compound by an order of 20%.Co-cultures B. cepaceaPenicillium sp., R. pickettii-Penicillium sp., and P. aeruginose-Penicillium sp. exhibited synergism for phenanthrene removal, reaching 72.84 ± 3.85%, 73.61 ± 6.38% and 69.47 ± 4.91%; in 18 days, respectively. 5.3 Application of Immobilized Cells Immobilized cells have been used and studied for the bioremediation of numerous toxic chemicals. Immobilization not only simplifies separation and recovery of immobilized Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 235 cells but also makes the application reusable which reduces the overall cost [107] used free suspension and immobilized Pseudomonas sp. to degrade petrol in an aqueous system. The study indicated that immobilization resulted in a combination of increased contact between cell and hydrocarbon droplets and enhanced level of rhamnolipids production. Immobilization can be done in batch mode as well as continuous mode. Packed bed reactors are commonly used in continuous mode to degrade hydrocarbons. It can be concluded that immobilization of cells is a promising application in the bioremediation of hydrocarbon contaminated site. 5.4 Commercially Available Bioremediation Agents Microbiological cultures, enzyme additives, or nutrient additives that significantly increase the rate of biodegradation to mitigate the effects of the discharge were defied as bioremediation agents by U.S.EPA [108]. Bioremediation agents are classified as bio- IJOART augmentation agents and bio-stimulation agents based on the two main approaches to oil spill bioremediation. The U.S. EPA compiled a list of 15 bioremediation agents [100, 101] as a part of the National Oil and Hazardous Substances Pollution Contingency Plan (NCP) Product Schedule, which was required by the Clean Water Act, the Oil Pollution Act of 1990(Table 7) Studies showed that bioremediation products may be effective in the laboratory but significantly less so in the field. However, However, due to the limitations of common fertilizers (e.g., being rapidly washed out due to tide and wave action), several organic nutrient products, such as oleophilic nutrient products, have recently been evaluated and marketed as bioremediation agents. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 236 Table 7: Bioremediation agents in NCP product schedule [16] Name or Trademark Product Type Manufacture BET BIOPETRO MC BioEnviro Tech, Tomball, TX BILGEPRO NA International Environmental Products, LLC, Conshohocken, PA. INIPOL EAP 22 NA Societe, CECA S.A., France LAND AND SEA NA Land and Sea Restoration LLC, San Antonio RESTORATION MC MICRO-BLAZE Verde Environmental, Inc., Houston, TX IJOART OIL SPILL EATER II NA/EA Oil Spill Eater International, Corporation, Dallas, TX OPPENHEIMER FORMULA PRISTINE SEA II MC Oppenheimer Biotechnology, Inc., Austin, TX MC Marine Systems, Baton Rouge, LA SYSTEM E.T. 20. MC Quantum Environmental Technologies, Inc(QET), La Jolla, CA VB591TMWATER, NA VB997TMSOIL, BioNutraTech, Inc., Houston,TX AND BINUTRIX WMI-2000 MC WMI International, Inc Abbreviations of product type: MC: Microbial Culture,EA: Enzyme Additive, NA: Nutrient Additive Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 237 5.5 Phytoremediation Phytoremediation is an emerging technology that uses plants to manage a wide variety of environmental pollution problems, including the cleanup of soils and groundwater contaminated with hydrocarbons and other hazardous substances. The different mechanisms, namely, hydraulic control, phytovolatilization, rhizoremediation, and phytotransformation. could be utilized for the remediation of a wide variety of contaminants. Advantages of using phytoremediation include cost-effectiveness, aesthetic advantages, and long-term applicability. 5.6 Genetically Modified Bacteria and Use of Plasmids IJOART Studies on PAH metabolism are entering a new era with the application of genetically engineered microorganisms (GEM) s for bioremediation processes. Modified microorganisms have shown potential for bioremediation of many chemical contaminants. However, ecological and environmental concerns and regulatory constraints are major obstacles for testing GEM in the field. The use of genetically engineered bacteria was applied to bioremediation process monitoring, strain monitoring stress response, end-point analysis and toxicity assessment. A bacterium needs the appropriate catabolic genes in order to be a degrader of a compound. Many of the genes involved in the degradation of PAHs are often located on plasmids [109]. Plasmids that carry structural genes that code for the degradation of many naturally occurring organic compounds and xenobiotics, are referred to as degradative or catabolic plasmids. A plasmid may encode a complete degradative pathway or partial degradative step. Some other plasmids code for enzymes that have specificity for several substrates. For example, the genes encoding the upper and lower pathways of naphthalene in the NAH plasmids of several pseudomonads have broad specificities, allowing the host to grow on several two and three -ring PAHs, as sole carbon and energy sources [110]. Dunn &Gunsalus [111] reported for the first time, the involvement of plasmids in the degradation of PAHs. All the genes involved in the degradation of naphthalene by Pseudomonas putida PpG7 are now known to be plasmid borne and transmissible [112],[113] Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 238 demonstrated that the genes encoding the enzymes of the first 11 steps of the naphthalene oxidation pathway are located on the NAH7 plasmid. In plasmid NAH7, the naphthalene catabolic genes are organized into two operons; nah and sal. The naphthalene oxidation genes are organized in two operons. The first operon includes genes nahABCDEF, coding for the conversion of naphthalene to salicylate, and the second operon includes genes nahGHIJK, coding for the oxidation of salicylate via the catechol meta-cleavage pathway. The naphthalene dioxygenase enzyme encoded by the NAH7 genes is known today to be a highly versatile enzyme system, encoding a wide range of reactions [114], [115],[102] produced the first report which provides direct biochemical evidence that the naphthalene plasmid degradative enzyme system is involved in the degradation of higher-molecularweight polycyclic aromatic hydrocarbons other than naphthalene. Zhou. et.al [116] successfully cloned a bio-degradative gene encoding catechol 2,3dioxygenase (C23O) from Pseudomonas sp. CGMCC2953, isolated from oil-polluted soil, into the plasmid pK4 derived from pRK415 with a broad host range. The apparent IJOART phenanthrene biodegradation parameters of the recombinant microorganism (Pseudomonas sp. CGMCC2953-pK) were determined and compared with those of the wild type. As the key enzyme of phenanthrene degradation, C23O, could be expressed constitutively in the recombinant strain, Pseudomonas sp. CGMCC2953-pK showed an increased ability to degrade phenanthrene. The excessive production of C23O in Pseudomonas sp. CGMCC2953-pK could serve as an effective approach to construct genetically engineered microorganisms for the bioremediation of environmental contaminations The combination of microbiological and ecological knowledge, biochemical mechanisms and field engineering designs are essential elements for successful in situ bioremediation using genetically modified bacteria. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 Table 8: Selected PAH degrading bacterial 239 plasmids and their hosts.[117] IJOART Table 9: Genes borne on the NAH7 plasmid and enzymes encoded by them. [117] Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 240 Protoplast fusion is another practically useful technology which has significant engineering applications in generating microbial strains with specific properties for environmental bioremediation. [118] constructed a high-efficiency phenanthrene-degrading bacterial fusant strain F14 protoplast fusion between Sphingomonas sp. GY2B (GenBank DQ139343) and Pseudomonas sp. GP3A (GenBank EU233280). Results showed within 24 hours Phenanthrene could be almost completely degraded by the fusant strain F14, which was much quicker than GY2B and GP3A.The fusant strain F14 had a wider range of temperature (25-30 °C) and pH value (6.5-9.0) than GY2B did. Phenanthrene was metabolized through a pathway having less accumulation of potentially toxic metabolites than GY2B. The results demonstrated the feasibility of accelerating the phenanthrene degradation, enhancing the adaptability of bacteria, and accumulating less potentially toxic metabolite(s) by using the protoplast fusion technology. 5.7Bioinformatic Approach: PAHbase IJOART Bioinformatics based analysis and prediction is playing a pivotal role in understanding and capturing the in-depth knowledge of biological molecules particularly with reference to proteomics and genomics.[119]. Bioinformatics technology has been developed to identify and analyse various components of cells such as gene and protein functions, interactions, metabolic and regulatory pathways. Bioinformatics analysis will facilitate and quicken the analysis of bioremediation processes [120]. There is constantly increasing need for new ways of comparing multiple sets of data and information related to the occurrence and potential of PAH degrading bacteria [121]. Due to the generation of huge number of sequences and information from the fast and user friendly implementations of bioinformatics and in order to access and use the information about PAH degrading organisms details from the research papers were extracted, analysed and presented in form of a precise informative database: PAHbase: reflecting the diversity and functional analysis of PAHs degrading bacteria PAHbase (URL: www.pahbase.in.) is a freely available functional database of Polycyclic Aromatic Hydrocarbons (PAHs) degrading bacteria. The database consists of relevant information obtained from scientific literature and databases (i.e. NCBI, DDBJ and EMBL.). The database provides a comprehensible representation of PAH degrading bacteria Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 241 with reference to its occurrence, extremophilic nature (Halophilic/Thermophilic/Mesophilic), taxonomy and phylogenetic relatedness with nearby species, and stress adaptation, preferred PAH source of utilization as carbon source, biodegradative ability, media used in laboratory physical, chemical and environmental conditions provided for degradation, metabolic pathways , enzymes involved in degradation, genetic basis of degradation, gene/s involved and gene location,16S ribosomal gene sequence and references. 5.8Bacterial Biosensors Biosensors are analytical tools, which use the biological specificity in sensing the target molecule. Many studies demonstrate the design and application of molecular biosensors for use in bioremediation.[122]. The genetic information, located on a plasmid vector, is inserted into a bacterial strain so that the engineered fusion replicates along with the cell’s normal DNA. Biosensor systems include a wide range of integrated devices that IJOART employ enzymes, antibodies, tissues, or living microbes as the biological recognition element. Bacterial biosensors uniquely measure the interaction of specific compounds through highly sensitive bio-recognition processes and offer great sensitivity and selectivity for the detection and quantification of target compounds. Whole-cell biosensors, constructed by fusing a reporter gene to a promoter element induced by the target compound, offer the ability to characterize, identify, quantify, and determine the biodegradabilty of specific contaminants present in a complex mixture without pre treatment of the environmental samples [72]. The presence of toxic compounds and the potential associated ecological risks can be determined by using bacterial biosensor and toxicity tests. Several biosensors have been developed for the detection of many petrochemical waste compounds including PAH. [123],[124]constructed a biosensor for detecting the toxicity of PAHs in contaminated soilswith an immobilized recombinant bioluminescent bacterium, GC2 (lac::luxCDABE), which constitutively produces bioluminescence. The monitoring of phenanthrene toxicity was achieved through measurement of the decrease in bioluminescence when a sample extracted with the rhamnolipid biosurfactant was injected into a mini-bioreactor. This system was proposed to be used as an in situ system to detect the toxicity of hydrophobic contaminants in soils and for the performance evaluation of PAH degradation in soils. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 242 6) Conclusion and Future Prospects. The increasing incidents of oil spills and production of PAHs demand the degradation of complex hydrocarbons. The complex hydrocarbons which are otherwise harmful to soil and aquatic flora and fauna, must be degraded to simpler nontoxic compounds. A better understanding of the mechanisms of biodegradation has a high ecological significance that depends on the indigenous microorganisms to transform or mineralize the organic contaminants and the potential benefits of using genetically modified bacteria. Although bioremediation is generally regarded as an economical remediation option for the clean-up of PAH-Contaminated soil, the successful application of this technology is restricted to low molecular weight PAHs, however PAHs containing five or more fused benzene rings, such as BaP, is limited. Hence there is an urgent need to address this issue and to direct future research on expanding our knowledge on the practical application of co-metabolic process, bioaugementation, application of GEMs etc. IJOART Petroleum microbiology research is advancing on many fronts, spurred on most recently by new knowledge of cellular structure and function gained through molecular and protein engineering techniques combined with more conventional microbial methods. Improved systems for biodegradation of PAH compounds are being commercialized with positive economic and environmental advantages. Systems biology is an integrated research approach to study complex biological systems. Modern tools of genomics, transcriptomics, proteomics, metabolomics, phenomics and lipidomics have been applied to investigate systems biology of microbial communities in a myriad of environments .Currently, user friendly bioinformatics tools including “omics” tools (Qiime-qiime.sourceforge.net and Phylotrac-www.phylotrac.org/) provide comprehensive database of all available genomics, proteomics and metabolomics information from bioremediation research for scientists to exchange information leading to generation of judicious predictive models and strategies for successful implementation of bioremediation applications in future [125]. Ground breaking research is being done to engineer new biocatalysts and biosensors for achieving complete mineralization of high molecular weight PAH compounds Future research exploiting molecular techniques and metagenomic studies are expected to explore and harness microbial potential for effective Bioremediation. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 243 References [1] C.Balachandran, D.Duraipandiyan,K.Balakrishnan and IgnacimuthuS,“Petroleumand polycyclic aromatic hydrocarbons (PAHs) degradationand naphthalene metabolism in Streptomyces sp. (ERI-CPDA-1) isolated from oil contaminated soil”, J. BioRrech,10.10, 02-059, 2012. [2] A.L.Juhasz andR. Naidu,“Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: a review of the microbial degradation of benzo[a ]pyrene,” International Biodeterioration& Biodegradation 45,pp. 57-88 2000. [3] M.Vincent,“Microbial Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHs) in Oily Sludge Wastes”.J. Middle East, pp.1-13,2006. IJOART [4] S.Mishra, J.Jyot, R.C Kuhadand B Lal“Evaluation of Inoculum Addition To Stimulate In Situ Bioremediation of Oily-Sludge-Contaminated Soil.” Appl. Environ. Microbiol.,67,2001. [5] Q.D.Bach, S.J.Kim, S.C Choi and Y.S Oh, “Enhancing the Intrinsic Bioremediation of PAHContaminated Anoxic Estuarine Sediments with BiostimulatingAgents”,J. Microbiol. 43, 319,2005. [6] C.S.Holliger,G.Gaspard, Glod et al., “Contaminated environments in the subsurface and bioremediation: organic contaminants,” FEMS Microbiology Reviews, vol. 20, no. 3-4,pp. 517–523,1997. [7] J.I.Medina-Bellver, P.Marın, A. Delgado, et al.,“Evidence for in situ crude oil biodegradation after the Prestige oil spill,” Environmental Microbiology, vol. 7, no. 6, pp. 773–779,2005. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 244 [8] T.M.April, J.M. Foghtand R.S.Currah RS, “Hydrocarbon degradingfilamentous fungi isolated from flare pit soils in northern and western Canada,” Canadian Journal ofMicrobiology, vol. 46, no. 1, pp. 38–49,2000. [9] W. Ulrici, “Contaminant soil areas, different countries and contaminant monitoring of contaminants,” EnvironmentalProcess II. Soil Decontamination Biotechnology, vol. 11, pp. 5–42,2000. [10] J.G. Leahy and R.R. Colwell ,“Microbial degradation of hydrocarbons in the environment,” Microbiological Reviews, vol. 54, no. 3, pp. 305–315,1990. [11] C.E.Zobell, “Action of microorganisms on hydrocarbons,” Bacteriological Reviews, vol. 10, pp. 1–49,1946 [12] R.M.Atlas, “Microbial degradation of petroleum hydrocarbons: IJOART an environmental perspective,” MicrobiologicalReviews, vol. 45, no. 1, pp. 180–209, 1981. [13] R.M. Atlas and R.Bartha, “Hydrocabon biodegradation and oil spill bioremediation,” Advances in Microbial Ecology, vol. 12, pp. 287–338,1992. [14] J.M.Foght and D.W.S.Westlake, “Biodegradation of hydrocarbons in freshwater,” in Oil in Freshwater: Chemistry,Biology, Countermeasure Technology, pp. 217–230, Pergamon Press, New York, NY, USA, 1987 [15] N Das and P Chandran, “Microbial Degradation of PetroleumHydrocarbon Contaminants: An Overview”,Biotechnology Research International Volume 2011, Article ID 941810, 13 pages,2011. [16] U.Národní, ”Bioremediation Of Persistent Organic Pollutants - A Review” , Technologie a biotechnologieinventura POPs v ÈR Èást VII(2002). Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [17] 245 A. Mrozik. Z. Piotrowska-seget and S. LabuzekS,“Bacterial Degradation and Bioremediation of Polycyclic AromacticHydrocarbons”,Polish journal of Environ.Studies vol.12, No.1,pp.15-25(2003). [18] S.C.Wilson and K.C.Jones, “Bioremediation of soils contami-nated with polynuclear aromatic hydrocarbons (PAHs): a review.” Environmental Pollution 88, 229-249,1993. [19] J.Sikkema, J.A.M.Bont, B. Poolman“Mechanism of membrane of hydrocarbons”, Microbiol.rev 59,201,1995. [20] R.C.Sims and M.R. OvercashMR.,“Fate of polynuclear aromatic compounds (PNAs) in soilplant systems,” Residue Reviews 88, 1-68,1983. [21] A. Bjorseth, J. Knutzen and J.Skei, “Determination of polycyclic aromatic IJOART hydrocarbons in sediments and mussels from Saudafjord, W. Norway, by glass capillary gas chromatography.” Science of the Total Environment 13, 71-86,1979. [22] K.C.Jones, J.A. Stratford, K.S. Waterhouse, E.T. Furlong, W. Giger, et al., “Increases in the polynuclear aromatic hydrocarbon content of an agricultural soil over the last century” Environmental Science and Technology 23, 95-101,1989. [23] D.J. Freeman and F.C.R.Cattell, “Woodburning as a source of atmospheric polycyclic aromatic hydrocarbons”, Environmental Science and Technology 24, 15811585.1990. [24] M.P. Shiarisand S.D.Jambard“Polycyclic aromatic hydro- carbons in surgicial sediments of Boston Harbour, MA, USA.Marine Pollution Bulletin 17, 469-472.1986. [25] W. Lijinsky, “The formation and occurence of polynuclear aromatic hydrocarbons associated with food”, Mutation Research 259, 251-262.1991. [26] M. Vidali, “Bioremediation. An overview,” Pure Appl. Chem., Vol. 73, No. 7, pp. 1163–1172,2001. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [27] 246 J.G.Mueller, S.E.Lantz, B.O. Blattmann, P.J. Chapman, “Bench-scale evaluation of alternative biological treatment process for the remediation of pentachlorophenol and creosote contaminated materials: slurry phase bioremediation”, Environmental Science and Technology 25, 1055-1061.1991. [28] R. Piskonenand M. Itävaara, “ Evaluation of Chemical Pretreatment of Contaminated Soil for Improved PAH Bioremediation”, Appl. Microbiol. Biotechnol.,65, 627, 2004. [29] J.Sabate, M.Vinasand A.M. Solanas, “ Bioavailability Assessment and Environmental Fate of Polycyclic Aromatic Hydrocarbons in Biostimulated Creosote-contaminated Soil”, Chemosphere, 63, 1648, 2006. [30] L.Nylund, P.Heikkila, M.Hameila, L.Pyy, K.Linnainmaa,andM.Sorsa, IJOART “Genotoxic effects and chemical composition of four creosotes,” Mutation Research 265, 223-236, 1992. [31] M. Malawska M and B. Wilkomrski, “An Analysis of Soil and Plant (Taraxacumofficinale) Contamination with Heavy Metals and Polycyclic Aromatic Hydrocarbons (PAHs) in the Area of the Railway Junction IŁawaGŁowna, Poland”, Water, Air, and Soil Pollution, 127, 339, 2011. [32] J.J. Perry, “Microbial metabolism of cyclic alkanes,” Petroleum Microbiology,pp61–98, NY, USA 1984. [33] R.A. Kanalyand S. Harayama, “Biodegradation of High-molecular Weight Polycyclic Aromatic Hydrocarbons by Bacteria,” J. Bacteriol., 182, 2000. [34] W. Ward, A. Singh and H.J.van,“Accelerated Biodegradation of Petroleum Hydrocarbon Waste. J. Ind. Microbiol. Biotechnol.,30, 260,2003. [35] J. Jones, M. Knight and J.A. Byron, “Effect of gross population by kerosene hydrocarbons on the microflora of a moorland soil,” Nature, vol. 227, p. 1166,1970. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [36] Y.Pinholt, S. Struweand A.Kjoller, “Microbial 247 changes during oil decomposition in soil,” Holarctic Ecology, vol. 2, pp. 195–200,1979. [37] S.L. Hollaway; G.M. Fawand R.K. Sizemore, “The bacterial community composition of an active oil field in the Northwestern Gulf of Mexico,”Marine Pollution Bulletin, vol. 11, no. 6, pp. 153–156,1980. [38] G.J.Mulkins, Phillips and J.E Stewart JE., “Distribution of hydrocarbon utilizing bacteria in Northwestern Atlantic waters and coastal sediments,” Canadian Journal of Microbiology, vol. 20, no. 7, pp. 955–962,1974. [39] K.S.M. Rahman, T.J. Rahman, I. Kourkoutas I et al.,“Enhanced bioremediation of n-alkane in petroleum sludge using bacterial consortium amended with rhamnolipid and micronutrients,” BioresourceTechnology, vol. 90, no. 2, pp. IJOART 159–168,2003. [40] R.J.W. Brooijmans, M.I. Pastink and R.J. Siezen, “Hydrocarbon-degrading bacteria: the oil-spill clean-up crew,” Microbial Biotechnology, vol. 2, no. 6, pp. 587– 594,2009. [41] C.E. Cerniglia, “Microbial metabolism of polycyclic aromatic hydrocarbons”, Advances in Applied Microbiology 30, 31-71,1984. [42] I. Kelly, J.P. Freeman, F. E. Evans, C.E. Cerniglia, “Identification of a carboxylic acid metabolite from the catabolism of phenanthere by Mycobacterium sp,” Applied andEnvironmental Microbiology 57, 636-641,1991. [43] M.A. Heitkamp, W.Franklin, C.E. Cerniglia CE.,“Microbial metabolism of polycyclic aromatic hydrocarbons: isolation and characterisation of a pyrenedegrading bacterium,” Applied and Environmental Microbiology 54, 2549-2555,1988. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [44] 248 J. Albaiges, R.W. Frei, E. Merian, “Chemistry and Analysis of Hydrocarbons in the Environment,” Gordon and Breach Science Publishers, New York, pp. 177190,1983. [45] C.E. Cerniglia, “Biodegradation of polycyclic aromatic hydrocarbons”, Biodegradation 3, 351-368,1992. [46] R.M. Atlas, R. Bartha R.,Microbial Ecology; Fundamentals and Applications”, Addison-Wesley, MA, pp. 423-427,1981. [47] C.E. Cerniglia CE.,“Biodegradation of polycyclic aromatic hydrocarbons,” Curr.OpinionBiotechnol. 4:331-338,1993. [48] M. Kastner, Breuer-Jammali; B. Mahro B.,“Enumeration and characterisation of the soil microflora from hydrocarbon contaminated soil sites able to mineralise IJOART polycyclic aromatic hydrocarbons (PAH),” Applied Microbiology and Biotechnology 41, 267-273,1994. [49] M.R. Smith, “The physiology of aromatic hydrocarbons degrading bacteria.(in Biochemistry of microbial degradation”, Kluver Academic Publishers, Netherlands, pp 347-378, 1994. [50] A.H. Neilson, A.S. Allard, “Microbial metabolism of Pahs and heteroarenes”, The handbook of environmental chemistry vol.3, part J, Springer,pp 1-64, 1998 [51] R. Karthikeyan and A. Bhandari , Anaerobic Biotransformation of aromatic And Polycyclic Aromatic Hydrocarbons In Soil Microcosms: A Review journal Of Hazardous Substance Research 3-19, 2001 [52] Jing Lu J; Guo C; Li1 J; Zhang H; et al, “A fusant of Sphingomonassp. GY2B and Pseudomonas sp. GP3A with high capacity of degrading phenanthrene”, J.of Hazard. Mat.12-01083,2012. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [53] 249 Berdugo-Clavijo C; et al, “Methanogenic biodegradation of two-ringed polycyclic aromatic hydrocarbons”,Federation of European Microbiological Societies01328,1574-6941, 2012. [54] A. Janbandhu and M.H. Fulekar , “Biodegradation of phenanthrene using adapted microbial consortium isolated from petrochemical contaminated environment”, Journal of Hazardous Materials 187,333–340,2011. [55] Mouttaki H., et al., (2012),“Identification of naphthalene carboxylase as a prototype for the anaerobic activation of non-substituted aromatic hydrocarbons”, Environmental MicrobiologyVol14, Issue 10, pp.2770–2774, 2012. [56] Meckenstock. And Eva Annweiler, Anaerobic Degradation of 2 Methylnaphthalene by a Sulfate-Reducing Enrichment Culture Appl Environ Microbiol., 66(12): 5329–5333. 2000. [57] IJOART S.M. Bamforth and Singleton.,“Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions”,JChemTechnolBiotechnol80:723–736, 2005. [58] Annweiler E, Materna A et al, Anaerobic degradation of 2-methylnaphthalene by a sulfate-reducing enrichment culture., Appl Environ Microbiol.66(12):532933,2000. [59] Seo J.S; Keum Y S ; Li Q X, Bacterial Degradation of Aromatic Compounds, Int J Environ Res Public Health. 6(1): 278–309.2009. [60] Analysis of PAHs and their transformation products in contaminated soil and remedial processes (Staffan Lundstedt-2003) Soil Remediation in a Cold Climate” (COLDREM)Department of Chemistry Environmental Chemistr-ISBN 91-7305-4526 -2003. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [61] 250 Mahajan et al , Evidence for the involvement of multiple pathways in the biodegradation of 1- and 2-methylnaphthalene by Pseudomonas putida CSV86, Arch Microbiol.161(5):425-33,1994. [62] Chaillan F; Fleche AL; Bury et al, “Identification and biodegradation potential of tropical aerobic hydrocarbon degrading microorganisms,” Research in Microbiology, vol. 155, no. 7, pp. 587–595,2004. [63] Singh H.,“Mycoremediation: Fungal Bioremediation”, Wiley- Interscience, New York, NY, USA,2006. [64] Bogusławska W and Browski WD, “The seasonal variability of yeasts and yeast-like organisms in water and bottom sediment of the Szczecin Lagoon,” InternationalJournal of Hygiene and Environmental Health, vol. 203, no. 5-6, pp. 451–458,2001. [65] IJOART Canet R; Lopez-Real; Beck AJ, “Overview of polycyclic aromatic hydrocarbon bioremediation by white-rot fungi”, Land Contam. Reclam. 7(3):19119,1999. [66] Sanglard D; Leisola MSA; Fiechter A, “Role of extracellular liginases in biodegradation of benzo[a ]pyrene by Phanerochaetechrysoporium,” Enzyme Microbial Technology 8,209-212,1986. [67] Gibson DT; SubramanianV, “Microbial degradation of aromatic hydrocarbons,” Microbial Degradation of Organic Compounds. Marcel Dekker, New York, pp. 181-252,1984. [68] Warshawsky D; Keenan TM; Reilman R; et al.,“Conjugation of benzo[a]pyrene metabolites by freshwater green alga Selenastrumcapricornutum,” Biochem. Biophys.Res.Commun. 152: 540-544,1990. [69] Walker JD;Colwell RR; Vaituzis Z; Meyer SE.,“Petroleum degrading achlorophyllous alga Protothecazopfii,” Nature, vol. 254, no. 5499, pp. 423–42,2003. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [70] 251 Csutak O; Stoicai; Ghindea R; Tanase1 Am; Vassu T.,“Insights on yeast bioremediation processes”, Romanian Biotechnological Letters Vol. 15, No.2, 2010. [71] Jerger DE and Woodhull PM.,“Full-scale bioslurry reactor treatment of wood preserving wastes at a superfund site”, Emerging Technologies in Hazardous Waste Management VI, American Chemical Society, Vol. II, pp. 1370-1373,1994. [72] HammeV;Singh JD; Ward OP, “Recent Advances in Petroleum Microbiology”. Microbiol. Mol. Rev., 67, 649,2003. [73] Brusseau MA., “The impact of physical, chemical and biological factors on biodegradation,” Proceedings of theInternational Conference on Biotechnology for Soil Remediation:Scientific Bases and Practical Applications, pp. 81–98,1998. [74] IJOART Weissenfels WD; Klewer H; Landhoff J.,“ Adsorption of polycyclic aromatic hydrocarbons (PAHs) by soil particles: influence on biodegradability and biotoxicity,” Applied Microbiology and Biotechnology 36, 689-696,1992. [75] Erickson DC; Loehr RC; Neuhauser EF, “PAH loss during bioremediation of manufactured gas plant site soil”, Water Research 27, 911-919.1993. [76] Guthrie EA; Pfaender FK, “Reduced pyrene bioavailability in microbially active soil,” Environmental Science and Technology 32, 501-508,1998. [77] Broderick LS and Cooney JJ, “Emulsification of Hydrocarbons by Bacteria from Freshwater Ecosystems”, Dev. Ind. Microbiol., 23, 425.1982. [78] Zavalskii.L.Y; Marchenko AI and Borovik R V, “The study of bacterial Chemotaxis to Naphthalene”, Microbiology Vol 72; 3: 363-378,2002. [79] Tiehm A, “Degradation of Polycyclic aromatic hydrocarbons in the presence of synthetic surfactants”,Appl. Environ. Microbiol.60,258,1994. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [80] 252 Lee PH; ONG SK; Golchin J; Nelson GL, “Use of solvent to enhance PAH bioremediation of coal-tar contaminated soils,” Wat. Res. 35, 3941,2001. [81] Calvo Ortega et al, Chemotaxis in polycyclic aromatic hydrocarbon-degrading bacteria isolated from coal-tar- and oil-polluted rhizospheres , FEMS Microbiology Ecology 44 (2003) 373-381 [82] Margesin R and Schinner F., (2001), “Biodegradation and bioremediation of hydrocarbons in extreme environments”.ApplMicrobiolbiotechnol 56:650-663,2001. [83] Kastner M; Maren BJ; MahroB., (1998), “Impact of Inoculation Protocols, Salinity, and pH on the Degradation of Polycyclic Aromatic Hydrocarbons (PAHs) and Survival of PAH Degrading Bacteria Introduced into Soil”, Applied And Environmental Microbiology, p. 359–362 Vol. 64, No. 1,1998. [84] IJOART Stapleton RD; Savage DC; Sayler GS; Stacey G., (1998), “Biodegradation of aromatic hydrocarbons in an extremely acidic environment”, Appl Environ Microbiol 64:4180–4184,1998. [85] FoghtJM ;Westlake DWS; Johnson WM; Ridgway HF., (1996), “Environmental gasoline-utilizing isolates and clinical isolates of Pseudomonas aeruginosaare taxonomically indistinguishable by chemotaxonomic and molecular techniques,” Microbiology, vol. 142, no. 9, pp. 2333–2340,1996. [86] Gibb A ; Chu A; Wong RCK; Goodman RH,“Bioremediation Kinetics of Crude Oil at 5°C”;.J. Environ. Engineering,127, 9, 818,2001. [87] Siron R; Pelletier E; Brochu C, “Environmental factors influencing the biodegradation of petroleum hydrocarbons in cold seawater. Arch Environ ContamToxicol 28:406–416,1995. [88] Bartha R and Bossert I, “The treatment and disposal of petroleum wastes,” Petroleum Microbiology, 553–578, NY, US,1984. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [89] 253 Cooney JJ.,“The fate of petroleum pollutants in fresh water ecosystems,” Petroleum Microbiology, 399–434, NY, USA,1984. [90] Mueller JG; Chapman PJ; Pritchard PH.,“Creosote contaminated sites”, Environmental Science and Technology 23, 1197- 1201,1989. [91] Chaillan F; Chaıneau CH; Point V; Saliot A ;Oudot J, “Factors inhibiting bioremediation of soil contaminated with weathered oils and drill cuttings,” Environmental Pollution, vol. 144, no. 1, pp. 255–265,2006. [92] Oudot J; Merlin FX; Pinvidic P, “Weathering rates of oil components in a bioremediation experiment in estuarine sediments,” Marine Environmental Research, vol. 45, no. 2, pp. 113–125, 1998. [93] Chaıneau CH; RougeuxG; Y´epr´emian C; Oudot J, “Effects of nutrient IJOART concentration on the biodegradation of crude oil and associated microbial populations in the soil,” Soil Biology and Biochemistry, vol. 37, no. 8, pp. 1490–1497,2005. [94] Carmichael LM and Pfaender FK,“The effect of inorganic and organic supplements on the microbial degradation of phenanthrene and pyrene in soils,” Biodegradation, vol. 8, no. 1, pp. 1–13,1997. [95] Ashok BT; Saxena S; Musarrat J.,“Isolation and characterization of four polycyclic aromatic hydrocarbon degrading bacteria from soil near an oil refinery”, LettApplMicrobiol 21:246–248,1995. [96] Rhykerd RL; Weaver RW; McInnesK, “Influence of salinity on bioremediation of oil in soil”.EnvironPollut 90:127–130,1995. [97] Coates JD; Anderson RT; Lovley DR, “Oxidation of polycyclic aromatic hydrocarbons under sulphate –reducing conditions”.Appl. Environ.Microbiol. 62, 1099,1996. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [98] 254 Suflita JM; Horowitz A.; Shelton DR; Tiedje JM.,“Dehalogenation: A Novel Pathway for the Anaerobic Biodegradation of Haloaromatic Compounds” Science, 218, 1115,1982. [99] Boyd SA and Shelton DR.,“Anaerobic Biodegradation of Chlorophenols in Fresh and Acclimated Sludge”, Appl. Environ. Microbiol.,47, 272.1984. [100] Zhang X; Sullivan ER ; Young LY.,Evidence for aromatic ring reduction in the biodegradation pathway of carboxylatednaphthalene by a sulphate-reducing consortium. Biodegradation11:117–124 ,2000. [101] Chakrabarty AM.,“Plasmids in Pseudomonas”, Annu. Rev. Genet., 10, 7,1976. [102] Menn FM; Easter JP;Sayler GS, “Genetically Engineered Microorganisms and Bioremediation”, Book ,Biotechnology Set, Second Edition Wiley, USA pp.443- IJOART 457,2008. [103] Mlynarz T; Ward OP.,“Degradation of Phenanthene in a soil matrix by indigenous and introduced bacteria” ,Biotechnology Letters 18, 181-186,1996. [104] Juhasz AL; Britz ML; Stanley GA, “Degradation of phenanthene, pyrene, benz[a] anthracene and dibenz[a,h ]anthracene by Burkholderiacepacia.”, Journal of Applied Microbiology 83, 189- 198,1997. [105] Hammel KE; Kalyanaraman B; Kirk TK., “Oxidation of polycyclic aromatic hydrocarbons and dibenzo[ p ]dioxins by Phanerochaetechrysosporiumliginase,” Journal of Biological Chemistry 261, 16948-16952,1986. [106] Gomez BC; Quintero R; Esparza-García F; Howard AM; et al., (2009), “Removal of phenanthrene from soil by co-cultures of bacteria and fungi pregrown on sugarcane bagasse pith” Bioresourcs tech.89 (2): 177-186,2009. Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [107] 255 Wilson NG and Bradley G.,“The effect of immobilization on rhamnolipid production by Pseudomonas fluorescens,” Journal of Applied Bacteriology, vol. 81, no. 5, pp. 525–530,1996. [108] Nichols WJ, “The U.S. Environmental Protect Agency: National Oil and Hazardous Substances Pollution Contingency Plan, Subpart J Product Schedule (40 CFR300.900),” Proceedings of the International Oil SpillConference, pp. 1479–1483, 2001. [109] Johnsen AR; Wick LY; Harms H.,“Principles of microbial PAH degradation in soil”.Environ.Poll., 133: 71-84.2005. [110] Foght JM and Westlake DW, “Transposon and spontaneous deletion mutants of plasmid-borne genes encoding polycyclic aromatic degradation by a strain of Pseudomonas fluorescens”, Biodegradation 7(4): 353-366,1996. [111] IJOART Dunn NW and Gunsalus IC.,“Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida,” J. Bacteriol., 114: 974-979,1973. [112] Chakrabarty.,“ Microorganisms having multiple compatible degradative energy- generating plasmids and preparation thereof “ United States Patent USP4259444,1981 [113] Yen KM andGunsalus IC, “Plasmid gene organization: naphthalene/salicylate oxidation”. Proc. Natl. Acad. Sci. USA, 79: 874- 878,1982. [114] Ensley BD; Ratzkin BJ; Osslund TD; Simon MJ; et al.,“Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo”; Sci., 222: 167-169,1983. [115] Parales RE; Bruce NC; Schmid A; Wackett LP., (2002), “Biodegradation, biotransformation, and biocatalysis (B3)”, Appl. Environ. Microbiol., 68: 46994709,2002 Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [116] 256 Zhou Y, Wei J, Shao N, Wei D, Construction of a genetically engineered microorganism for phenanthrene biodegradation. J Basic Microbiol. 53(2):188-94. 2013 [117] OluwafemiS, Obayori and Lateef B. Salam,”Degradation of poly cyclic aromatic hydrocarbons: Role of plasmids Scientific Research and Essays vol 5(25), pp 4093-4106 (2010). [118] Lu J, Guo C, Li J, Zhang H, Lu G, Dang Z, Wu R., “A fusant of Sphingomonas sp. GY2B and Pseudomonas sp. GP3A with high capacity of degrading phenanthrene”. World J Microbiol Biotechnol. 2013. [119] Surani JJ; Akbari VG; Purohit MK; Singh SP.,“Pahbase, a Freely Available Functional Database of Polycyclic Aromatic Hydrocarbons (Pahs) Degrading Bacteria”, J. Bioremed Biodegrad, 2:1/10.4172/2155-6199, 2011. [120] IJOART Fulekar MH and Sharma J.,“Bioinformatics Applied In Bioremediation”, Innovative Romanian Food Biotechnology Vol. 2 No. 2, 2008. [121] Singh SP; Purohit MK; Raval VH ; Pandey S; et al.,“Capturing the potential of Haloalkaliphilic bacteria from the saline habitats through culture dependent and metagenomic approaches”, Current research, technology and education topics in applied microbiology and microbial biotechnology 81-87,2010. [122] Purohit HJ,“Biosensors as molecular tools for use in bioremediation”, Journal of Cleaner Production 11, 293–301,2003. [123] Gu MB; Gil GC; Kim JH.,“A two-stage minibioreactor system for continuous toxicity monitoring”, BiosensBioelectron 14:355–61,1999. [124] gas Gil GC ; Mitchell RJ; Chang ST ; Gu MB.,“A biosensor for the detection of toxicity using a recombinant bioluminescent bacterium”, Biosens Bioelectron;15:23–30,2000 Copyright © 2013 SciResPub. IJOART International Journal of Advancements in Research & Technology, Volume 2, Issue 7, July-2013 ISSN 2278-7763 [125] 257 Chakraborty R; Cindy HW; Hazen TC.,“Systems biology approach to bioremediation”, Current Opinion in Biotechnology 23:1–8,2012. [126] Angelidaki I; Mogensen AS; Ahring BK.,“Degradation of Organic Contaminants Found in Organic Waste”, Biodegradation, 11, 377,2000. [127] Al-Daher R; Al-Awadhi N; El-Nawawy A.,“Bioremediation of Damaged Desert Environment with the Windrow Soil Pile System in Kuwait”, Environ. Int., 24, 175,1998. [128] Bouchez M; Blanchet D; Vandecasteele JP,“Degradation of Polycyclic aromatic hydrocarbons by pure strains and by defined strain asssociations: inhibition phenomena and cometabolism”, Appl. Microbiol. Biotechnol. 43, 156,1995. [129] IJOART Guerin WF and Jones GE.,“Two stage mineralisation of phenanthrene by estuarine enrichment cultures,” Applied and Environmental Microbiology 54, pp.929936,1988. [130] Kieran J;Germaine; Elaine K; et al.,“Bacterial endophyte-mediated naphthalene phyto protection and phytoremediation “, FEMS Microbiol Lett 296 (2009) 226–234,2009. [131] Kiyohara H; Nagao K; Kouno K; Yanko K.,“Phenanthrene –degrading phenotype of Alicaligenesfaecalis AFK2”, Appl. Environ. Microbiol 43,458,1982. [132] MadhuriKaushish Lily MK; Bahuguna1 A; Dangwal1 K; Garg V. “Degradation Of Benzo [A] Pyrene By A Novel Strain Bacillus subtilisBMT4i (MTCC 9447)”, Brazilian Journal of Microbiology, 40: pp.884-892 ,2009. [133] Robert A. K and Shigeaki H., “Biodegradation of High-Molecular-Weight Polycyclic Aromatic Hydrocarbons by Bacteria,” J. Bacteriol, 182(8):20592067,2000. Copyright © 2013 SciResPub. IJOART