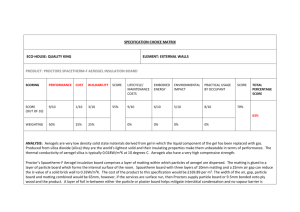
SYNTHESIS, CHARACTERIZATION AND CATALYTIC PROPERTIES OF TITANIUM CONTAINING SILICA AEROGEL
advertisement

SYNTHESIS, CHARACTERIZATION AND CATALYTIC PROPERTIES OF TITANIUM CONTAINING SILICA AEROGEL LEE SOON CHAI UNIVERSITI TEKNOLOGI MALAYSIA PSZ 19:16 (Pind. 1/97) UNIVERSITI TEKNOLOGI MALAYSIA BORANG PENGESAHAN STATUS TESIS♦ JUDUL : SYNTHESIS, CHARACTERIZATION AND CATALYTIC PROPERTIES OF TITANIUM CONTAINING SILICA AEROGEL SESI PENGAJIAN: 2005/2006 Saya : LEE SOON CHAI (HURUF BESAR) mengaku membenarkan tesis ( PSM / Sarjana / Doktor Falsafah )* ini disimpan di Perpustakaan Universiti Teknologi Malaysia dengan syarat-syarat kegunaan seperti berikut: 1. Tesis adalah hakmilik Universiti Teknologi Malaysia. 2. Perpustakaan Universiti Teknologi Malaysia dibenarkan membuat salinan untuk tujuan pengajian sahaja. 3. Perpustakaan dibenarkan membuat salinan tesis ini sebagai bahan pertukaran antara institusi pengajian tinggi. 4. **Sila tandakan ( √ ) √ SULIT (Mengandungi maklumat yang berdarjah keselamatan atau kepentingan Malaysia seperti yang termaktub dalam AKTA RAHSIA RASMI 1972) TERHAD (Mengandungi maklumat TERHAD yang telah ditentukan oleh organisasi/badan di mana penyelidikan dijalankan) TIDAK TERHAD Disahkan oleh ______________________________ (TANDATANGAN PENULIS) Alamat Tetap: 3, Jalan Gemilang 10, Taman UPC, 86100 Ayer Hitam, Johor Darul Tak’zim. Tarikh: 5.12.2005 ________________________________ (TANDATANGAN PENYELIA) PROF. DR. HALIMATON HAMDAN Nama Penyelia Tarikh: 5.12.2005 CATATAN: * Potong yang tidak berkenaan. ** Jika tesis ini SULIT atau TERHAD, sila lampirkan surat daripada pihak berkuasa/organisasi berkenaan dengan menyatakan sekali sebab dan tempoh tesis ini perlu dikelaskan sebagai SULIT atau TERHAD. ♦ Tesis dimaksudkan sebagai tesis bagi Ijazah Doktor Falsafah dan Sarjana secara penyelidikan, atau disertasi bagi pengajian secara kerja kursus dan penyelidikan, atau Laporan Projek Sarjana Muda (PSM). “I hereby declare that I have read this thesis and in my opinion this thesis is sufficient in terms of scope and quality for the award of the degree of Master of Science (Chemistry)”. Signature : …………………………. Name of Supervisor : Prof. Dr. Halimaton Hamdan Date 5.12.2005 : BAHAGIAN A ⎯ Pengesahan Kerjasama* Adalah disahkan bahawa projek penyelidikan tesis ini telah dilaksanalan melalui kerjasama antara _______________________ dengan ________________________ Disahkan oleh: Tandatangan : _________________________________ Tarikh: ______________ Nama : _________________________________ Jawatan : _________________________________ (Cop rasmi) * Jika penyediaan tesis/projek melibatkan kerjasama. BAHAGIAN B ⎯ Untuk Kegunaan Pejabat Sekolah Pengajian Siswazah Tesis ini telah diperiksa dan diakui oleh: Nama dan Alamat Pemeriksa Luar : Prof. Madya Dr. Wan Ahmad Kamil Bin Mahmood School of Chemical Sciences, Universiti Sains Malaysia, 11800 Minden, Pulau Pinang. Nama dan Alamat Pemeriksa Dalam I : Prof. Madya Dr. Hanapi Bin Mat Fakulti Kejuruteraan Kimia & Kejuruteraan Sumber Asli, Universiti Teknologi Malaysia, 81300 Skudai, Johor Darul Takzim. Nama Penyelia Lain (jika ada) :__________________________________________________ Disahkan oleh Penolong Pendaftar di Sekolah Pengajian Siswazah: Tandatangan : _________________________________ Tarikh: ______________ Nama : GANESAN A/L ANDIMUTHU SYNTHESIS, CHARACTERIZATION AND CATALYTIC PROPERTIES OF TITANIUM CONTAINING SILICA AEROGEL LEE SOON CHAI A thesis submitted in fulfilment of the requirements for the award of the degree of Master of Science (Chemistry) Faculty of Science Universiti Teknologi Malaysia DECEMBER 2005 ii I declare that this thesis entitled “Synthesis, Characterization and Catalytic Properties of Titanium Containing Silica Aerogel” is the result of my own research except as cited in the references. The thesis has not been accepted for any degree and is not concurrently submitted in candidature of any other degree. Signature : ____________________ Name : LEE SOON CHAI Date : 5.12.2005 iii Dedicated to My Parents iv ACKNOWLEDGEMENTS I would like to express my sincere gratitude and appreciation to my research supervisor, Prof. Dr. Halimaton Hamdan, for her guidance, support and patience towards the completion of this work. Synthesis and modification of porous materials have been an attractive topic for me as investigated intensively by the Zeolite and Porous Materials Group (ZPMG) of Universiti Teknologi Malaysia. Grateful acknowledgements are to Dr. Hadi Nur, Assoc. Prof. Dr. Zainab Ramli, Assoc. Prof. Dr. Salasiah Endud, and Dr. Bogdan Sulikowski for their advice and valuable suggestion particularly in the method of conducting a research. To my all lab mates, especially Didik Prasetyoko, Lim, Yong and Ng, thank you for their valuable discussion and friendship. My sincere appreciations also extend to lab assistants and others who have provided assistance at various occasions. I wish to thank the Ministry of Science, Technology and Innovation (MOSTI) for funding the research and my studies (UTM Fellowship Award; Project Vote: 74506). Lastly, I would like to acknowledge my family, for their love and care that convince me to always do my best. v ABSTRACT Silica aerogel and titania silica aerogel were synthesized by chemical means. The effect of titanium source, sulphuric acid and titanium loading were studied. The structure and properties of the aerogels were examined by X-ray diffraction (XRD), scanning electron microscopy (SEM), nitrogen adsorption (BET), energy dispersive X-ray analysis (EDX), Fourier transform infrared (FTIR), and ultra violet-visible diffuse reflectance spectroscopy (UV-Vis DRS). Both silica aerogel and titania silica aerogel are amorphous. The surface area of the resulting titania silica aerogel was significantly affected by the quantity of the acid used during synthesis. The physicochemical properties were found could be engineered by the change of acid loading and titanium loading. Isolated titanium in tetrahedral framework position, well dispersed titania particle or crystalline titania (anatase) were formed in-situ during the aerogel synthesis process. Catalytic reaction of cyclohexene and hydrogen peroxide was carried out at 70 ˚C in a fixed batch reactor. The effects of physicochemical properties of the catalyst, solvent, reaction temperature, oxidant content and alkene to the reaction have been investigated. Both allylic and nonallylic oxidation process have occurred in the reaction. 1,2-cyclohexanediol was formed as major compound in the reaction. vi ABSTRAK Aerogel silika dan aerogel titania-silika telah disintesis melalui pendekatan kimia. Pengaruh daripada sumber titanium, asid sufurik dan kepekatan titanium telah dikaji. Struktur dan sifat aerogel telah dikaji menggunakan pembelauan sinar-X (XRD), mikroskop imbasan elektron (SEM), penjerapan nitrogen, analisis penyerakan tenaga sinar-X (EDX), Fourier transform infra merah (FTIR), and spektroskopi pemantulan bauran ultra lembayung-nampak (UV-Vis DRS). Keduadua aerogel silika dan aerogel titania-silika bersifat amorfus. Luas permukaan aerogel titania silika didapati amat dipengaruhi oleh kuantiti asid yang digunakan semasa sintesis. Sifat fizikokimia didapati dapat dikawal dengan mengubah penggunaan asid dan penggunaan titanium. Titanium terpencil dalam keadaan rangka tetrahedral, partikel titania dalam penaburan sempurna and hablur titania (anatase) didapati terbentuk in-situ dalam proses sintesis aerogel. Tindakbalas pemangkinan bagi sikloheksena dengan hidrogen peroksida telah dijalankan dalam reaktor pukal. Pengaruh daripada sifat fizikokimia mangkin, pelarut, suhu tindakbalas, kuantiti pengoksida dan alkena terhadap keaktifan mangkin telah dikaji. Kedua-dua proses pengoksidaan allilik and bukan-allilik didapati telah berlangsung dalam tindakbalas. 1,2-sikloheksanadiol didapati terbentuk sebagai hasil utama dalam tindakbalas. vii TABLE OF CONTENTS CHAPTER TITLE PAGE DECLARATION OF THE STATUS OF THESIS SUPERVISOR’S DECLARATION CERTIFICATION OF EXAMINATION TITLE PAGE i DECLARATION OF ORIGINALITY AND ii EXCLUSIVENESS 1 DEDICATION iii ACKNOWLEDGEMENTS iv ABSTRACT v ABSTRAK vi TABLE OF CONTENTS vii LIST OF TABLES xi LIST OF SCHEMES xiii LIST OF FIGURES xiv LIST OF ABBREVIATIONS AND SYMBOLS xvi LIST OF APPENDICES xvii INTRODUCTION 1.1 General Introduction 1 1.2 Research Background and Problem Statement 2 1.3 Research Objectives and Scope 4 1.4 Hypothesis 4 viii 2 LITERATURE REVIEW 2.1 Sol-Gel Science 6 2.2 Silica 8 2.2.1 The Chemistry of Aqueous Silicates 10 2.3 Titania and the Chemistry of Aqueous Titania 13 2.4 The Chemistry between Silica and Titania 15 2.4.1 Titania-Silica in Catalysis 2.5 Aerogel 2.5.1 16 18 History and Development of 18 Aerogel 2.5.2 Aerogel Synthesis 19 (i) Drying Process 19 (ii) Elimination of Surface 19 Tension (iii) Freeze Drying 20 (iv) Supercritical Fluid 21 Extraction 2.5.3 Properties and Applications of 23 Aerogel 3 EXPERIMENTAL 3.1 Synthesis of Silica Aerogel 25 3.2 Synthesis of Titanium Containing Silica Aerogel 28 3.2.1 Post Synthesis: Synthesis of 29 Titania-Silica System (i) Grafting with Titinium 29 (IV) Tetrachloride (ii) Grafting with Titanium 29 (IV) Isopropoxide (iii) Precipitation of Titania on Amorphous Silica 29 ix 3.2.2 Direct Synthesis: Synthesis of 30 Titania-Silica Aerogel System 3.3 Parameter Study for Synthesis (Direct Synthesis) 30 of Titanium Containing Silica Aerogel 3.3.1 Sources of Titanium 30 3.3.2 Si:Ti Molar Ratio 31 3.3.3 Sulphuric Acid Loadings 32 3.4 Characterization 3.4.1 Nitrogen Adsorption: Brunauer, 33 33 Emmett, Teller (BET) method 3.4.2 XRD Measurement 35 3.4.3 UV-Vis Diffuse Reflectance 36 Spectroscopy 3.4.4 Fourier Transform Infrared 37 Spectroscopy 3.4.5 Scanning Electron Microscopy 3.5 Catalytic Properties: Oxidation of Alkene 4 39 39 RESULTS AND DISCUSSION 4.1 Synthesis of Silica Aerogel 42 4.2 Synthesis of Titanium Containing Silica Aerogel 45 4.2.1 Post Synthesis 45 4.2.2 Direct Synthesis 51 4.3 Parameter Study for the Synthesis (Direct 53 Synthesis) of Titanium Containing Silica Aerogel 4.3.1 The Effect of Titanium Source 53 4.3.2 The Effect of Si:Ti Molar Ratio 58 4.3.3 The Effect of Loading of Sulphuric 62 Acid 4.4 Catalytic Properties: Oxidation of Alkene 4.4.1 The Influence of the Type of 66 66 Titanium 4.4.2 The Influence of Solvent 70 x 4.4.3 The Influence of Hydrogen 72 Peroxide Loading 4.4.4 The Influence of Reaction 74 Temperature 4.4.5 The Influence of Alkene 4.5 The Mechanism of the Reaction 5 76 77 CONCLUSIONS AND SUGGESTIONS 5.1 Conclusions 82 5.2 Suggestions 84 REFERENCES 85 APPENDICES 95 xi LIST OF TABLES TABLE NO. TITLE PAGE 2.1 The solubility of silica in different solvent [54] 22 2.2 The critical point of different solvents [55, 56] 23 2.3 Some properties of aerogel [59, 60] 24 3.1 Temperature programme implemented in the 27 supercritical drying process [64] 3.2 3.3 Titanium sources that have been used in the synthesis of titanium containing silica aerogel IUPAC classification of pores [67, 68] 31 34 3.4 Some assignments of infrared frequencies [78] 38 3.5 GC-FID instrument setting 40 4.1 The surface area of the silica aerogel 44 4.2 The BET surface area of titanium containing 50 silica 4.3 Effect of titanium source on the surface 54 characteristics of the Ti-Si aerogels 4.4 Effect of concentration of titanium on the 58 surface characteristics of the Ti-Si aerogels. Titanium isopropoxide as titanium source, H+: NaOH molar ratio = 1.25. 4.5 Effect of concentration of acid on the surface 63 characteristics of the Ti-Si aerogels. Titanium isopropoxide as titanium source, Si:Ti molar ratio = 33 4.6 Sample used in the catalytic testing and their 67 xii characteristics 4.7 Catalytic activity of the titanium containing 69 silica aerogel, TS-1 and anatase. Reaction condition: 10 ml cyclohexene, 10 ml acetone, 8.35 ml H2O2 35%, 156.3 mg catalyst, and 1 ml toluene (internal standard) at 70 ˚C 4.8 Catalytic activity of the aerogel A250 as a 71 function of solvent. Reaction condition: 10 ml cyclohexene, 10 ml solvent, 8.35 ml H2O2 35%, 156.3 mg catalyst, and 1 ml toluene (internal standard) at 70 ˚C 4.9 Catalytic activity of the aerogel A250 as a 73 function of alkene: H2O2 molar ratio. Reaction condition: 10 ml cyclohexene, 10 ml acetone, respective amount of H2O2 35%, 156.3 mg catalyst, and 1 ml toluene (internal standard) at 70 ˚C 4.10 Catalytic activity of the aerogel A250 as a 75 function of reaction temperature. Reaction Condition: 10 ml cyclohexene, 10 ml acetone, 8.35 ml H2O2 35%, 156.3 mg catalyst, and 1 ml toluene (internal standard) 4.11 Catalytic activity of the aerogel A250 as a function of amount of hydrogen peroxide. Reaction condition: 10 ml alkene, 10 ml acetone, 8.35 ml H2O2 35%, 156.3 mg catalyst, and 1 ml toluene (internal standard) at 80 ˚C 76 xiii LIST OF SCHEMES SCHEME NO. 1 TITLE The reactions in the oxidation of cyclohexene PAGE 78 [95, 96, 97] 2 Reaction mechanism of the oxidation of cyclohexene using hydrogen peroxide as oxidant [102, 103, 109] 81 xiv LIST OF FIGURES FIGURES NO. 2.1 TITLE PAGE Polymerisation behaviour of aqueous silica and 12 followed by the formation of gels and powders 2.2 The freeze drying process path (bolded arrows) 20 in pressure-temperature (P-T) phase diagram of a pure substance 2.3 The supercritical drying process path (bolded 22 arrows) in pressure-temperature (P-T) phase diagram of a pure substance 3.1 Synthesis of sodium silicate from rice husk ash 26 3.2 Synthesis of aerogel from sodium silicate 28 4.1 XRD diffractogram of silica aerogel 42 4.2 SEM micrograph showing the surface 43 morphology of silica aerogel 4.3 FTIR spectrum of silica aerogel 44 4.4 The X-ray diffractograms of titanium modified silica aerogels The X-ray diffractograms of titanium modified 45 4.5 46 amorphous silica (RHA) 4.6 The FTIR spectra of titanium modified RHA 47 4.7 The FTIR spectra of titanium modified aerogel 48 4.8 The UV-Vis spectra of titanium modified RHA 49 4.9 The UV-Vis spectra of titanium modified silica 49 aerogels 4.10 XRD diffractogram of titanium modified silica 52 xv aerogels (Aph6) 4.11 FTIR spectrum of titanium modified silica 52 aerogels (Aph6) 4.12 UV-Vis spectra of titanium modified silica 53 aerogels (Aph6) and silica aerogel 4.13 The effect of titanium source on the 56 physicochemical characteristics of the Ti-Si aerogels by UV-Vis DRS. (a) Titanium(III) sulphate, (b) Titanium(IV) chloride, (c) Titanium(IV) alkoxide, (d) Titanium(IV) oxide in anatase form 4.14 X-ray diffractograms of aerogel samples with 60 various Si:Ti molar ratios compared with anatase TiO2 4.15 UV-Vis spectra of samples synthesized with 61 various Si:Ti molar ratios 4.16 UV-Vis spectra of samples synthesized with 65 various H+: NaOH molar ratio 4.17 Time course study for the reaction mixture 10 ml cyclohexene, 10 ml acetone, 8.35 ml H2O2 35%, 156.3 mg TS-1, and 1 ml toluene (internal standard) at 80 ˚C 79 xvi LIST OF ABBREVIATIONS λ Wavelength 2θ Bragg angle BET Brunauer, Emmet, Teller Cu Kα X-ray diffraction from copper K energy level EDX Energy dispersive X-ray analysis etc Etcetera FTIR Fourier Transform Infrared GC-FID Gas Chromatography – Flame Ionisation Detector iep Isoelectric point IUPAC International Union of Pure and Applied Chemistry KBr Potassium bromide MCM Mobil Crystalline Material MS Mass Spectroscopy m/z Mass-to-charge ratio NMR Nuclear Magnetic resonance ppm Part per million RHA Rice husk ash Si:Ti Silicon to titanium molar ratio of starting material TMOS Tetramethylortosilicate TOF Turnover frequency UV-Vis DRS Ultra Violet-Visible diffuse reflectance spectroscopy XRD X-ray diffraction xvii LIST OF APPENDICES APPENDIX 1 TITLE Component Table for GC-FID peaks PAGE 95 identification 2 Chromatogram of the reaction mixture analysed 96 using gas chromatography 3 Calibration curve for quantify the concentration 97 of cyclohexene 4 Calibration curve for quantify the concentration 98 of cyclohexene oxide 5 Calibration curve for quantify the concentration 99 of 2-cyclohexen-1-ol 6 Calibration curve for quantify the concentration 100 of 2-cyclohexen-1-one 7 Calibration curve for quantify the concentration 101 of 1,2-cyclohexenediol 8 FTIR spectrum of 1,2-cyclohexanediol that has 102 been synthesized as standard 9 Mass spectrum of 1,2-cyclohexanediol that has 103 been synthesized as standard 10 Reaction mechanisms involving hydroxy radical and cyclohexene [95, 96] 104 CHAPTER 1 INTRODUCTION 1.1 General Introduction Aerogel is a gel in which the liquid phase has been replaced by air without damaging the solid phase. Aerogel is a novel space-age super material. It is inert, non-toxic, and environmental friendly new material. It has been used as a catcher’s mitt in spacecraft to capture dust from a comet [1]. Silica aerogel is a very interesting material. It is extremely light (specific gravity as low as 0.025 g/cm3), with the lowest thermal conductivity known to solid material, high surface area and high porosity. This makes it suitable for many applications. It has been applied as heat storage systems, catalysts and catalyst supports. Silica aerogel is dielectric with air filled pores (can be as small as 10 nanometers in diameter) offers a better way to keep the interconnecting wires from shorting across the narrow dividing space between transistors [2]. Many physical and chemical properties of a metal oxide can be modified by interaction with a second oxide. Silica–alumina, for example, has stronger acidity than both silica and alumina [3]. A screening study of silica-supported catalysts was conducted by Hisao Yoshida et al. and they found that silica supported Ti system was the most effective catalyst for epoxidation of propene [4]. It strongly suggests that silica-titania mixed oxide might be the best combination to become the best catalyst for the oxidation reaction compared to other oxides. 2 1.2 Research Background and Problem Statement Titania (TiO2) is a technologically important material as catalyst and as support. With its special properties, TiO2 attracts more attention recently, especially for hydrodesulphurisation (HDS) or hydrodenitrogenation (HDN) in the petroleum refining process [5, 6, 7]. The character of the catalyst with TiO2 carrier is superior to that with γ-Al2O3 carrier. However, TiO2 is seldom used as a catalyst carrier in commercial process due to two disadvantages. TiO2 has a small specific surface area (usually 10 m2/g) and the mechanical strength is five times less than γ-Al2O3. In addition, TiO2 in high surface area form has low mechanical strength, limited extrudability and low thermal stability. Therefore, effort has been devoted in recent years to coat titania onto high surface area supports such as silica and alumina to improve the thermal stability and the surface area of TiO2 [8]. Despite the disadvantages, titania has the ability to modify catalytic properties of the metal, thus attracts the studies of the interaction between titaniametal interfaces [9]. Since, it is very difficult to obtain high surface area titania (>100 m2/g); its use has been limited. It is now established that nanoscale engineering of sol–gel TiO2–SiO2 mixed oxides provides excellent epoxidation catalysts. The area of titanosilicate-catalyzed epoxidation of olefins with hydrogen peroxides is largely because of the discovery of TS-1 where Ti has been substituted for Si in the MFI framework by Shell in 1971 [10]. This molecular sieve was reported to be active in the following oxidation reactions [11]: (i) oxidation of primary and secondary alcohols to the corresponding aldehydes and ketones, (ii) hydroxylation of aromatics to phenol derivatives, (iii) epoxidation of alkenes to epoxides, (iv) oxyfunctionalization of alkanes to alcohol and ketones, (v) ammoximation of carbonyl compounds aldoxymes or ketoximes, (vi) oxidation of thioethers to sulfoxides and sulfones, and (vii) oxidation of primary and secondary amines to oximes or azoxy compounds and hydroxylamines. TS-1 is the most prominent representative of epoxidation catalyst [12]. However, the use of TS-1 is limited by inherently small pore size and only relatively few substrates can be oxidized. Moreover, an obstacle in the commercialisation of TS-1 is that it is not possible to be moulded. 3 The search for large pore analogues of TS-1 has led to the study of Ti substituted into the framework or grafted onto the channels of zeolite beta or MCM type silicalites. A series of new preparation methods of materials containing highly dispersed titanium centres in a silica matrix were developed [13, 14, 15]. Smaller particles of metal oxide can be obtained when two oxide gel are mixed at the same time. However, phase separation may occur due to different rates of hydrolysis (sol-gel process) of silicon and titanium alkoxide, which results in formation of larger TiO2 particles and prevents the homolytic substitution of titanium in silica framework. Thus, Ti-MCM, Ti-aerogel or Ti-zeolite in several researches are fail to be engineered the Ti-O-Si bonding as in TS-1 [3, 13, 15]. However, high catalytic activity has been achieved by the use of organic based peroxide as oxidant if the TiO2 particle was small enough. Thus, most studies avoid the use of hydrogen peroxide in their catalytic oxidation. In addition, Dusi [16] has synthesized 20% TiO2–80 wt% SiO2 aerogel from alkoxide sources and found that highly dispersed titania in the silica matrix was obtained, showed outstanding performance in the epoxidation of cyclic olefins with alkylhydroperoxides but inactive with hydrogen peroxide. This was due to the formation of TiO2 particles inside the silica matrixes. Therefore, it is a challenge to synthesize titania-silica aerogel to produce homogeneous or well-dispersed mixed oxide by using aqueous solution. In recent publications, there were several synthesis routes for the production of titania-silica mixed oxide but alkoxide precursors are used. As the alkoxide is commonly more expensive starting material, it will directly increase the cost of the final material and limit its commercial value. Recently, Chan [17] have successfully synthesized silica aerogel using organic waste precursor. Their innovation has resulted in a more economical production of silica aerogel. Therefore it is feasible to find a better path to synthesize well-dispersed titania-silica mixed oxide prepared from an organic waste. In addition, crystalline titanium oxide has great potentials in other various applications, such as in photocatalysis [18], making the study of the titania-silica aerogel more desirable. 4 Titania oxide is of interest as catalyst or support. A disadvantage of titania as support is its low surface area. Therefore, inert oxide like silica aerogel is selected as a support in order to obtain higher surface area dispersed titania. 1.3 Research Objectives and Scope The objectives of this research are: 1) To synthesize titanium containing silica aerogel. 2) To investigate and characterize the physical and chemical properties of titanium containing aerogel. 3) To identify the catalytic properties of the titanium containing silica aerogel in the oxidation of cyclohexene by using hydrogen peroxide as oxidant. 4) To identify the influence of reaction conditions in the oxidation of cyclohexene by titanium containing silica aerogel. 1.4 Hypothesis To overcome these problems, inert oxides like silica have been used as support to obtain high surface area dispersed titania. In this research work, direct synthesis, precipitation and grafting of titania were implemented on the silica aerogel as support. This approach not only increases the surface area of the titanium oxide but also strengthens the silica aerogel. Deposition or anchoring of Ti sites on silica circumvents the steric problem by avoiding narrow channels. Sol–gel process provides an attractive route to the preparation of multi-component oxide materials that show homogeneity in the distribution of heterometal oxide bonds [19]. Catalysts prepared by sol-gel contain accessible immobilized Ti within the silica framework. Since high specific surface area is obtained and the resulting porous structure is very open, larger substrates can 5 access the active sites. Better accessibility may be obtained by having the active component on the surface. CHAPTER 2 LITERATURE REVIEW 2.1 Sol-Gel Science Sol-gel process gives several advantages to material development as stated below [19]: • Increase chemical homogeneity in multi-component system; • Produce high surface area gel or powder, which lead to relatively low sintering temperatures; • Preparation of high chemical purity material due to the absence of grinding and pressing process; • Prepared with relative ease from simple solution. Sol-gel process involves sol, gel and colloidal chemistry. Colloid state comprises of particles with a size range of 1 nm to 1000 nm and not to be affected by gravitational forces. The interactions are dominated by short-range forces, such as Van der Waals and surface forces. The International Union of Pure & Applied Chemistry (IUPAC) defines colloid dispersion as a system in which the particles of the colloidal size (1-1000 nm) of any nature (solid, liquid or gas) are dispersed in a continuous phase of a different composition or state. In order to be treated as colloidal, not all three dimensions need to be in colloidal range or even only one dimension is in this range (e.g. fibre or thin film) may also be treated as colloidal. The region of suspension may begin at about 1000 nm [20, 21]. 7 Sol is a stable (does not settle or agglomerate at a significant rate) dispersion of solid colloidal particles in a liquid phase. The dispersion of solid in water is known as aquasol or hydrosol. Silica organosol can be obtained by transferring the aquasol to an organic solvent. An aerosol is a colloidal dispersion of particles in gas. Pyrogenic or fume oxides are powders made by condensing of precursor from a vapour phase at elevated temperature. Silica made using this method is called an Aerosil. Cryogel is a powder obtained by freeze-drying a sol. Sol is not stable against mechanical force, such as centrifugation. It may consist of weakly cross-linked and flexible polymer [22]. Colloidal particles can be linked together or be aggregated by gelation, coagulation or flocculation or coacervation. Gelation is a link of colloidal particles to form a continuous solid skeleton enclosing the liquid phase. Coagulation involves the formation of close pack clumps of sol and followed by precipitation. Flocculation occurs in the presence of flocculating agent that functions as a bridge to link the particles in groups while remain open structure. When an adsorbed layer of material that makes the colloidal particles becomes less hydrophilic, no bridge is formed between particles, hence forming a concentrated liquid phase that is immiscible with the aqueous phase. The process is termed as coacervation. Gel point is the time for the last bond to form, which completes the giant molecule. Gelation can occur after a sol is cast into mold, turning an object into a desired shape. If the gel is greater than a few millimetres, it is generally called a monolith. Aging is the process of change in structure and properties after gelation. Further, dissolution, condensation and re-precipitation may occur during the aging process. Shrinkage during aging process may due to syneresis, which attraction or bond formation between particles induces the expulsion of the liquid from the pores. Xerogel (xero means dry) is obtained by drying a gel under normal condition and capillary pressure causes shrinkage of the gel network (often reduced in volume by a factor of 5 to 10 compared to the original wet gel). Wet gel dried under 8 supercritical condition of the solvent may prevent the collapse of the wet gel structure to produce a matter with low volume fraction of solid but high volume fraction of air, known as aerogel. Since supercritical liquid has no interfacial between liquid and vapour, there is relatively little shrinkage of the gel due to the absence of capillary pressure. Aerogel has a very low particle coordination number, which is usually macroporous and has high surface area. Nevertheless, they are usually mechanically weak and unstable when exposed to water vapour. Both xerogel and aerogel have high porosity and surface area that make them useful as filter, catalytic substrates, or catalyst support. They are also useful in the preparation of dense ceramics. 2.2 Silica Silicon (Si) constitutes about 28% of the earth's crust. While, silica (SiO2) is the most abundant elements in the earth's crust, viz. 59% mass of the earth's crust is silica. The combination of silica with other oxide forms the silicate minerals in our rock and soil. Silica can be in a form of crystalline (i.e. quartz, cristobalite, tridymite, coesite) or amorphous. The tetrahedral silicate, [SiO4]4- is the building block of silica. Four oxygen ions are in mutual contact and the silicon ion is located in the tetrahedral hole [23]. The Si-O bond length is about 0.162 nm. The bond length is shorter than the sum of the covalent radii of silicon and oxygen atoms (0.191 nm) due to partial ionic and relatively high stability of the siloxane bond. The polymorphism of silica is based on different linkages of the [SiO4]4units. Amorphous silica is formed by random packing of [SiO4]4- unit and it has lower density compared to that of crystalline silica. Opal is one type of the natural amorphous silica. Tridymite and cristobalite have much open structure. Meanwhile, quartz has the densest structure and it is present in sand as a major component with some metal oxides as impurities. 9 Silanol groups are formed on the silica surface during synthesis or due to the rehydroxylation of thermally dehydroxylated silica. There are several types of silanol. They are isolated (single or free silanol), vicinal (hydrogen bonded silanolsilanol), geminal (silanediol), and silanetriol. These silanols can be identified by the 29 Si NMR and Infrared spectroscopy. Internal silanols are present within the colloidal particle during synthesis. H2O is physically adsorbed to all types of silanol groups through hydrogen bonds. Adsorbed water molecules have direct effect on the neighbouring weakened siloxane group, result in splitting of the group and formation of new OH group on the surface. Removal of physically adsorbed water may be completed by heating to 190 ± 10 °C. By about 450-500 °C, all the vicinal groups condense, yielding water vapour and strained siloxane (Si-O-Si) bond. Strained siloxane bond may transform to stable siloxane bond due to the calcinations at above 500 °C. Sintering at temperature higher than 600 ± 10 °C may result in the loss of surface area of the silica. Internal silanols start to condense at 600-800 °C. Above 800 °C [24], geminal silanols are condensed. At temperature 1000-1100 °C, only isolated silanol groups remain on the silica surface [25]. The protons on the silanol group may be exchanged with alkaline ions such as Na+, K+, and NH4+ during synthesis in an alkaline medium. Silanol group can also be esterified as a basis of silica in analytical and chromatography process. Since the seventeenth century, it is known that sand and sodium or potassium carbonate reacts at red heat to form a water soluble glass called water glass. Water glass has been commercially manufactured in 1855 in Europe and America after systematic investigation by Johann Nepomuk von Fuchs in 1850 [26]. Manufacture has generally been carried out in large open-hearth furnaces above 1300 ºC by the following reactions: 10 SiO2 + Na2SO4 + 1/2 C 1/2CO2 + SO2 + SiO2.Na2O (2.1) SiO2 + Na2CO3 CO2 + SiO2.Na2O (2.2) Recently, low temperature method has been employed to synthesize sodium silicate from amorphous silica. In this thesis, amorphous RHA has been used as silica source. Rice husk ash is widely available in Asia from the rice industry as a waste product. This method is made possible by the high solubility of amorphous silica under high pH [17]. Soluble silicates produced from silica are widely used in the glass, ceramics, and cement as a major component. It also been used as bonding and adhesive agents in pharmaceuticals, cosmetics, and detergents industries [27]. Silica has been used as a major precursor for a variety of inorganic and organometallic materials, which have applications in synthetic chemistry as catalysts, thin films or coatings for electronic and optical materials [19]. 2.2.1 The Chemistry of Aqueous Silicates The oxidation state and the coordination number of silicon are +4 and four respectively. Silicon (ionic radius = 0.42 Å) is less susceptible to nucleophilic attack and coordination expansion does not spontaneously occur with nucleophilic reagents [28]. Hence, the kinetic of hydrolysis and condensation of silicon system are slower than in transition metal systems and in Group III systems. The active silica is defined as one that will depolymerise completely to soluble silicate in 100 min at 30 ºC in an excess of 10-2 M NaOH solution (pH 12). Such solution contains monomeric silica and particles up to 10-20 Å. 11 Silicic acid, Si(OH)4 is predominant mononuclear species below pH 7. Silicic acid can be formed by acidifying a soluble silicate or hydrolysing ester (e.g. Si(OEt)4) in excess of water [28]. The earlier approach has been applied in making colloidal silica, which involves making an acidic sol and precipitation of sodium salt (by adding alcohol or acetone) in a strongly acidic medium (about pH 2). The polysilicic acid can be made alkaline that colloidal particles are grown to desired size and stabilize the product. Anionic species (e.g. Si4O8(OH)62-, Si4O8(OH)44-, SiO8(OH)3-, SiO(OH)22-) are the predominant species above pH 7. The negative charges are due to the adsorption of the hydroxyl ions above pH 7, but silica loses the charge in acid solution. When a dilute solution of sodium silicate is partially neutralized with acid to a pH of 8-9, a silica sol rather than a gel is obtained if the concentration of the sodium salt is less than 0.3 M. Na2SiO3 + 2HCl + H2O Si(OH)4 + 2NaCl (2.3) Refer to Figure 2.1, polymerisation (gelation) of silicic acid occur in three states as below [29]: • Polymerisation of monomer, Si(OH)4 to form particles. • Growth of particles. • Particles are linked together in branched chains; the overall medium becomes viscous (thickening), and then solidifies to a coherent network of particles that retains the liquid by capillary action. After gelation, condensation and particle growth (Figure 2.1) may proceed in the aging process. Condensation takes place to maximize the number of Si-O-Si bonds and minimize the number of terminal hydroxyl groups through internal condensation. Ostwald ripening mechanism further the growth of particle size due to the solubility difference between particles especially at higher temperature and above pH 7. At pH> 7, the dissolution of silica is more favoured, nucleation and growth is the predominant mechanism [30, 31]. Particles grow in size and decrease in number as highly soluble small particle dissolve and precipitate on larger nuclei (less 12 soluble). Growth stop when the difference in the solubility between the largest and the smallest particles becomes only a few ppm. Due to the above reasons, Ostwald ripening may result in larger particles formed and reduction in surface area. Figure 2.1 Polymerisation behaviour of aqueous silica and followed by the formation of gels and powders [32]. The classic silica aquasols with particle size 5-100 nm in diameter may be prepared in aqueous medium. By autoclaving the solution, the particle size can exceed 300 nm. Large particle silica sol (2000-3000 nm) can be prepared in organic medium through Stöber Process in an alcohol-ammonia system with enough water [33]. On the other hand, high porosity or high surface area can be prepared on the basis of small dimension of building units. Small particles, “reverse” system (small pores between units) or porosity generated by aggregation of small particles are required in the model of high-surface-area materials. The upper limiting surface area of silica composed of discrete primary particles would be about 2000 m2/g [32]. 13 2.3 Titania and the Chemistry of Aqueous Titania Titanium was first discovered in 1791 by William Gregor [34]. It is the fourth most abundant metal in the earth’s crust, after iron, aluminium and magnesium. It is the first member of the 3d transition series and has four valence electrons, 3d24s2. The most stable oxidation state is +4, which involves the loss of all these electrons. However, titanium may also exist in lower oxidation states, i.e. +3, +2, +1, 0, -1, and -2. Titanium dioxide is important in paint industry as white pigment due to its high opacity, relative chemical inertness and the comparative abundance (and hence cheapness) of titanium ores. The titanium dioxide also possesses a wide range of semi-conductor and dielectric properties, which are highly depending on the density of the point defects. Titanium dioxide exists in three crystalline structure, anatase, brookite and rutile. They have been prepared synthetically. Titanium dioxide precipitated from sulphate or chloride solution at room temperature is essentially amorphous even after drying at 110 °C. Precipitated from the boiling sulphate solutions is in a form of anatase. Rutile may be separated from boiling chloride solution. Meanwhile, brookite crystals may be grown from amorphous TiO2 under hydrothermal conditions with the presence of sodium hydroxide. Both anatase and rutile are tetragonal, whereas brookite is orthorhombic. In all three forms, each titanium atom is coordinated to six almost equidistant oxygen atoms, and each oxygen atom to three titanium atoms [34]. Titanates do not contain discrete TiO44- ions (except barium salt) but are more correctly regarded as mixed metal oxides. There are two types of titanate. They are metatitanate MI2TiO3 or M IITiO3 and orthotitanate MI4TiO4 or M II2TiO4. Titanates are usually water insoluble and are crystalline. The metatitanate of the type MI2TiO3 are prepared by fusion of titanium dioxide with the alkali metal carbonate. Meanwhile, metatitanate MIITiO3 (MII =Mg, Co, Mn, etc.) are prepared by heating the metal oxide with the stoichiometric quantity of TiO2 in a seal tube at temperature of 1000-1300 °C for several hours. 14 Metal oxo species involves metal-oxygen multiple bonds. The TiO2+ exists discretely in the titanyl sulphate, TiOSO4.H2O. The species present in aqueous solutions of titanium (IV) are TiO2+, TiOH3+, Ti(OH)22+, and TiO(OH)+depends on the pH of the solution [34]. Peroxo complexes may be formed in the presence of peroxide solution. The addition of hydrogen peroxide to an acid solution of titanium (IV) will cause the formation of an intense yellow-orange colour. They are being orange in acid solutions, yellow in solutions of pH ~ 8 and colourless in strong alkaline media. The red solid formed when H2O2 is added to a solution of oxotitanium (IV) sulphate in concentrated sulphuric acid is Ti(O2)SO4.3H2O as monomer. The addition of alcohol to a solution of oxotitanium sulphate containing H2O2 and adjusted to pH 8.6 with potassium carbonate may give a yellow solid of a probable formula TiO3.2H2O. There is one peroxo group per titanium atom in the molecule. Solid peroxo titanates of the type MI4Ti(O2)4.H2O have been prepared by adding ice-cold solutions of the alkali-metal hydroxide and H2O2 to the TiO3.2H2O. They are decomposed by aqueous acid and are tetraperoxo species in the solid state, although no more than two peroxo groups would bound to the metal in alkaline solution. The alkoxides of titanium are prepared from titanium (IV) tetrachloride and react with sodium alkoxides (NaOR) in alcohol or with alcohol (ROH) in excess of anhydrous ammonia. The metal alcoxides are rapidly hydrolysed to metal hydroxide in water. The alkoxides of titanium are most widely studied group of organic compound of this element. This is because of their possible application to the development of new polymeric materials with useful properties. For instance, tetra-nbutoxide has been used in the production of heat resistant paints due to the capability of formation of highly dispersed titanium oxide [34]. The solubility of titania in water between pH 3 and 12 is only approximately -6 10 mol/dm3 [35]. The growing particles are very sensitive to shear-induced aggregation. Agitation at low level may produce uniform particle [22]. In an acidic 15 condition (0.001 molar H+), the particle surface charge increases, the final titania particle size decreases, and larger shear rate is required to induce aggregation. 2.4 The Chemistry between Silica and Titania There are three main approaches that can be applied to synthesize titaniasilica material. They are ionic interaction due to opposite surface charge of the particles, reaction of the silicic acid with target oxide, and reaction with the silanol group on the silica surface (grafting). However, the second method is only applied to iron, uranium, chromium and aluminium but not yet explored to titania. The other two methods involve surface reaction but not monomeric silica species. The isoelectric point (iep, electrical mobility of silica particles is zero) and point of zero charge (surface charge is zero) of silica is at pH 2 ± 0.5. Therefore, silica is negatively charged above pH 2 [36]. It has been assumed that the catalyst below pH 2 is the H+ ion, which forms an active cationic complex. Also, above pH 2 the OH- ion is the catalyst in that active anionic silica is generated. Vysotskii and Strazhesko [37] have pointed out that in the presence of acid such as sulphuric acid, the iep is not only the point of minimum rate of gelling but also the point which gels of maximum strength and maximum specific surface area are obtained. This is because the rate of aggregation is minimum at the iep and the rate of growth of the ultimate particles from monomer at minimum, so that the ultimate particles are the smallest as they form the gel. In contrast, titania has an iep around pH 5-6 [38]. To coat sol carrying positive charge, like Fe2O2 or Al2O3, it is necessary to reverse the charge by adding the dilute sol into dilute (10%) sodium silicate under intense agitation. Alternatively, chelating agent such as citrate could be applied before adding silicate. The surface thus covered with a negatively charged molecular layer of adsorbed silicate on which a layer of SiO2 can be applied [39]. For example, 16 cation-coated silica sol can be synthesized by adding basic salts of Al, Zr or Ti (e.g. AlCl3) to an acidic or basic silica sol while stirring. Followed by addition of NaOH until pH 4-6 and finally aged under 80-100 °C. This cationic-coated silica sol can mix easily with water-soluble organic solvent and may be used in acidic condition [40]. The negative charge on silica can be reversed by adsorbing an excess of positively charged material on the surface. The coating included oxides of tri- and tetravalent metals such as aluminium, chromium, gallium, titanium, and zirconium. In making these products, some researchers mixed acidified silica sol with a basic metal salts, which contained extremely small colloidal particle of metal oxide and adsorbed on the silica surface. For instance, a titania-coated sol was made by hydrolysing an organic titanium compound in an acid-stabilized silica sol at the pH of less than 2 and heating the mixture to cause the titania to be deposited on the surface of the particles [41, 42]. 2.4.1 Titania-Silica in Catalysis Since the development of the first heterogeneous titania/silica catalyst for the epoxidation of olefins by Shell in 1971, a series of new preparation methods were developed to synthesize materials containing highly dispersed titanium centres in a silica matrix. Titanosilicate-catalyzed epoxidation of olefins with hydrogen peroxides was made possible by the discovery of TS-1 in which Ti has been substituted for Si in the MFI framework. The search for large pore analogues of TS-1 has led to the study of Ti substituted into the framework or grafted onto the channels of zeolite beta or MCM type silicalites. Several researches have been directed at homogeneous analogues of TS-1, such as CpTi-silsesquioxane immobilized in the mesopores of MCM-41. Deposition or anchoring of Ti sites on silica circumvents the steric problem by avoiding narrow channels. Catalysts prepared by sol-gel [43] contain accessible immobilized Ti 17 within the silica framework. Since high specific surface area is obtained and the resulting porous structure is very open, larger substrates can access the active sites. Better accessibility may be obtained by having the active component on the surface. In this arrangement, the pore diffusion is better and no size selection takes place. In search of a preparation method that would produce this geometric arrangement and a high surface concentration of the active site, i.e. isolated Ti within a silica environment, we investigated the preparation of titania/aerosilica catalysts by sol-gel synthesis. It is now established that nanoscale engineering of sol–gel TiO2–SiO2 mixed oxides provides excellent microporous or mesoporous epoxidation catalysts. A comparison of the catalytic performances of these amorphous oxides with molecular sieve materials has been reported. Highly dispersed titania in the silica matrix, mesoporous structure, and high surface area are the key characteristics of the 20 wt% TiO2–80 wt% SiO2 aerogel obtained by this method. This catalyst showed outstanding performance in the epoxidation of cyclic olefins with alkylhydroperoxides [16]. Yoshida et al. [4] has conducted a screening study of silica-supported catalysts. Silica supported Ti system was found to be the most effective catalyst for the epoxidation of propene. All of these findings strongly suggest that silica-titania mix oxides might be the best combination to become the best catalyst for the oxidation reaction compared to others. Besides as catalyst, TiO2–SiO2 mixed oxide materials are widely used in optical films because of their chemical stability and large refractive index difference. Optical coatings of TiO2–SiO2 can be produced as anti reflective thin films with tailored refractive indices. Sol–gel process provides an attractive route to the preparation of multi-component oxide materials that show homogeneity in the distribution of heterometal oxide bonds [44]. 18 2.5 Aerogel 2.5.1 History and Development of Aerogel Aerogel was first discovered in 1931 by physicist Steven S. Kistler of the College of the Pacific, Stockton, California, who wanted to prove that a ‘gel’, once dried, contained a continuous solid network the same size and shape as the wet gel [45]. His attempts with aqueous silica or alumina gels (hydrogels) from which water was removed at above critical conditions (374.0 ˚C, 22.1 MPa) did not produce satisfactory results. Eventually, he obtained silica aerogels by: 1. Preparation of a hydrogel in reaction of sodium silicate with hydrochloric acid, 2. Careful removal of sodium and chlorine ions, 3. Converting the hydrogel into alcogel by replacing water with ethyl alcohol in a lengthy process of multifold solvent replacement, and 4. Drying at above critical conditions for ethyl alcohol. Later, Fricke obtained aerogels from alumina, tungsten, tin, and iron oxides, as well as from organic gels such as gelatine, proteins, and cellulose. Rediscovery of aerogels took place in the 1960s. In the late 1970s, the French government approached Stanislaus Teichner at Universite Claud Bernard in Lyon seeking a method for storing oxygen and rocket fuels in porous materials. Teichner et al. substantially simplified the procedure by carrying out the sol-gel transition in the vary solvent which was then removed at supercritical conditions. Because water and alkoxysilanes are immiscible, a mutual solvent such as alcohol is normally used as a homogenizing agent [45]. 19 2.5.2 Aerogel Synthesis (i) Drying Process At a normal drying process at ambient condition, gel will form a xerogel once dried. This is due to the surface tension of the pore liquid causing the shrinkage and fracture of the gel structure. Three stages are occurring in the drying process. First stage is decreasing in gel volume (shrinkage) due to large capillary forces exerted by the pore liquid during evaporation. Once the gel network may not be compressed further, pore liquid starts to evaporate and causes the capillary force reduces in second stage. Final stage starts with the evaporation of the liquid within the pores and the vapour diffuse to the surface [46]. The increase of solvent tension will cause a linear decrease in the xerogel surface area, pore volume and pore size [47, 48]. Hence, approaches to obtain lower surface tension become significant in order to produce a dried gel with higher surface area. (ii) Elimination of Surface Tension. Capillary pressure, Pc is the factor that drives shrinkage of a gel during drying process. By assuming that the contact angle is zero, Pc is given by equation as shown below [49]. Pc = 2γ LV (rp − t ) t = Thickness of a surface adsorbed layer γ LV = Surface tension of the pore liquid rp = Pore radius (2.4) 20 Thus, capillary pressure could be minimized through reduce the surface tension of the pore liquid by chemical additive or prepare gel with larger pore size. Other alternative is dry the sample when the liquid has no surface tension, as happened in freeze drying and supercritical drying methods where surface tension ceases and meniscus no longer form. Aging is another approach to reduce fracture. It strengthens the network and thereby the gel skeleton becomes stiffer and stronger [50]. (iii) Freeze Drying Freeze drying is a way to avoid the presence of the liquid vapour interface. In this method, the pore liquid is first frozen and thereafter dried by sublimation under vacuum as shown in Figure 2.2. The materials obtained are termed cryogels. The gel network may be destroyed by the nucleation and growth of the solvent crystals. Thus, surface area and mesopore volume tend to be smaller than that of aerogel as well as larger pores are formed in cryogels. Flash freezing has been developed to overcome these problems. Solvent that has low expansion co-efficient and high pressure of sublimation is recommended in order to reduce the time of the process and to obtain better properties in cryogels [51]. Pressure Liquid Solid Gas Temperature Figure 2.2 The freeze drying process path (bolded arrows) in pressuretemperature (P-T) phase diagram of a pure substance [52]. 21 (iv) Supercritical Fluid Extraction Figure 2.3 shows the pressure-temperature (P-T) phase diagram of a pure substance. The vapour pressure curve starts at the triple point and ends at the critical point. The melting pressure curve starts at triple point and rises with increasing temperatures and pressures. Meanwhile, the sublimation curve starts at triple point and goes down with temperatures and pressures. In the region below the critical point, phase transition take place by changing temperature and pressure of the substance. Each phase can co-exist in the same system with clearly separated phases. In the region where the temperature above the critical point, the substance cannot be liquefied by pressure increased. Critical temperatures and pressures (both minima) show new “phase”: supercritical gas/fluid has a density like that of the liquid but its flow properties and ability of molecules to be separated from one another like a gas. There is no phase transition between gas and liquid. The substance in this region is termed supercritical fluid. It is the gas-like, high diffusion coefficients and low viscosities. In addition to the liquid-like high solvating properties, that makes supercritical fluids good solvents. In the view of solvent properties, the surface tension of the solvent will be reduced with the increase of temperature. Once critical point achieved, there is no more surface tension exists. Therefore, ethanol can be extracted at this stage without the collapse of the gel structure due to the surface tension. The bolded arrows show the path how a critical point can be reached in the aerogel synthesis process. Supercritical extraction is an extraction using a supercritical fluid (must be above critical point). The solvent is heated in a pressure reactor until exceeds its critical point, viz. formation of the supercritical fluid. The fluid is released slowly from the system and then causes the decrease of pressure. When the pressure is lower than the critical point, all the ethanol will be converted into gas form. When the pressure reaches ambient pressure, the reactor will be 22 flushed with inert gas, e.g. nitrogen gas to remove the ethanol that remains as vapour in the system. Clearly, the whole process to remove ethanol does not involve surface tension. Hence, aerogel can be produced. Pressure Solid Supercritical Fluid Liquid Pc Critical Point Ptp Gas Triple Point Tc Ttp Figure 2.3 Temperature The supercritical drying process path (bolded arrows) in pressuretemperature (P-T) phase diagram of a pure substance [52]. Ethanol, an alcohol, is used as a medium in the supercritical drying process. Ethanol has lower surface tension compared to the other alcohols and other solvents. In addition, ethanol is cheap and abundant. It is a good solvent that can dissolve in both polar and nonpolar solvents [53]. At the same time provide low silica solubility (Table 2.1), thus protect from damage of the silica skeleton along the synthesis process. Table 2.1: The solubility of silica in different solvent [54]. Solvent Solubility of silica (mg/L) Methanol 1890 Ethanol 164 Propanol 8 23 Nevertheless, once the critical point is approached, aerogel may be formed. For example, supercritical carbon dioxide, CO2 (Tc = 31 ˚C, Pc = 73 atm) has been used in many aerogel syntheses. Besides used in supercritical extraction, critical point also can be applied in liquefaction of gases, e.g. fuels and air conditioning (must be below critical point). Table 2.2 shows the critical point of some solvents. Table 2.2: The critical point of different solvents [55, 56]. Substance Formula Tc (˚C) Pc (MPa) Pc (g/cm3) Vc (cm3/mol) Carbon Dioxide CO2 31.0 7.38 0.47 94 Methanol CH3OH 239.4 8.08 0.27 117 Ethanol C2H5OH 240.9 6.14 0.28 168 Water H2O 374.0 22.1 0.32 56 Note: 1 MPa = 106 Pa = 9.87 atm. Tc = critical temperature, Pc = critical pressure, Vc = critical volume. 2.5.3 Properties and Applications of Aerogel Aerogels composed of 65 to 90% of air, are the lightest solids ever produced. Silica aerogel is a porous material with optical and thermal properties that makes the material very interesting as an insulation material [3, 57]. Table 2.3 shows the properties of silica aerogel. Besides being the best thermal, electrical, and acoustic insulators known, aerogels are finding its application as filters for seawater desalination, micrometeoroid collectors, and subatomic particle detectors. In the future, aerogels could be used in windows, building insulation, automobile catalytic converters, and high-efficiency battery electrodes [58]. 24 Aerogel catalysts are prepared by the sol-gel method associated with the supercritical drying procedure. The resulting catalysts, in the form of simple or mixed oxides and supported metals exhibit interesting high surface areas and large pore volumes. Their very good resistance to heat treatments allow them to be used for all types of catalysed reactions up to 450¯500 °C. Aerogel catalysts show in general greater activity and selectivity than the corresponding xerogels. Their stability with time on stream is also remarkable [43]. Table 2.3: Some properties of aerogel [59, 60]. Property Value Thermal conductivity 0.018- 0.3 Wm-1K-1 Bulk density 80- 140 kgm-3 Particle size 5- 500 µm Specific surface area 400- 1000 m2/g Temperature stability Up to 600 ˚C Pore size < 50 nm CHAPTER 3 EXPERIMENTAL The experiment is generally divided into the following stages: 3.1 • Synthesis of silica aerogel • Synthesis of titanium containing silica aerogel • Characterization of titanium containing silica aerogel • Catalytic testing of the titanium containing silica aerogel Synthesis of Silica Aerogel The white RHA taken from Sabak Bernam, Selangor was used in the synthesis of sodium silicate. The synthesis is depicted in Figure 3.1. As the type of silica in the RHA is present in amorphous form, this gives an advantage to synthesize sodium silicate through low temperature alkali extraction method i.e. below 100 °C. The solubility of amorphous silica is very low at pH < 10 and increases sharply above pH 10. This unique solubility behaviour makes silica extractable from RHA by dissolution under alkaline conditions and subsequently precipitating it at a lower pH. This process of obtaining silica is an alternative method to the current high-energy method that manufactured by smelting quartz sand with sodium carbonate at 1300 °C [61]. 26 Sodium hydroxide 14.55 g RHA 39.13 g Filtrate 95 °C Distilled water 450 ml 1 day Screw cap Teflon bottle Figure 3.1 Supernatant (Sodium silicate) Synthesis of sodium silicate from rice husk ash. 200 g of sodium silicate was reacted with 167.32 g H2SO4 96% to form a gel. The gel was crushed and kept in a Teflon bottle for 1 day (Figure 3.2). Later the gel was washed with distilled water to remove soluble salt. Soxhlet process was carried out to replace the water in the aquagel with ethanol forming an alcohol filled gel (is termed alcogel) [62]. Supercritical extraction (Figure 3.2) was carried out using Parr instrument autoclave fitted with a thermocouple, a pressure gauge and a temperature controller. Ethanol was used as the supercritical drying solvent in this unit. Ethanol has a critical temperature and pressure of 239 ˚C and 1200 psi, respectively. The volume of ethanol that needs to be loaded is calculated using the equation 3.1 below. y = (2000-Vgel)/3.72 where, Vgel = volume gel loaded (ml) If y < 500, 500 ml ethanol is used If y > 500, y ml ethanol is used (3.1) 27 The reactor was heated according to the heating programmed as shown in Table 3.1. When the reactor reached 275 °C and kept for an hour, the pressure was isothermally released at a rate of 20 psi/min. The reactor condition was maintained slightly above the critical condition of ethanol to ensure that the whole mixture was supercritical. The exiting solvent was collected in the condenser. The nitrogen gas was flushed slowly through the autoclave for 15 minutes after the pressure reached ambient pressure. Then, the reactor was left to cool overnight [63]. Table 3.1: Temperature programme implemented in the supercritical drying process [64]. Time (hour) Temperature (°C) 1st 50 2nd 100 3rd 150 4th 200 5th 225 6th 250 7th 275 28 Magnetic stirrer Sodium silicate solution Washed and filtered Aging + H2SO4 Aquagel Gel AEROGEL Supercritical extraction Figure 3.2 3.2 Alcogel Soxhlet with ethanol Synthesis of silica aerogel from sodium silicate. Synthesis of Titanium Containing Silica Aerogel In this work, three methods of preparation of titanium containing silica aerogel were implemented. They were direct synthesis, grafting and precipitation. Grafting and precipitation involved heterogeneous synthesis condition, where between the silica (silica aerogel made) and the titanium source. In contrast, direct synthesis involved homogeneous mixture of the sodium silicate with the titanium precursor. 29 3.2.1 Post Synthesis: Synthesis of Titania-Silica System (i) Grafting with Titanium(IV) Tetrachloride 30 g of the silica support (RHA, or aerogel) was added to a solution containing 12.56 g of TiCl4 in 200 ml n-hexane. After stirring for 4 hours, the solvent was removed by evaporation in a rotary evaporator at 50 °C and then at 80 °C for 1 hour. The resulting solid was dried at 120 °C for 12 hours and calcined in air at 500 °C for 20 hours [64]. (ii) Grafting with Titanium(IV) Isopropoxide 40 g of the silica support was added to a solution containing 25.11 g of titanium(IV) isopropoxide in 350 ml of hexene. After stirring, the alcohol was removed by evaporation in a rotary evaporator at 70 °C. The resulting solid was dried at 120 °C for 12 hours and calcined in air at 500 °C for 20 hours [65]. (iii) Precipitation of Titania on Amorphous Silica The supports used were RHA (BET surface area of 30.06 m2/g), and silica aerogel (BET surface area of 374.44 m2/g). The silica was first calcined at 600 °C for 12 hours before deposition of titania. 16.76 g pure TiCl4 was added to a diluted solution of HCl (pH = 0.5-1.0, HCl 30% 8 g + H2O 432 g). 40 g of silica support were then added and ammonium hydroxide was added under continuous agitation until a final pH of 7.5 was reached. The resulting solid was dried at 120 °C for 12 hours and calcined in air at 500 °C for 20 hours [65]. 30 3.2.2 Direct Synthesis: Synthesis of Titania-Silica Aerogel System Titanium(IV) ethoxide (4 mmol Ti) and sulphuric acid were mixed with sodium silicate (400 mmol Si) solution to get a transparent solution. The solution was adjusted to pH 6 by adding NaOH solution or sulphuric acid. The gel was aged for 3 days. This synthesis method was developed during this research work through several trial and errors process. After that, the silica gels were dispersed in distilled water and filtered to remove the soluble salts. The washing step was repeated two more times. The aquagel was then solvent exchanged with ethanol using soxhlet technique to remove water from the gel matrix. Supercritical drying process was conducted in order to synthesis an aerogel. 3.3 Parameter Study for Synthesis (Direct Synthesis) of Titanium Containing Silica Aerogel 3.3.1 Sources of Titanium Various titanium sources were used to prepare the titanium containing silica aerogel as listed in Table 3.2. In a typical preparation, titanium(IV) isopropoxide (10 mmol Ti) and sulphuric acid solution (H+:NaOH molar ratio = 1) were mixed with sodium silicate solution (330 mmol Si) to obtain a homogeneous mixture solution. The solution is left to gel and aged for 3 days. After that the silica gels were dispersed in distilled water and filtered to remove the soluble salts. The washing step was repeated until the filtered cake near to neutral. The aquagel was then solvent exchanged with ethanol using soxhlet 31 technique to remove water from the gel matrix. Supercritical drying process was performed to remove ethanol in order to synthesis aerogel (Figure 3.2). The same preparation procedure was implemented for other titanium source. The loading of titanium was fixed to Si:Ti = 33 in the gel mixture. The gel matrixes were obtained by adding stoichiometric volume of sulphuric acid for neutralizes the sodium silicate. After supercritical dried, the sample was dried in vacuum oven. The samples were characterized using nitrogen adsorption, X-ray diffraction (XRD), Fourier transform infrared (FTIR), and UV-Vis diffuse reflectance spectroscopy (UV-Vis DRS). Table 3.2: Titanium sources that have been used in the synthesis of titanium containing silica aerogel 3.3.2 Titanium Source Formula Titanium(IV) ethoxide Ti(OCH2CH3)4 Titanium(IV) isopropoxide Ti[OCH(CH3)2]4 Titanium(IV) propoxide Ti(OCH2CH2CH3)4 Titanium(III) sulphate Ti2SO3 Titanium(IV) chloride TiCl4 Titanium(IV) oxide TiO2 Si:Ti Molar Ratio Various silica per titania ratios have been used to prepare the titanium containing silica aerogel. The titanium(IV) isopropoxide was used in this part of the experiment. Si:Ti molar ratios studied are 1, 6, 33, and 49. In a typical preparation for molar ratio Si:Ti = 1, titanium(IV) isopropoxide (33 mmol Ti) and sulphuric acid solution (H+: NaOH molar ratio = 1.25) were mixed 32 with sodium silicate solution (330 mmol Si) to obtain a homogeneous mixture. The solution was left to gel and aged for 3 days. After that, the silica gels were dispersed in distilled water (500 ml) and filtered to remove the soluble salts. The washing step was repeated until the filtered cake was almost neutral. Subsequently, the aquagel was soxhlet with ethanol to replace water with ethanol. Supercritical drying process was carried out to remove ethanol in order to synthesis an aerogel. The same preparation procedure was implemented for other Si:Ti molar ratios. The gel was obtained by adding 2.5 times the volume of acid that was needed to neutralize the sodium silicate. After supercritical drying, the sample was dried in vacuum oven. The samples were characterized using nitrogen adsorption, X-ray diffraction (XRD), Fourier transform infrared (FTIR), and UV-Vis diffuse reflectance spectroscopy (UV-Vis DRS). 3.3.3 Sulphuric Acid Loadings Various loading of the sulphuric acid were used to prepare the titanium containing silica aerogel. The acid was not only used to neutralize the sodium silicate, but also to induce the formation of silicic acid and to change in aging pH. The titanium(IV) isopropoxide was used for this purpose. The molar ratio of Si:Ti = 33 was selected. The acid loadings (mol H+: mol NaOH) were varied from 0.75 to 2.5, i.e. 0.75, 1.00, 1.25, 1.50, 1.75, and 2.50 times the volume to neutralize sodium silicate. In a typical preparation, titanium(IV) isopropoxide (10 mmol Ti) and sulphuric acid solution (H+: NaOH molar ratio = 2.50) were mixed with sodium silicate solution (330 mmol Si) to obtain a homogeneous mixture. The solution was left to gel and aged for 3 days. 33 After that the silica gels were dispersed in 500 ml of distilled water and filtered to remove the soluble salts. The washing step was repeated until the filtered cake was near to neutral. The aquagel was then solvent exchanged with ethanol using soxhlet technique to remove water from the gel matrix. Supercritical drying process was performed to remove ethanol in order to synthesis an aerogel. After supercritically dried, the sample was dried in vacuum oven. The samples were characterized using nitrogen adsorption, X-ray diffraction (XRD), Fourier transform infrared (FTIR), and UV-Vis diffuse reflectance spectroscopy (UV-Vis DRS). 3.4 Characterization The structure of the solid samples were characterized using nitrogen adsorption, X-ray powder diffraction (XRD), Ultra Violet-Visible diffuse reflectance spectroscopy (UV-Vis DRS), Fourier Transform Infrared Spectroscopy (FTIR), and scanning electron microscopy (SEM). 3.4.1 Nitrogen Adsorption: Brunauer, Emmett, Teller (BET) method Specific surface area can be determined through BET equation [66]: p 1 c −1 p = + , Vads (p ° − p) Vm c Vm c p ° Where, po = atm pressure p = partial pressure c = BET constant. Vads = volume of gas adsorbed. Vm = monolayer capacity. (3.2) 34 The effective molecular area of nitrogen (am) is taken as 16.2 Å2 (16.2 x 10-20 m2/molecule), and NA is Avogadro number (6.0223 x 10-23 molecule/mole), which is used to calculate the specific surface area of the sample (ABET). ABET = Vm.NA.am or ABET = Vm(4.53) m2/g (3.3) Total pore volume can be calculated using formula: Vp = V0.95 (0.00156), where V0.95 is volume adsorbed at pressure relative, p/p o = 0.95. By using these data, the pore diameter, d can be calculated using formula: d = 4 (Vp/ABET). The classification of the pores is shown as in Table 3.3. Table 3.3: IUPAC classification of pores [67, 68]. Type of Pore Pore Diameter (nm) Micropores 0-2 Mesopores (also intermediate pore or transitional pores) 2-50 Macropores 50-7500 Megapores > 7500 Note: 1 nm = 10 Å = 10-9 m The BET method is not valid for the calculation of surface area for isotherm Type I and Type III. On the other hand, both Type II and Type IV isotherms are amenable to the BET analysis, provided that the value of c is not too high and the BET plot is linear for the region of the isotherm containing “Point B”. “Point B” was taken by Emmett and Brunauer to indicate the completion of the monolayer, and was the point that often displayed a rather long straight portion started from this point in the isotherm. By assuming that the solid composed of similar size spheres [67], the mean diameter (l) may be calculated from the surface area (A) using formula l = 6 , ρA where ρ is the density of the spheres. According to the Globular theory [68], the 35 particle size of silica can be estimated through the same equation with following concerns: l= 6 ρA BET (3.4) With l is the particle size (nm), ABET is the specific surface area (m2/g), and ρ is the density (density of amorphous silica 2.2 x 106 g/m3). Experimental: The BET specific surface area and total pore volume (measured at p/po = 0.95) were obtained from the isotherms of nitrogen adsorption at 77 K, using ThermoFinnigan S.p.A. Qsurf Surface Area Analyser M1. The flow rate for the gases was set at 20 ml per minute or 40 psi (i.e. 3.5 unit on the flow meter scale) and room temperature was set to the instrument. The sample (ca. 0.05 g) was previously degassed at 200 ºC under flowing nitrogen for 30 minutes. Then, it was transferred to test channel and analysis was started after nitrogen gas flushed for 25 minutes. Standard calibration reading was kept in the range of 35 to 40 seconds. After analysis, the total weight of the sample holder and sample (after degassed) was keyin to get the surface area or total pore volume. 1 cm3 nitrogen gas adsorbed is equivalent to 2.84 m2 of surface area is considered for the instrument measurement. 3.4.2 XRD Measurement XRD is a technique used to characterize solid materials. It is powerful in determining the phase of the materials. The crystalline materials will have their own diffraction pattern, which can be considered as their “fingerprint”. In contrast, small particles or amorphous phases give either broad or weak diffractogram or no diffraction at all. In particular, the surface region, where catalytic activity resides is virtually invisible for XRD [69]. 36 Powder diffraction pattern is a plot of the intensity of the diffracted beams, which represents a map of reciprocal lattice parameter or Miller indices (hkl) as a function of 2θ, which satisfies the Bragg condition: nλ = 2d sin θ Where, (3.4) n = order of the reflection (n = 1, 2, 3, …) d = distance between two lattice planes λ = wavelength of the X-rays θ = diffraction angle Commonly, first order diffraction (n = 1) is implemented. The Bragg relation gives the corresponding lattice spacing, which are characteristic for a certain compound [70]. Experimental: All the samples were characterized by X-ray powder diffraction using Bruker Advance D8 with Siemens 5000 diffractometer and the Cu Kα (λ = 1.5405 Å) radiation as the diffracted monochromatic beam at 40 kV and 40 mA. Silicon powder was used as an internal standard. Typically, powder samples were grounded and spread on a sample holder. The diffraction pattern was scanned in the range between 2º to 60º at a step of 0.020º and step time 1.0 s (scanning speed of 1.2º/min). 3.4.3 UV-Vis Diffuse Reflectance Spectrometry Electronic transitions of substrate can be studied by the UV-Visible light. The UV-Vis spectra are very broad [71]. In the diffuse reflectance mode, samples can be measured as loose powders. Diffuse reflectance is also the indicated technique for strongly scattering or absorbing particles. The reflectance spectrum is described the Kubelka-Munk function [72]: 37 K (1 − R∞ ) = S 2 R∞ Where, 2 (3.5) K S = absorption coefficient, a function of the frequency v = scattering coefficient R∞ = reflectivity of a sample of infinite thickness, measured as a function of v. Experimental: UV-Vis DRS measurements under ambient conditions were performed using Perkin-Elmer Lambda 900 UV/VIS/NIR Spectrometer equipped with a diffuse reflectance attachment with a 76 mm integrating sphere using BaSO4 as a reference. The samples were previously outgased at 120 °C for 12 hours before analysis in order to eliminate the adsorbed water. The reflection in the percentage was measured and presented by Kubelka-Munk function. 3.4.4 Fourier Transform Infrared Spectroscopy Infrared has a wavelength in the range of 1-1000 µm. Infrared is classified to far (10-200 cm-1), mid (200-4000 cm-1), and near (4000-10000 cm-1) infrared that used for the detection of lattice vibrations, molecular vibrations, and overtones accordingly. Mid infrared region is that of interest to us. The infrared region between 4000 and 200 cm-1 can roughly be divided into four regions: 1. The X-H stretch region (4000 - 2500 cm-1), where strong contributions from OH, NH, CH and SH stretch vibrations are observed, 2. The triple bond region (2500 - 2000 cm-1), where contributions from gas phase CO (2143 cm-1) and linearly adsorbed CO (2000 - 2200 cm-1) are seen, 38 3. The double bond region (2000 -1500 cm-1), where in catalytic studies bridge bonded CO, as well as carbonyl groups in adsorbed molecules (around 1700 cm-1). The fingerprint region (1500 - 500 cm-1), where all single bonds between carbon and elements such as nitrogen, oxygen, sulphur and halogens absorb, 4. The M-X or metal-adsorbate region (around 200 - 450 cm-1), where the metal-carbon, metal-oxygen and metal-nitrogen stretch frequencies in the spectra of adsorbed species are observed. Correlation charts should be consulted for more precise assignments [73, 74, 75, 76]. The hydroxyl range between 3000 and 3800 cm-1 contains contributions from adsorbed water and several hydroxyl groups on the SiO2 surface. The broad absorption band around 3550 cm-1 is due to hydrogen-bonded OH groups. The sharp peak at 3740 cm-1 corresponds to single OH group, which has no interaction with other hydroxyls. The peak around 3660 cm-1 could belong to OH groups inside the silica. Similar correlations exist for the O-H stretch frequencies of OH groups on alumina and titania supports [77]. The assignments of some infrared frequencies in silicon compounds are showed in Table 3.4. Table 3.4: Some assignments of infrared frequencies [78]. Group -SiOH Range (cm-1) and Intensity Assignments and Remarks 3700-3200 (s) OH stretch, similar to alcohols 900-820 (s) Si-O stretch ca. 1430(m-s) Ring mode 1100 (vs) Ring mode 1100-1050 (vvs) Si-O-C antisymmetric stretch Si-O-Ar 970-920 (vs) Si-O stretch Si-O-Si 1100-1000 (s) Si-O-Si antisymmetric stretch Si-Ar Si-O-C (aliphatic) Note: w = weak, m = medium, s = strong, vs = very strong, vvs = very very strong. 39 Experimental: Infrared spectra of the samples were collected using a Perkin-Elmer Fourier transform infrared (FTIR), with a spectral resolution of 2 cm-1, scanned for 10 s at 20 ºC by KBr pellet method. The framework spectra were recorded in the region of 1500 – 400 cm-1. 3.4.5 Scanning Electron Microscopy Electron microscopy gives straightforward determination of morphology of the surface. Scanning electron microscopy (SEM) is carried out by rastering a narrow electron beam over the surface. The yield of either secondary or backscattered electrons is detected as a function of the position of the primary beam. An electron microscope offers additional possibilities for analysing the sample. Emitted X-rays are characteristic for an element and allow for a determination of the chemical composition of a selected part of the sample, i.e. energy dispersive X-ray analysis (EDX). Experimental: The SEM stub was cleaned, and then placed double-sided tape on the top. The sample is dispersed on the surface. A tin layer of gold coating is deposited on the sample by gold spattering. Then, it is analysed using XL 40 Phillips type instrument. 3.5 Catalytic Properties: Oxidation of Alkene Oxidation was carried out batch wise in a mechanically stirred 250 ml thermostated glass reactor equipped with reflux condenser. In a typical run, 10 ml of fresh distilled cyclohexene, 10 ml of acetone and 156 mg of the catalyst were mixed in the reactor and the suspension was heated at 70 ºC. Then, 8.35 ml of 35% 40 hydrogen peroxide was added to the reaction mixture while maintaining vigorous stirring. After the reaction, the mixture was cooled to room temperature. The organic layer was separated by centrifugation and/or by extraction with diethyl ether and then analysed by a Thermo Finnigan, Trace Gas Chromatography (GC) using a capillary column (Equity-1, 30 m x 0.25 mm x 0.25 µm). The oventemperature programme was tabulated as in Table 3.5. A flame ionisation detector (FID) was applied. Table 3.5: GC-FID instrument setting Oven Parameters Setting Initial temperature 40 ˚C Initial time 2.00 min Rate 10.0 ˚C/min Final temperature 200 ˚C Carrier mode Constant pressure Detector Parameters Setting FID base temperature 250 ˚C H2 flow 35 ml/min Air flow 350 ml/min Makeup gas flow 30 ml/min Injection Port Parameters Setting Base temperature 250 ˚C Split flow 100 ml/min Injection volume 1.0 µl Products were identified by comparing with authentic samples (internal standard) that have been checked using gas chromatography- mass spectroscopy (GC-MS). Authentic samples that implemented in this study were cyclohexene oxide, 2-cyclohexen-1-ol and 2-cyclohexen-1-one supplied by Fluka. While, standard 41 1, 2-cyclohexanediol has been synthesized through homogeneous synthesis as described below [79]. 140 ml of 30% hydrogen peroxide (1.4 moles) was added to 600 ml of 88% formic acid (13.7 moles) in a three-necked flask equipped with a thermometer and a motor-driven stirrer. Freshly distilled cyclohexene (82 g, 1.0 mole) was added slowly from a dropping funnel over a period of 20–30 minutes while the temperature of the reaction mixture was maintained between 40 °C and 45 °C by cooling with an ice bath and by controlling the rate of addition. The reaction mixture was kept at 40 °C for 1 hour after all the cyclohexene has been added. Then it was left overnight at room temperature. The formic acid and water were removed by distillation from a steam bath under a reduced pressure. An ice-cold solution of 80 g of sodium hydroxide in 150 ml of water was added in small portions to the residual viscous mixture of the diol and its formats. The temperature of the mixture was controlled so that it did not exceed 45 °C. The alkaline solution was warmed to 45 °C, and an equal volume (350 ml) or more of ethyl acetate was added. After thorough extraction, the lower layer was separated and extracted at 45 °C six times with equal volumes of ethyl acetate. The seven ethyl acetate solutions were combined (total volume about 2.1 liter), and the solvent was distilled from a steam bath until the residual volume was 300–350 ml and the solid product begins to crystallize. The mixture was cooled to 0 °C, and the product was separated by filtration (77–90 g, melted in the range of 90– 98 °C). The mother liquor was concentrated on a steam bath to a volume of 65–75 ml, and more solid crystallized. The mixture was cooled and filtered as before and yielded an additional 4–15 g of crude product melted in the range of 80–89 °C. Trans-1, 2-cyclohexanediol, boiling point 120–125 °C/ 4 mm, was obtained by distillation of the combined crude products, using an oil bath, a flask having a side arm, with an air condenser sufficiently wide that they will not become plugged as the product solidifies. The yield of product of melting point 101.5–103 °C was 75–85 g (65–73%). CHAPTER 4 RESULTS AND DISCUSSION 4.1 Synthesis of Silica Aerogel The silica aerogel is a semi-transparent and very light fluffy powder. The XRD pattern of silica aerogel in Figure 4.1 shows it is completely amorphous. This result is well agrees with other reported study [45]. 800 700 600 Lin (Counts) 500 400 300 200 100 2 Figure 4.1 10 20 30 40 50 2θ (degrees) XRD diffractogram of silica aerogel. 60 70 80 90 43 The SEM micrograph in Figure 4.2 shows the surface morphology of the silica aerogel. The sample was found having a rough and homogeneous surface. It was similar with the morphology of the aerogel that prepared using TMOS as silica source [80]. Figure 4.2 SEM micrograph showing the surface morphology of silica aerogel. The EDX analysis of silica aerogel shows the presence of silicon and oxygen as major elements. Trace amount of aluminium, which comes from the RHA source was also detected. RHA sample taken from Bagan Sekincan, Perak was also found to contain trace amount of alumina content [81]. This is resulted from the dissolution of the alumina by the sodium hydroxide solution; similar to the Bayer process in the extraction of alumina from bauxite mineral [82]. Al2O3 + 2OH- + 3H2O 2Al(OH)4- (4.1) FTIR spectrum of silica aerogel (Figure 4.3) shows a band at 969 cm-1, which was attributed to the stretching of terminal silanol group, namely Si-OH or SiO-H 44 group. The band at 799 cm-1 was associated with symmetric Si-O-Si stretching. The most intense band at 1090 cm-1 was assigned as the asymmetric Si-O-Si stretching [83]. 100 80 60 %T 40 799 969 20 1090 Figure 4.3 1100 1300 1500 cm -1 900 700 400 FTIR spectrum of silica aerogel. The highest and the lowest surface area of the silica aerogel achieved in this study are 882 m2/g and 387 m2/g respectively (Table 4.1). The particle sizes of the silica in samples A1.0 and A2.5 are 7.03 nm and 3.09 nm respectively according to the Globular theory [68]. Table 4.1: The surface area of the silica aerogel. Sample Aerogel BET Surface Area (m2/g) Particle Size (nm) A1.0 387 7.03 A2.0 784 3.48 A2.5 882 3.09 A3.0 867 3.14 A14.8 607 4.49 45 4.2 Synthesis of Titanium Containing Silica Aerogel 4.2.1 Post Synthesis X-ray diffractograms in figures 4.4 and 4.5 indicate that both titanium- modified aerogel and titanium-modified RHA are amorphous even after grafting with titanium(IV) chloride and titanium(IV) isopropoxide. This properties also been observed in the diffractograms for titania that precipitated in the silica matrixes (aerogel and RHA). Crystalline form of titania was neither present in anatase, rutile nor brookite. These results indicate that the titanium may either be in amorphous form or dispersed as small crystalline titania with short-range order, which could not be detected by XRD. Intensity Si aerogel Si aerogel precipitated titanium(IV) chloride Si aerogel grafted titanium(IV) chloride Si aerogel grafted titanium(IV) isopropoxide 2 10 20 30 40 50 60 70 80 2θ (degrees) Figure 4.4 The X-ray diffractograms of titanium-modified silica aerogels. 90 46 Intensity RHA RHA precipitated titanium(IV) chloride RHA grafted titanium(IV) chloride RHA grafted titanium(IV) isopropoxide 2 10 20 30 40 50 60 70 80 90 2θ (degrees) Figure 4.5 The X-ray diffractograms of titanium-modified amorphous silica (RHA). The infrared spectra in Figure 4.6 show the vibration characteristic of RHA and titanium-modified amorphous RHA. The FTIR spectra of RHA and titaniummodified RHA by grafting with titanium(IV) chloride and titanium isopropoxide show the presence of Si-O-Si asymmetric stretching at 1099 cm-1 (internal asymmetric Si-O-Si range: 1250-900 cm-1). The FTIR spectra of RHA and titaniummodified RHA precipitation of titanium(IV) chloride (Figure 4.6) also shows the presence of Si-O-Si asymmetric stretching at wave number same as in grafting method. The broad absorption band around 3450 cm-1 is due to hydrogen-bonded OH groups due to the adsorption of the moisture from air [77]. 47 RHA 3445 467 RHA grafted titanium(IV) isopropoxide 1099 3451 1097 462 RHA grafted titanium(IV) chloride %T 3431 467 RHA precipitated titanium(IV) chloride 1097 466 1100 4000 Figure 4.6 3000 2000 cm -1 1500 1000 800 The FTIR spectra of titanium-modified RHA. Additional weak band at 968 cm-1 was due to the presence of terminal silanol in silica aerogel and titanium-modified aerogels by grafting with titanium(IV) chloride and titanium isopropoxide as shown in Figure 4.7. Silica aerogel and titanium-modified aerogel by precipitation (Figure 4.7) also show absorption at 968 cm-1 due to the presence of large amount of silanol groups. This band is absent in RHA and titanium-modified RHA, as a result of high temperature burning process during the combustion of rice husk. Adsorbed water and silanol groups on the aerogels surface contribute the hydroxyl absorption band in the range between 3000 and 3700 cm-1 [77]. Si-O-Si asymmetric and Si-O-Si symmetric stretching is also present in all aerogel samples. 400 48 Si aerogel 968 3463 Si aerogel grafted titanium(IV) isopropoxide 1100 3423 Si aerogel grafted titanium(IV) chloride %T 1100 968 3449 Si aerogel precipitated titanium(IV) chloride 1099 3451 1100 4000 Figure 4.7 3000 2000 cm-1 1500 1000 800 The FTIR spectra of titanium-modified aerogels. UV-Vis spectroscopy has been extensively used to characterize the coordination and the nature of titanium substituted in molecular sieves. The ultraviolet peak position of the Ti4+ ion depends on its coordination and the size of the extra framework TiO2 particles. UV-Vis spectra (Figure 4.8 and Figure 4.9) of RHA and aerogel showed a peak at 242 nm due to minor impurities present in the silica matrix. Post synthesis samples showed the presence of extra framework titanium oxide (280-330 nm) [78]. It is in the range of the charge transfer of titanium in octahedral coordination for the anatase titania. While, no diffraction peak was detected in their X-ray diffractograms. These results support the hypothesis that the titania is present as small crystalline anatase particles. 49 RHA RHA grafted titanium(IV) isopropoxide K-M RHA grafted titanium(IV) chloride RHA precipitated titanium(IV) chloride 190 0 Figure 4.8 250 300 350 400 nm 450 500 550 600 0 The UV-Vis spectra of titanium-modified RHA. Si aerogel Si aerogel grafted titanium(IV) isopropoxide K-M Si aerogel grafted titanium(IV) chloride Si aerogel precipitated titanium chloride 190 0 Figure 4.9 250 300 350 400 450 500 nm The UV-Vis spectra of titanium-modified silica aerogels. 550 600 0 50 The BET surface areas of the sample were determined from nitrogen adsorption analysis as listed in Table 4.2. The surface area of the RHA was found to increase after the titania was deposited on the silica. This mean that the titania has higher surface area than that of RHA and the titania is located in the external surface of the RHA. The use of titanium(IV) isopropoxide as starting material (in modifying RHA) had result in the highest surface area, showing that it produce smaller titania particle. Grafting with titanium(IV) isopropoxide also gave the best external dispersion of titania and allowed the formation of small crystallites at its external surface as observed by other study [78]. Table 4.2: The BET surface area of titanium containing silica. Sample BET Surface Area (m2/g) RHA 30 RHA grafted titanium(IV) isopropoxide 105 RHA grafted titanium(IV) chloride 39 RHA precipitated titanium(IV) chloride 60 Silica aerogel 391 Silica aerogel precipitated titanium(IV) chloride 381 Silica aerogel grafted titanium(IV) isopropoxide 383 Silica aerogel grafted titanium(IV) chloride 397 In the case of aerogel, precipitation and grafting with titanium showed little change to the surface area of the original aerogel. The titania is well dispersed on the silica aerogel. If compared to the titanium-modified RHA, this results shows that the dispersion of the titania in aerogel is better than in RHA. It is due to aerogel has thirteen times larger surface area than RHA, which provided much more space for the deposition of titania and contributed to less agglomeration of the titania in the silica matrix. Moreover, titania was dispersed in the inside of the pores rather than distributed on the outer surface as titania has been trapped in the silica matrix during 51 the gel formation step. Aerogel shows it is so good as a support that it can still maintain its high surface area (391 m2/g) even after treatment in the post synthesis process (381-397 m2/g). 4.2.2 Direct Synthesis The XRD pattern in Figure 4.10 shows titanium-modified silica aerogel is similar to that of silica aerogel (Figure 4.4). No crystalline phase was observed for the titanium compound. FTIR spectrum of titanium-modified silica aerogel (Figure 4.11) shows a band at 972 cm-1, which attributed to the Si-O-Ti bonds stretching. The stretching band for Si-OH group was superimposed onto that 972 cm-1 peak. The most intense band at 1090 cm-1 was assigned as the asymmetric Si-O-Si stretching. The Si-O-Si symmetric stretching was indicated by the absorption at 796 cm-1 [83]. An UV-Vis spectrum of titanium-modified silica aerogel is shown in Figure 4.12. A band presents in the region of 210 to 225 nm (λ ≤ 230 nm) which was corresponded to oxygen to tetrahedral titanium Ti(IV) ligand-to-metal charge transfer, assigned to isolated Ti in tetrahedral framework position [84]. The reason is that siloxane is a strong electron withdrawing ligand which results in charge transfer from oxygen to titanium centre and causes blue-shifted to lower wavenumber. The absence of absorption at about 340 nm indicates the absence of large TiO2 crystalline particles [12]. The BET surface area and total pore volume of the titanium-modified silica aerogel were 336 m2/g and 0.88 ml/g respectively. The particle size was less than 8.11 nm. This titanium-modified silica aerogel sample was a mesoporous material because the pore size was about 10.43 nm. 52 400 Lin (Counts) 300 200 100 0 2 10 20 30 40 50 60 70 80 90 2θ (degrees) Figure 4.10 XRD diffractogram of titanium-modified silica aerogel (Aph6). 100 80 60 %T 799 972 40 20 1090 1500 Figure 4.11 1300 1100 cm -1 900 700 FTIR spectrum of titanium-modified silica aerogel (Aph6). 400 53 222 Aph6 K-M Silica aerogel 190 300 400 500 600 700 800 900 1000 1100 1200 nm Figure 4.12 UV-Vis spectra of titanium-modified silica aerogel (Aph6) and silica aerogel. 4.3 Parameter Study for Synthesis (Direct Synthesis) of Titanium Containing Silica Aerogel 4.3.1 The Effect of Titanium Source The BET surface area of the titanium containing silica aerogel prepared using various titanium sources is listed in Table 4.3. The parameters that have been fixed in this experiment are Si:Ti molar ratio = 33 (2.94 %mol Ti) and H+: NaOH molar ratio = 1. The surface area of the titanium-modified silica aerogels were not significantly altered compared to that of unmodified silica aerogel. In general, the 54 incorporation of the titanium in silica reduced the bulk specific surface area by 16%. However, the surface area of anatase-modified sample was reduced by 28%. From this data, a trend can be observed, i.e., higher surface area would provide larger total pore volume of the titanium–modified aerogel. Consequently, it gives a similar trend in the pore diameter of the titania silica aerogel matrix. Therefore, the source of the titanium had shown significant influence to the titania form especially in particle size and homogeneity. If the titania was not bonded to the silica, the silica could be a separator to avoid agglomeration of the titania particle. Hence, produce fine and well-dispersed titania particles were produced. Table 4.3: Effect of titanium source on the surface characteristics of the Ti-Si aerogels. Titanium Source BET Surface Total Pore 2 Area (m /g) Titanium(IV) ethoxide Pore Diameter Volume (ml/g) (nm) 394 0.89 9.01 Titanium(IV) chloride 352 0.77 8.80 Titanium(III) sulphate 347 0.73 8.46 Titanium(IV) 343 0.69 8.02 Titanium(IV) propoxide 332 0.69 8.26 Titanium(IV) oxide 285 0.55 7.71 395 1.98 20.00 (A250) isopropoxide (anatase) Blank silica aerogel (unmodified) The highest surface area was obtained in titanium(IV) ethoxide prepared aerogel. It was followed by titanium(IV) chloride, titanium(III) sulphate, titanium(IV) isopropoxide, and titanium(IV) oxide. Since the other parameters were same in the preparation, the silica structure may remain the same for all these 55 samples. Thus, it suggests that titanium(IV) ethoxide is the best titanium source to produce high surface area and well dispersed titania in the silica matrixes. This phenomenon is supported by the increase of the pore volume where smaller titania particle may be formed in the silica matrix compared to other titania sources. As the density of the titania is higher than silica, thus the mean particle size (l = 6/(ρ.ABET)) is less than 8.5 nm except for anatase (less than 10 nm). The reduction of the surface area supported the presence of Ti in the aerogel. There was a significant reduction of pore diameter and pore volume upon introduction of titanium in the pore that reduced the total pore volume. This suggests that titania presents as a separated phase from the silica and well dispersed in the silica matrixes. Not only the surface properties of the sample were altered, the physicochemical properties of the sample could be varied by the changes in the synthesis processes. Their physicochemical properties due to the changes in titanium sources were figured out using UV-Vis DRS technique as shown in Figure 4.13. The spectra in Figure 4.13(a) show that titanium(III) gives two types of titanium. Homogeneity of the gel was poor as indicated by the formation of two layers. A very strong absorption centred at 245 nm was observed for the precipitate matter. The upper layer represents the tetrahedral form of titanium, indicated by absorption of lambda max at 219 nm. This phenomenon resulted from phase separation due to unsuitable pH condition. In general, trivalent titanium compound is a preferred titanium oxide source in the synthesis of large-pored crystalline titanium molecular sieve zeolites [85] and small pore titano-silicate [86], where initial pH of 10.5 or higher were employed in their synthesis. 56 31. 0 Upper layer 27.5 Lower layer 25 TiCl4 246nm 25 20 245nm 20 15 K-M 15 219nm K-M 10 10 5 5 0. 2 0.1 190. 0 250 300 350 400 450 500 550 nm 190.0 600. 0 250 300 350 250nm 247nm 400 450 500 550 600.0 (b) (a) 37.0 35 nm Ti-ethoxide Ti propoxide 30 245nm Ti isopropoxide 25 20 K-M 15 10 5 0.1 190.0 300 400 500 600.0 nm (c) Anatase onlyadded in aerogel synthesis) Anatase (before 6 .0 5 .0 Anatase 4 .0 K -M 3 .0 334nm 2 .0 1 .0 0 .0 1 9 0 .0 300 400 500 6 0 0 .0 nm (d) Figure 4.13 The effect of titanium source on the physicochemical characteristics of the Ti-Si aerogels by UV-Vis DRS. (a) Titanium(III) sulphate, (b) Titanium(IV) chloride, (c) Titanium(IV) alkoxide, (d) Titanium(IV) oxide in anatase form. 57 When titanium(IV) chloride was used as starting material, UV-Vis spectra show a band at 246 nm (Figure 4.13 (b)). It is due to the presence of hydrated titanium in the silica matrix as a result of the hydrolysis of Ti-Cl by the water to form Ti(SiO)3OH [87]. In the case of titanium alkoxides used as starting material, the spectrum shows a predominant band centred at 245 nm, 247 nm, and 250 nm assigned to titanium(IV) isopropoxide, titanium(IV) propoxide and titanium(IV) ethoxide respectively (Figure 4.13 (c)). The absorption centred at 240-260 nm is likely due to the presence of [Ti(SiO)3O]- species attributed to the higher electron density of the negatively charged oxygen than the siloxane bond [87]. This implies that more OH ligands were bonded to the titanium centre and caused blue shifting to higher wavenumber. The absorption edges (at about 330 nm) are lower compared to other titanium source. Anatase is a crystalline titanium oxide that presents in octahedral coordination, which each titanium atom is coordinated to six almost equidistant oxygen atoms. Anatase powder has been recorded using UV-Vis spectroscopy. The spectrum of anatase its alone (Figure 4.13(d)) shows a λmax at 335 nm and a shoulder at 238 nm. Significant absorption above 300 nm is a good indication of the presence of large TiO2 particles. It has been reported that bulk anatase would show an absorption edge above 360 nm. Meanwhile, the absorption edge below 360 nm is assigned to nanoparticles [13]. When the titanium oxide (anatase form) was dispersed in the gelling solution, fast gelling process (less than 15 seconds) at pH 7 cause these particles to be trapped and well-dispersed in the silica matrixes. The spectrum for this sample shows a band centred at 334 nm with the absorption edge at 400 nm similar to that of starting material, i.e. anatase powder. This confirms the presence of anatase form of titanium oxide. While, the shoulder at 238 nm has disappeared in the spectrum after the anatase powder was used in the aerogel synthesis. The reason is that the amorphous titanium oxide that present in the starting material have been dissolved and transformed to new anatase TiO2 that was induced by anatase crystal seed. It was 58 made possible by the ability of amorphous TiO2 to dissolve in sulphuric acid, similar in the extraction of the titania from the titanium ore. In this case, silica aerogel performed as a support for the anatase powder, improved the quality of the anatase powder and immobilized foreign particle. 4.3.2 The Effect of Si:Ti Molar Ratio The influence of the titania content on the dispersion of the species in silica matrixes was examined. A series of samples has been prepared with H+: NaOH molar ratio is 1.25 and titanium isopropoxide as titanium source. Nitrogen adsorption has been carried out and the data are listed in Table 4.4. Table 4.4: Effect of concentration of titanium on the surface characteristics of the Ti-Si aerogels. Titanium isopropoxide as titanium source, H+: NaOH molar ratio = 1.25. Si:Ti BET Surface Area Total Pore Volume Pore Diameter Molar Ratio (m2/g) (ml/g) (nm) 1 (A350) 469 0.90 7.29 6 743 1.66 8.94 33 947 1.75 7.39 49 917 1.72 7.50 Data in Table 4.4 shows that surface area of the bulk aerogel is significantly reduced due to higher titanium loading. For the sample with Si:Ti = 1, the specific surface area is the lowest (469 m2/g) and total pore volume (0.90 ml/g). This is due to the formation of the extra framework titanium(IV) oxide in the silica matrix. The titania is possibly located inside the pore of the aerogel as proven by the decrease in the pore volume. The surface area and total pore volume are all increase or decrease with the amount of titanium loading. Pore diameter for the Si:Ti = 6 shows such an anomaly result that differ from the trend. It is not only give larger pore diameter than 59 those with lower titanium loadings but also sample with higher titanium loading. Further study is necessary to clarify its reason, whether titania is combined together with the silica wall building unit or is it caused by any other reasons. X-ray diffractograms in Figure 4.14 of samples Si:Ti = 1, Si:Ti = 6 and anatase modified-silica aerogel show diffraction peaks at the same position. All peaks correspond to crystalline titanium(IV) oxide, synthetic anatase (PDF pattern number: 04-0477), which matches the sample anatase; originally used as the starting material in this study. This confirms that the titania in the silica matrix are in crystalline form for samples with Si:Ti less than 6. This further supports the fact that titania is located in the pore of the silica aerogel matrix with a reduction in pore volume. The intensities of the peaks in Si:Ti = 6 diffractogram are much lower than those in sample with Si:Ti = 1, implies less crystalline and lower amount of anatase in the sample. No peak was observed for lower loading of titanium. Amorphous titania or possibly very small size of crystalline titania may be formed in those with low Ti-loading samples. 60 Intensity Anatase Si:Ti = 1 Si:Ti = 6 Si:Ti = 33 2 10 20 30 40 50 60 70 80 2θ (degrees) Figure 4.14 X-ray diffractograms of aerogel samples with various Si:Ti molar ratios compared with anatase TiO2. 90 61 UV-Vis DRS spectra in Figure 4.15 shows the aerogel samples that were synthesized with various Si:Ti molar ratios. The UV-Vis DRS of Si:Ti = 1 has further confirmed the formation of anatase with high titania loading. However, λmax of Si:Ti = 1 is shown at 246 nm, suggesting the presence of [Ti(SiO)3O]- species. All samples in this series have the same absorption edge at ca. 370 nm. This is an indication of the presence of anatase in the system. The spectra for Si:Ti = 33 and Si:Ti = 49 are similar to that of Si:Ti = 6, suggesting that similar phase is present. These samples show a band centred at 251 nm and suggest the presence of [Ti(SiO)3O]- species. Some literature suggested that the absorption bands centred in the range of 240 to 280 nm were due to the charge transfer between framework oxygen to octahedral coordinated Ti(IV) centre, and highly dispersed TiO2 with particle size less than 5 nm (octahedral titanium species) [88]. 251nm Si:Ti = 33 32.4 30 Si:Ti = 6 25 20 K-M Si:Ti = 45 15 246nm 10 5 Si:Ti = 1 305nm Anatase 0.0 190.0 250 300 350 400 450 500 550 600.0 nm Figure 4.15 UV-Vis spectra of samples synthesized with various Si:Ti molar ratios. Virtually, assignment for the bands that centred below 280 nm is due to highly dispersed titania (either in nanosize segregated TiO2 particle or titania in silica framework). The smaller the wavelength of the absorption may indicate smaller particle size, lower Ti coordination number (λmax < 240 nm for tetrahedral Ti(IV)) and better dispersion of titania has formed in the silica matrix. The siloxane ligand 62 has lower electron density than OH ligand (or other ligands that have high electron density), thus Ti(IV) bonded to siloxane bond will adsorb at lower wavenumber. Titanium(IV) is possibly bonded to silica with coordination number more than 4 (240-260 nm). This may resulted from the interaction between silica and titania to form binary particle. In addition, there is also possible attribute to the presence of very small titania particles that cannot be detected by the XRD, specifically those with low titanium loading. This provides an alternative path for the synthesis of nanosize particle anatase crystals in the silica matrix. 4.3.3 The Effect of Loading of Sulphuric Acid From the nitrogen adsorption studies (Table 4.5), the loading of the acid has significantly modified the surface physical properties of the titanium containing silica aerogel. It is a combination of three effects: the effect of pH to silica, the effect of pH to titania and the effect of interaction between titania and silica. Soluble silica and titania were present in different form at different pH (or different concentration of acid) as stated in Chapter 2. At high pH values, the washing process will flush out the silica because amorphous silica can easily dissolve at pH above 12. Practically, gel may be able to form at pH below pH 12. However, washing process (for flushing out the salt formed) dissolves the silica because of the increase in solubility of silica above neutral condition. Thus, synthesis of the titanium containing silica aerogel above pH 7 is not favourable. The aerogel that was synthesized under the neutral condition gives the lowest surface area. This is due to the effect of gelling and aging at high pH, which was affected by the solubility of the silica. At this pH, most of the silicates are present as polynuclear and anionic species. At high pH values, particles are charged by ionisation, therefore aggregation was reduced. In addition, particle growth by monomer deposition and faster Ostwald ripening process. This factor causes bigger 63 primary particle to form and convex surface dissolves quickly during aging process. Hence, aerogel with the lowest surface area (263 m2/g) in this series is produced. Table 4.5: Effect of concentration of acid on the surface characteristics of the Ti-Si aerogels. Titanium isopropoxide as titanium source, Si:Ti molar ratio = 33. H+: NaOH BET surface area Total pore Pore diameter molar Ratio (m2/g) Volume (g/cm3) (nm) 1.00 (A215) 263 0.51 7.70 1.25 791 1.44 7.30 1.50 1003 2.80 11.16 1.75 786 2.09 10.65 2.50 864 1.76 10.34 At lower pH value i.e. with excess sulphuric acid loading in the reaction, the system is in the pH range that the particle growth is minimum. Most of the sodium silicate exists in form of mononuclear silicic acid at pH lower than 7. It is recommended that the size of the particle is limited to about 2 nm in pH below 7 due to the solubility of silica [75]. Subsequently, the aggregation of small particle during gelation produces high surface area gel. The highest surface area of the pure silica aerogel that can be synthesized in this research was 882 m2/g as in Section 4.1. However, the highest surface area in titanium containing silica aerogel is 1003 m2/g, which greater than silica aerogel. This may be caused by the effect of titania, which acts as a space separator between silica particles in the gel matrixes. This phenomenon has been observed in other materials such as Ti-MCM-41 [89]. Therefore, the addition of titania in silica aerogel created opportunity to synthesize higher surface area aerogels. The isoelectric point (iep, electrical mobility of silica particles is zero) and point of zero charge (surface charge is zero) of silica and titania may play a significant rule to the gel obtained. The iep of the silica and titania is at pH around 64 2.0 ± 0.5 and at pH 5-6 respectively [36, 90, 91]. Therefore, silica is negatively charged above pH 2 [92]. It is assumed that the catalyst below pH 2 is the H+ ion, which forms an active cationic complex. Also, above pH 2 the OH- ion is the catalyst in that active anionic silica is generated. Vysotskii and Strazhesko [37] have pointed out that in the presence of acid such as sulphuric, the iep is not only the point of minimum rate of gelling but also the point where gels of maximum strength and maximum specific surface area were obtained. This is because the rate of aggregation is minimum at the iep and the rate of growth of the ultimate particles from monomer at minimum, so that the ultimate particles are smallest as they form the gel. Thus, excess acid in the gel synthesis normally results in higher surface area aerogels. They are above 700 m2/g in this experiment. However, high loading of sulphuric acid produced lower surface area. This could be due to excessive heat formed during the reaction that induced higher solubility of silica, resulting in faster rate of coarsening process in the primary aging stage. Moreover, the heat could cause evaporation of some portion of water from the gel and fracture of the gel skeleton. According to the UV-Vis spectra for different acid loading (Figure 4.16), the absorption edge are above 360 nm, indicating the presence of anatase in the sample except for the aerogel synthesized with H+: NaOH molar ratio is 1. Interestingly, acid loading had modified the chemical properties of the titania. Increase in acid loading resulted in blue shifting to higher wavenumber. The peak centred at 227 nm indicates the presence of tetrahedral titanium in the silica framework. The gel that formed at H+: NaOH molar ratio 1 had shown pH 6 in the experiment. Since slightly excess acid has been used. In this case, it is believed that ion-ion interaction between positively charged titania with negatively charged silica in the reaction mixture has occurred due to the surface charge at pH 4. The gel was later aged at pH 6 for 48 hours before washing process that provided low aggregation environment for the titania. Thus, there was no absorption at 250 nm and no absorption edge at above 360 nm. 65 25.8 227nm 27.4 257nm 25 20 20 15 15 K-M K-M 10 10 340nm 375nm 5 5 0.2 190.0 0.3 300 400 nm 500 600.0 190.0 300 (a) 26.4 25 400 nm 500 600.0 500 600.0 (b) 18.8 258nm 270nm 16 20 14 12 15 10 K-M K-M 8 10 6 380nm 280nm 4 5 2 0.2 190.0 0.1 300 400 nm (c) Figure 4.16 500 600.0 190.0 300 400 nm (d) UV-Vis spectra of samples synthesized with various H+: NaOH molar ratios. (a) H+: NaOH molar ratio = 1.0, (b) H+: NaOH molar ratio = 1.25, (c) H+: NaOH molar ratio = 1.5, (d) H+: NaOH molar ratio = 1.75. The samples which were synthesized at H+: NaOH molar ratio =1.25 and 1.50 showed same absorption at 258 nm. They have the same type of titania, as in the samples that studied in Section 4.4.2. Higher acid loading of acid showed absorption 66 centred at 270 nm, indicates size of the titania particle is larger particle than those showed absorption at 250 nm. Therefore, this study suggests the most important parameter for engineering of the surface area of the aerogel and chemical properties of the titania is the acid loading in the gelling process. 4.4 Catalytic Properties: Oxidation of Alkene The synthesized TiO2-SiO2 aerogels were tested in the oxidation reaction of cyclohexene using hydrogen peroxide as oxidant. Various parameters have been studied; including catalyst, solvent, loading of hydrogen peroxide, reaction temperature and different alkene. 4.4.1 The Influence of the Type of Titanium. Various materials have been tested for their catalytic properties in this study. It is known that materials with the same physicochemical properties should have similar chemical properties. Experimental results in previous section have shown a trend that preparation at different concerntration of titanium is able to produce sample with similar physicochemical properties. Thus, the samples used in this testing are classified into four groups based on the type of titania due to the UV-Vis absorption wavelength as stated in Table 4.6. The catalytic test data is shown in Table 4.7. Silica aerogel without titanium shows very low conversion (7%) and low 1,2-cyclohexanediol selectivity (31%). Blank sample also have very low reaction activity with it low conversion of cyclohexene [93]. 67 A350, is a sample synthesized with Si:Ti = 1, shows the presence of anatase in its X-ray diffractogram. TiO2 anatase powder was used as a standard. Sample A350 with the highest titanium loading in the study shows the highest activity among the aerogel samples with 26% conversion. However, A350 has the lowest selectivity. The high conversion is enhanced by the presence of anatase in the sample. Anatase powder above shows the highest activity in this series of samples but relatively has lower selectivity in the reaction. It shows the catalytic properties of A350 is due to the presence of crystalline TiO2 (anatase) in the aerogel sample [94]. Table 4.6: Sample used in the catalytic testing and their characteristics. Characteristics Sample UV-Vis Absorption TS-1 215 nm (2 mol% Ti as supplied) A215 (as in Table 4.5) (as in Table 4.3) (as in Table 4.4) (as in Table 4.3) Highly dispersed TiO2 particles in silica matrix 350 nm silica aerogel Isolated tetrahedral titanium in silica framework 250 nm A350 Isolated tetrahedral titanium in silica framework 227 nm A250 Type of Titania Crytalline TiO2 (anatase) in silica matrix no absorption Silica matrix (Blank silica aerogel) Sample A250 shows the highest selectivity towards the formation of 2cyclohexen-1-one and shows the lowest epoxide content in the reaction mixture. The formation of 2-cyclohexen-1-one is a result of allylic oxidation [95, 96, 97]. Lei revealed that base and acid additives are able to afford the formation of 2-cyclohexen-1-one and 2-cyclohexen-1-ol respectively [98]. Thus, the higher selectivity of 2-cyclohexen-1-one in sample A250 was believed to be caused by trace amount of NaOH that remained in the aerogel which was synthesized with H+: NaOH molar ratio =1. A250 used in other studies also indicated similar trend, where 68 the yield of 2-cyclohexen-1-one was always higher than 2-cyclohexen-1-ol. In fact, it was the oxidation of the 2-cyclohexen-1-ol by hydrogen peroxide that produced additional 2-cyclohexen-1-one [99]. The results suggest that A250 can potentially undergo allylic oxidation and can be further studied by modifying its basicity. TS-1 is known as the most prominent epoxidation catalyst [10]. It has an absorption at 215 nm (210-230 nm), which indicates the presence of tetrahedral Ti sites; isolated by SiO “ligands” and acts as Lewis acidic centres to activate the peroxide [100, 101]. Results in Table 4.7 shows TS-1 has the highest epoxide selectivity among the other catalysts. Although sample A215 that has the same UVVis absorption and shows the highest epoxide selectivity among the aerogel samples, it is lower than TS-1. It proves that epoxidation is catalysed by the Ti-O-Si bonding (adsorb at 210-230 nm). At the same time, A215 shows the most prominent glycol selectivity. This may be due to the presence of alumina in aerogel sample, which favours the formation of glycol. The conversion of the TS-1 is lower compared to A215. In this case, the small pore size in TS-1 with the dimensional channel system of 5.3 x 6.1 Å has caused diffusion limitation to the substrate and result in lower conversion [102]. In contrast, aerogel is mesoporous and has relatively larger pore volume. Both TS-1 and A215 have selectively converted cyclohexene to epoxide and diol as the main mixture (total selectivity is about 80%) with H2O2, thus confirming the importance of the titanium structure in the silica framework. Table 4.7: Catalytic activity of the titanium containing silica aerogel, TS-1 and anatase. Reaction conditions: 10 ml cyclohexene, 10 ml acetone, 8.35 ml H2O2 35%, 156.3 mg catalyst, and 1 ml toluene (internal standard) at 70 ˚C. TOF Catalyst (mM cyclohexene /g catalyst/ hour) Blank Selectivity (%) Conversion (%) Cyclohexene 2-cyclohexen-1-ol 2-cyclohexen-1-one oxide 1,2- others cyclohexanediol - 7 9 9 5 28 50 117 7 9 10 5 31 44 200 15 18 7 4 61 10 A215 260 20 12 6 6 70 6 Anatase 502 38 8 7 3 56 26 A350 342 26 9 9 7 46 29 A250 261 20 4 8 14 58 17 Silica aerogel TS-1 (2 mol% Ti) 69 70 4.4.2 The Influence of Solvent It is known that solvent have great effect on the activity and selectivity in the liquid phase oxidations on the titanium silicates [103, 104, 105, 106]. The oxidation of cyclohexene is conducted on the sample A250 using several solvents having different polarity. The polarity of the solvent used in this study is in the order of toluene< ethyl acetate < acetone [107]. The influence of solvent is presented in Table 4.8. It should be noted that the glycol (1,2-cychohexanediol) selectivity is the highest when ethyl acetate is present in the reaction. It is observed that reaction without the use of solvent has a two-fold turn over frequency higher than with toluene. It is due to dilution of cyclohexene by the organic phase of toluene, reducing the contact with catalyst that is present mostly in the aqueous phase. In contrast to high polarity solvent, the mass transfer problems associated with the presence of different liquid phase are minimized and are able to form a single phase with the organic substrate and hydrogen peroxide. Therefore, it is as espected that acetone will give the highest conversion among other solvents. The activities of the sample A250 are suited with the polarity of the solvent used in the reaction as proposed by other workers [104, 108]. Whereby the activity of TS-1 in the oxidation of alkenes is enhanced by the use of polar solvents. The same phenomena was also reported in the oxidation study of the Ti-Beta zeolite synthesized by dry gel conversion [103]. However, it is contradict to those observed by Corma that the activity of oxidation of alkene over hydrothermal synthesized Tibeta was higher in aprotic solvent acetonitrile than in protic solvent in the order of MeCN> MeCOMe > MeCOEt [106]. They ascribed it is related to the hydrophilicity of the hydrothermally synthesized Ti-beta which differs from TS-1. In this case, TiSi aerogel synthesized under high temperature supercritical extraction is relatively hydrophobic as the case of TS-1. Table 4.8: Catalytic activity of the aerogel A250 as a function of solvent. Reaction conditions: 10 ml cyclohexene, 10 ml solvent, 8.35 ml H2O2 35%, 156.3 mg catalyst, and 1 ml toluene (internal standard) at 70 ˚C. TOF Solvent (mM cyclohexene /g catalyst/ hour) Without Selectivity (%) Conversion (%) Cyclohexene 2-cyclohexen-1-ol 2-cyclohexen-1-one oxide 1,2- others cyclohexanediol 184 14 0 8 16 76 0 Acetone 261 20 4 8 14 58 17 Toluene 99 7 0 0 23 77 0 Ethyl acetate 250 19 0 7 9 84 0 solvent 71 72 4.4.3 The Influence of Hydrogen Peroxide Loading The alkene to oxidant dependance of cyclohexene oxidation was investigated and the reaction performance are given in the Table 4.9. There was no obvious influence in the selectivity of allylic oxidation by changing this parameter. In equilibrium amount of oxidant, the conversion was 20%. When the amount of oxidant was doubled, the catalytic activity was increased more than 150%. At the same time, the side products increased 2-fold to 30% selectivity. These significant changes resulted from the excess oxidant that favoured additional oxidation to the glycol and allylic oxidation products. The main side products would be accounted for the formation of 2-hydroxycyclohexanone and adipic acid. This phenomenon has also been observed in the oxidation of cyclooctene, where conversion and side products increased as the molar ratio of alkene to oxidant decreased [93]. In contrast, the conversion of the alkene was reduced when the amount of oxidant was decreased. However, the selectivity to glycol was apparently increased up to 77%. The selectivity and activity of catalyst A250 did not differ much when the molar ratio of alkene to oxidant was more than 5. At the same time, no quantitative amount of side product was formed when hydrogen is used as the limiting reagent. This implies that the molar ratio of alkene to hydrogen peroxide 10:1 is a suitable condition to produce glycol with less side product in the reaction mixture. Aerogel sample show it is so good as catalyst in catalyzing cyclohexene to 1,2cyclohexenediol. Table 4.9: Catalytic activity of the aerogel A250 as a function of alkene: H2O2 molar ratio. Reaction conditions: 10 ml cyclohexene, 10 ml acetone, respective amount of H2O2 35%, 156.3 mg catalyst, and 1 ml toluene (internal standard) at 70 ˚C. TOF Selectivity (%) Alkene/H2O2 (mM Conversion molar ratio cyclohexene /g (%) Cyclohexene 2-cyclohexen-1-ol 2-cyclohexen-1-one oxide 1,2- others cyclohexanediol catalyst/ hour) 0.5 658 49 4 6 7 54 30 1.0 261 20 4 8 14 58 17 5.0 156 12 5 9 10 76 0 10.0 165 12 5 9 9 77 0 73 74 4.4.4 The Influence of Reaction Temperature By using the same catalyst (A250), the influence of the reaction temperature was tested at 30 ˚C, 50 ˚C, 70 ˚C, and 80 ˚C. Table 4.10 shows that the catalytic activity decreased with an increase of reaction temperature. This could resulted from the higher temperature favour the decomposition of hydrogen peroxide to form water and oxygen gas. At 80 ˚C, the reaction activity was the lowest. Even though, higher temperatures (70 ˚C, and 80 ˚C) gave higher yields of expoxide and glycol; products of nonradical reaction. In contrast, radical based oxidation reaction (allylic oxidaton) was favoured at lower reaction temperature, which produced larger amount of 2-cyclohexen-1-ol and 2-cyclohexen-1-one. This implies that a large number of peroxy radicals was formed at lower temperatures (30 ˚C, and 50 ˚C). Therefore, there is a strong indication that the formation of large amount of other side products (up to 30%) was induced by these reactive radicals. On the other hand, reaction carried out at 70 ˚C was prefered for higher amount of glycol yield, with maintained activity. Table 4.10: Catalytic activity of the aerogel A250 as a function of reaction temperature. Reaction conditions: 10 ml cyclohexene, 10 ml acetone, 8.35 ml H2O2 35%, 156.3 mg catalyst, and 1 ml toluene (internal standard). Temperature (˚C) TOF (mM cyclohexene /g catalyst/ hour) Selectivity (%) Conversion (%) Cyclohexene 2-cyclohexen-1-ol 2-cyclohexen-1-one oxide 1,2- others cyclohexanediol 30 276 21 5 15 17 33 30 50 224 17 7 16 19 42 16 70 261 20 4 8 14 58 17 80 41 3 13 9 11 67 0 75 76 4.4.5 The Influence of Alkene The catalytic activity and selectivity of the A250 in the oxidation of linear alkene i.e. 1-octene, was tested and the results are summarized in Table 4.11. The activity of the linear olefin showed a large drop in conversion compared to cyclic olefin. This complies to the previous study that the conversions are increased in the order of 1-decane < 1-octene < 1-hexene < cyclohexene [93]. This result clearly indicates that, besides the intrinsic reactivity of the double bond, olefin size and the accessibility to the active sites of the catalyst are the major limiting factors of the catalytic reaction. With this sample, the selectivity of the epoxide is as high as 64% in the oxidation of 1-octene. Glycol was the only side product present in the 1-octene oxidation reaction which indicates 1-octene oxide is less reactive to the hydrolysis compared to cyclohexene oxide. The results suggest that catalytic activity and selectivity of the catalyst (aerogel) are substrate dependent. Table 4.11: Catalytic activity of the aerogel A250 as a function of amount of hydrogen peroxide. Reaction condition: 10 ml alkene, 10 ml acetone, 8.35 ml H2O2 35%, 156.3 mg catalyst, and 1 ml toluene (internal standard) at 80 ˚C. TOF Alkene (mM cyclohexene /g catalyst/ hour) Selectivity (%) Conversion (%) Epoxide Diol Others Cyclohexene 41 3 13 67 20 1-octene 3 0.35 64 36 0 77 4.5 The Mechanism of the Reaction There are a few reactions that can occur in the oxidation of cyclohexene. Three reaction paths have been descried namely hydrolysis, epoxidation and allylic oxidation [91]. Epoxidation reaction is resulted from the interaction between oxometallic species and alkene. Other workers found cyclohexene converted into epoxide and diol as predominant mixture over titanium containing zeolites with aqueous H2O2 [102, 103]. The mechanism of the allylic oxidation involves radicals. These radicals come from decomposition of peroxo titanium species formed by the reaction of hydrogen peroxide with the titanium sites of catalyst as follows: Ti O H Ti O O H Ti O + + H2O2 H2O + H2O (4.2) Ti O + OH (4.3) Ti OH + OH (4.4) Ti O O H These peroxy radical may react with the alkene in several ways [95, 96, 97]. Allylic oxidation usually produces 2-cyclohexen-1-ol and 2-cyclohexen-1-one. In this study, it is noted that the products of the reaction mixture are combination of the yield from path 2 and path 3 as shown in Scheme 1. The experimental data as shown in the line chart below in Figure 4.17 shows that only epoxide is formed at the beginning of the reaction. The production and consumption of the epoxide has reached equilibrium at about 45 mM in the reaction mixture. It is followed by the formation of glycol which resulted from the epoxiran ring opening reaction to the epoxide according to Scheme 1. 78 OH O 1 OH 2 O OH 3 O OH The reactions in the oxidation of cyclohexene [95, 96, 97]. Scheme 1 This reaction may result from acid catalysed hydrolysis, basic hydrolysis, or direct hydrolysis process. The mechanism of acid catalysed hydrolysis is a hydronium (H3O+) ion catalysed process. There is the first addition of a hydrogen ion, and then an oxinium complex is formed. It is followed by the formation of carbocation. The attack from the water to the carbocation results in the formation of glycol and reproduces the hydroxonium ion. C O + C H+ O C C H+ OH C C + OH C C OH + H+ (4.5) H OH In comparison to the basic hydrolysis, the mechanism would be differ as follows: H OH R1 O C CH2 R2 - - + OH O C CH2 R2 OH R1 OH C CH2 + OHR2 OH R1 (4.6) 79 Meanwhile, the direct hydrolysis process is noted as below: C O + C OH C C OH H2O (4.7) The quantity of the glycol increased continuously during the first 40 hours. While, the glycol formation rate was slow down after the first 4 hours. It is resulted from the consumption of cyclohexene in allylic oxidation that forming 2-cyclohexen1-ol and later followed by 2-cyclohexen-1-one. These two allylic oxidation compounds were also detected in the same reaction as byproducts by other researchers [94]. Time Course Study for Oxidation of Cyclohexene 250 225 Concentration (mM) 200 175 Cyclohexene oxide 2-cyclohexene-1-ol 2-cyclohexen-1-one 1,2-cyclohanediol Others 150 125 100 75 50 25 0 -25 0 10 20 30 40 50 Time (hour) Figure 4.17 Time course study for the reaction mixture 10 ml cyclohexene, 10 ml acetone, 8.35 ml H2O2 (35%), 156.3 mg TS-1, and 1 ml toluene (internal standard) at 80 ˚C. Subsequently, when the reaction was left for a longer period, other side products with higher molecular weight were detected. At the same time, a significant 80 decrease of the amount of 1,2-cyclohexanediol was observed in the product mixture at the end of the reaction. This suggests that side products were formed only from further reaction of the glycol. It was found that glycol could be further oxidized and the six membered ring was able to be cleaved in the way to the formation of adipic acid, which can be explained by over-oxidation under the reaction condition [109]. Futhermore, minor portion of the side product may result from further oxidation of the allylic products, where some researchers have used 2-cyclohexen-1ol and 2-cyclohexen-1-one as reactants in the catalytic oxidation reaction for the formation of its epoxide and other derivatives, which directly proved the ability of these compounds for further reaction [94]. The mechanism of the reaction is proposed in Scheme 2. Path 1 is the formation of epoxide. In contrast, Path 2 indicates an allylic oxidation process. Due to the amount of the product formed, Path 1 is the predominant mechanism in this study. 81 Path 2 H2O 1/2 H2O2 [Ti] + OH Cyclohexene [Ti] H2O2 O2 Path 1 OO O Cyclohexene Oxide OO [Ti] H2O O2 OH OH 1,2-Cyclohexanediol [Ti] O OH H2O2 + O 2-Cyclohexen-1-ol 2-Cyclohexen-1-one OH 2-hydroxycyclohexanone [Ti] H2O2 O OH O Adipic Acid Scheme 2 Reaction mechanism of the oxidation of cyclohexene using hydrogen peroxide as oxidant [102, 103, 109, 110]. CHAPTER 5 CONCLUSIONS AND SUGGESTIONS 5.1 Conclusions Titanium containing silica aerogel has been synthesized through high temperature supercritical process. Sol-gel direct synthesis was demonstrated as the potential technique of preparing high surface area titanium containing silica aerogel. The surface area of the bulk aerogel system was increased when titanium was introduced to the silica aerogel matrixes. The study indicated that acid loading in the synthesis has the most important influence in directing the type of titanium formed in the silica aerogel matrixes. Homolytic substitution of the titanium in the silica framework was successfully carried out proven by the UV absorption at 215 nm. Well-dispersed fine particles of titanium oxide in the silica matrixes has also been obtained, which showed UV absorption in the range of 240-290 nm. Crystalline TiO2 (anatase) was synthesized in-situ during the sol-gel process when a high loading of titanium (Si:Ti = 6, 1) was implemented. 83 The catalytic tests showed that titanium containing silica aerogel was active to the oxidation of cyclohexene using hydrogen peroxide as oxidant. The products from both radical (allylic oxidation) and non-radical oxidation (epoxidation) were present. Meanwhile, the product mostly came from the non-allylic oxidation that produces epoxide and 1,2-cyclohexanediol. The selectivity of 1,2-cyclohexanediol was relatively high compared to other products and it always presented as the major product. The aerogel containing highest amount of titanium(IV) oxide (Sample A350) gave the highest conversion of 26%. However, the selectivity of the catalysts was highest when sample A215 was applied. The mechanism of reaction is proposed as in Scheme 2 of Section 4.5. The catalytic oxidation reaction was greatly influenced by the solvent, temperature, amount of oxidant, and type of alkene. The parameters were optimised for selectivity to the glycol. The study established that favoured conditions for the reaction were using oxidant as a limiting reagent, ethyl acetate as the solvent, and the reaction conducted at 70 ˚C. Combination of high surface area, greater strength of the mixed oxide as well as tuneable physical and physicochemical properties of titania in the silica aerogel matrix, results in titania-silica aerogel potential as heterogeneous catalysts. Another advantage of aerogel is able to be molded to desired shape and size, which in most cases are not possible in other systems. 84 5.2 Suggestions Some suggestions for future work: 1. Incorporation of third oxide to the titania silica aerogel either during the sol-gel synthesis or via post synthesis. The properties of the physical and chemical properties of the ternary oxide system formed may be varied. 2. Application of titania silica aerogel in photocatalytic reaction. The advantage of in-situ formation of the crystalline anatase during aerogel synthesis, in addtion to immobilization of anatase in the silica matrix may overcome the anatase powder lost during the application. REFERENCES 1. Donald, E., Atkins, K. L., Yen, C.W., Vellinga, J. M., Price S., and Benton, C. C. Stardust: Finessing Expensive Cometary Sample Returns. Acta Astronautica. 1996. 39(1-4). 51-60. 2. Domínguez, G., Westphal, A. J., Jones, S. M. and Phillips, M. L. F. Energy Loss and Impact Cratering in Aerogels: Theory and Experiment. Icarus. 2004. 172(2). 613-624. 3. Hu, S. W., Willey, R. J. and Notari, B. An Investigation on the Catalytic Properties of Titania–Silica. Journal of Catalysis. 2003. 220: 240–248. 4. Yoshida, H., Murata, C. and Hattori, T. Screening Study of SilicaSupported Catalysts for Photoepoxidation of Propene by Molecular Oxygen. Journal of Catalysis. 2000. 194: 364-372. 5. Tauster, S. J. and. Fung, S. C. Strong Metal-Support Interactions: Occurrence Among the Binary Oxides of Groups IIA–VB. Journal of Catalysis. 1978. 55(1): 29–35. 6. Baker, R. T. K., Prestridge, E. B. and Garten, R. L. Electron Microscopy of Supported Metal Particles: I. Behavior of Pt on Titanium Oxide, Aluminum Oxide, Silicon Oxide, and Carbon. Journal of Catalysis. 1979. 56(3): 390406. 7. Jones, H. B. and Smith, R. Hydrogen Recovery from Gaseous Product of Fluidized Catalytic Cracking. U. S. Patent. 4,206,038. 1980. 8. Hanprasopwattana, A., Rieker, T., Sault, A. G. and Datye, A. K. Morphology of Titania Coatings on Silica Gel. Catalysis Letters. 1997. 45: 165-175. 9. Bond, G. C. and Burch, R. Catalysis (Specialist Periodical Report). Chemical Society. 1983. 6: 27-90. 86 10. Taramasso, M., Perego, G., Notari, B. Preparation of Porous Crystalline Synthetic Material Comprised of Silicon and Titanium Oxides. U.S. Patent. 4,410,501. 1983. 11. Mantegazza, M. A., Petrini, G., Spano, G., Bagatin, R. and Rivetti, F. Selective Oxidations with Hydrogen Peroxide and Titanium Silicalite Catalyst. Journal of Molecular Catalysis A: Chemical. 1999. 146: 223–228. 12. Reddy, J. S. and Sayari A. Oxidation of Propylamine over Titanium Silicate Molecular Sieves. Applied Catalysis A: General. 1995. 128: 231-242. 13. On, D. T., Nguyen, S. V., Hulea, V., Dumitriu, E. and Kaliaguine, S. Monoand Bifunctional MFI, BEA and MCM-41 Titanium-Molecular Sieves. Part 1. Synthesis and Characterization. Microporous and Mesoporous Materials. 2003. 57(2): 169-180. 14. Serrano, D. P., Grieken, R. V., Melero, J. A. and García, A. Liquid Phase Rearrangement of Long Straight-Chain Epoxides over Amorphous, Mesostructured and Zeolitic Catalysts. Applied Catalysis A: General. 2004. 269(1-2): 137-146. 15. Berlini, C., Ferraris, G., Guidotti, M., Moretti, G., Psaro R., and Ravasio, N. A Comparison Between [Ti]-MCM-41 and Amorphous Mesoporous Silica– Titania as Catalysts for the Epoxidation of Bulky Unsaturated Alcohols. Microporous and Mesoporous Materials. 2001. 44-45: 595-602. 16. Dusi, M., Mallat, T. and Baiker, A. Role of Amine Modifiers in the Epoxidation of Allylic Alcohols with a TiO2–SiO2 Aerogel. Journal of Catalysis. 1999. 187: 191–201. 17. Chan, K. Y and Hamdan, H. Aerogel from Rice Husk Ash: Synthesis, Characterization and Surface Modification. Master Thesis. Universiti Teknologi Malaysia; 2000. 18. Anpo, M. and Takeuchi M. The Design and Development of Highly Reactive Titanium Oxide Photocatalysts Operating under Visible Light Irradiation. Journal of Catalysis. 2003. 216(1-2): 505-516. 19. Brinker, C. J. and Scherer, G. W. Applications: Sol-Gel Science, The Physics and Chemistry of Sol-Gel Processing. San Diego: Academic Press. 839-880; 1990. 87 20. Everett, D. H. International Union of Pure and Applied Chemistry: Symbols and Terminology for Physicochemical Quantities and Units. London: Butterworths. 1971. 21. Verwey, E. J. W. and Overbeek, J. T. Theory of Stability of Lyophobic Colloids. New York: Elsevier. 1949. 22. Bergna, H. E. The Colloid Chemistry of Silica. Washington: American Chemical Society. 1994. 23. Gould, E. S. Inorganic And Structures. New York: Hendry Holt & Company. 1957. 24. Fyfe, C. A., Gobbi, G. C., Kennedy, G. J., Graham, J. D., Ozubko, R. S., Murphy, W. J., Bothner-By, A., Dadok, J., Chesnick, A. S. Detailed Interpretation of the 29Si and 27Al High-Field MAS N. M. R. Spectra of Zeolites Offretite and Omega. Zeolites. 1985. 5 (3): 179-183. 25. Kiselev, A. V. and Lygin, V. I. Infrared Spectra of Surface Compounds. New York: Wiley. 1975. 26. Vail, J. G. Soluble Silicates (ACS Monograph Series). Vols. 1 and 2. New York: Reinhold. 1952. 27. Anon. Soluble Silicates and Their Applications. UK: Crossfield Publication. 1997. 28. Freundlich, H. Colloid and Capillary Chemistry. London: Methuen. 1926. 29. Iler, R. K. The Chemistry of Silica. New York: Wiley. 1979. 30. Prabakar, S., Assink, R. A., Raman, N. K., Myers, S. A. and Brinker, C. J. Identification of Self- and Cross-Condensation Products in Organically Modified Silica Sols by 29Si and 17O NMR Spectroscopy. Journal of NonCrystalline Solids. 1996. 202 (1-2): 53-60. 31. Davis, P. J., Deshpande, R., Smith, D. M., Brinker, C. J. and Assink, R. A. Pore Structure Evolution in Silica Gel During Aging/Drying. IV. Varying Pore Fluid pH. Journal of Non-Crystalline Solids. 1994. 167(3): 295-306. 32. Bergna, H. E. The Colloid Chemistry of Silica. Washington: American Chemical Society. 1990. 33. Stöber, W., Fink, A. and Bohn, A. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. Journal of Colloid and Interface Science. 1968. 26(1): 62-69. 88 34. Clark, R. J. H. The Chemistry Of Titanium And Vanadium: An Introduction to the Chemistry Of The Early Transition Elements. The Netherlands: Elsevier Publishing. 1968 35. Girrels, R. M. and Christ, C. L. Solution, Minerals and Equilibria. New York: Harper and Row. 1965. 36. Furlong, D. N., Sing, K. S. W. and Parfitt, G. D. The Precipitation of Silica on Titanium Dioxide Surfaces: I. Preparation of Coated Surfaces and Examination by Electrophoresis. Journal of Colloid and Interface Science. 1979. 69(3): 409-419. 37. Vysotskii, Z. Z. and Strazhesko, D. N. Adsorption and Adsorbents. New York: Wiley. 63; 1974. 38. Furlong, D. N. and Parfitt, G. D. Electrokinetics of Titanium Dioxide. Journal of Colloid and Interface Science. 1978. 65(3): 548-554. 39. Iler, R. K. Relation of Particle Size of Colloidal Silica to the Amount of a Cationic Polymer Required for Flocculation and Surface Coverage. Journal of Colloid and Interface Science. 1971. 37(2): 364-373. 40. Gao, X. T. and Wachs, I. E. Titania-Silica as Catalysts: Molecular Structural Characteristics and Physico-Chemical Properties. Catalysis Today. 1999. 51: 233-254. 41. Mandick, M. and Thomson, A. C. Method of Producing a Stable Titania Coated Silica Sol. U. S. Patent. 3,244,639. 1996. 42. Mandick, M. and Thomson, A. C. Method of Producing Alumina-Coated Silica in Sol Form. U. S. Patent. 3,252,917. 1996. 43. Klein, S., Thorimbert, S. and Maier, W. F. Amorphous Microporous Titania–Silica Mixed Oxides: Preparation, Characterization, and Catalytic Redox Properties. Journal of Catalysis. 1996. 163(2): 476-488. 44. Jung, M. W. NMR Characterization on the Preparation of Sol–Gel Derived Mixed Oxide Materials. International Journal of Inorganic Materials. 2001. 3: 471–478. 45. Stolarski, M., Walendziewski, J., Steininger, M. and Pniak, B. Synthesis and Characteristic of Silica Aerogels. Applied Catalysis A: General. 1999. 177 (2): 139-148. 89 46. Phalippou, J., Scherer, G. W., Woignier, T., Bourret, D. and Sempéré, R. Ultraporous Materials with Low Permeability. Journal of Non-Crystalline Solids. 1995. 186(2): 64-72. 47. Brinker, C. J., Smith, D. M., Deshpande, R., Davis, P. M., Hietala, S., Frye, G. C., Ashley, C. S. and Assink, R. A. Sol-Gel Processing of Controlled Pore Oxides. Catalysis Today. 1992. 14(2): 155-163. 48. Smith, D. M., Deshpande, R., Brinker, C. J., Earl, W. L., Ewing, B. and Davis, P. J. In-Situ Pore Structure Characterization During Sol-Gel Synthesis of Controlled Porosity Materials. Catalysis Today. 1992. 14(2): 293-303. 49. Einarsrud, M. A. Light Gel by Conventional Drying. Journal of NonCrystalline Solids. 1998. 225: 1-7. 50. Prassas, M., Phalippou, J., Hench, L. L. and Zarzycki, J. Preparation of xNa2O-(1-x)SiO2 Gels for the Gel-Glass Process: I. Atmospheric Effect on the Structural Evolution of the Gels. Journal of Non-Crystalline Solids. 1982. 48(1): 79-95. 51. Shlyakhtin, O. A., Young, J. O. and Tretyakov, Y. D. Preparation of Dense La0.7Ca0.3MnO3 Ceramics from Freeze-Dried Precursors. Journal of the European Ceramic Society. 2000. 20(12): 2047-2054. 52. Karen, P. and Kjekshus A. Chapter 190: Phase Diagrams and Thermodynamic Properties. Handbook on the Physics and Chemistry of Rare Earths. 30: 229-373. 2000. 53. Demydov, D., and Klabunde K. J. Characterization of Mixed Metal Oxides (SrTiO3 and BaTiO3) Synthesized by a Modified Aerogel Procedure. Journal of Non-Crystalline Solids. 2004. 350: 165-172. 54. Davis, P. J., Brinker, C. J. and Smith, D. M. Pore Structure Evolution in Silica Gel during Aging / Drying Temporal and Thermal Aging. Journal of Non-Crystalline Solids. 1992. 142: 189-196. 55. Ambrose, D. Critical Constants, in D. R. Lide (Ed.): CRC Handbook of Chemistry and Physics. 77th ed. CRC Press, Boca Raton/FL, USA. 6-54ff. 1996. 56. Tewari, P. H., Hunt, A. J. and Lofftus, K. D. Ambient-Temperature Supercritical Drying of Transparent Silica Aerogels. Materials Letters. 1985. 3(9-10): 363-367. 90 57. Hermann, G., Iden, R., Mielke, M., Teich, F. and Ziegler, B. On the Way to Commercial Production of Silica Aerogel. Journal of Non-Crystalline Solids. 1995. 186: 380-387. 58. Pajonk, G. M., Elaloui, E., Achard, P., Chevalier, B., Chevalier, J. L. and Durant, M. Physical Properties of Silica Gels and Aerogels Prepared with New Polymeric Precursors. Journal of Non-Crystalline Solids. 1995. 186: 1-8. 59. Schmidt, M. and Schwertfeger, F. Applications for Silica Aerogel Products. Journal of Non-Crystalline Solids. 1998. 225(1): 364-368. 60. Smith, D. M., Maskara, A. and Boes, U. Aerogel-Based Thermal Insulation. Journal of Non-Crystalline Solids. 1998. 225(7). 254-259. 61. Iler, R. K. Silica Gels and Powders. New York: Wiley. 462-599; 1979. 62. Tang, Q. and Wang, T. Preparation of Silica Aerogel from Rice Hull Ash by Supercritical Carbon Dioxide Drying. The Journal of Supercritical Fluids. 2005. 35(1): 91-94. 63. Marzouk, S., Rachdi, F., Fourati, M. and Bouaziz, J. Synthesis and Grafting of Silica Aerogels. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2004. 234(1-3): 109-116. 64. Heley, J. R., Jackson, D. and James, P. F. Fine Low Density Silica Powders Prepared by Supercritical Drying of Gels Derived from Silicon Tetrachloride. Journal of Non-Crystalline Solids. 1995. 186: 30-36. 65. Castillo, R., Koch, B., Ruiz, P. and Delmon, B. Preparation of Titania Supported on Silica Catalyst: Study of the Dispersion and the Texture of Titania. Preparation of Catalysts VI. 1995. 91: 291-297. 66. Brunauer, S., Emmett, P. H. and Teller, E. Adsorption of Gases in Multimolecular Layers. Journal of American Chemical Society. 1938. 60: 309-319. 67. Gregg, S. J., Sing, K. S. W. Adsorption, Surface Area and Porosity. 2nd eddition. London: Academic Press. 1982. 68. Vansant, E. F., Voort, P. V. D. and Vrancken, K. C. Characterization and Chemical Modification of the Silica Surface. Studies in Surface Science and Catalysis. Vol. 93. The Netherlands: Elsevier Science. 1995. 69. Niemantsverdriet, J. W. Spectroscopy in Catalysis. New York: VCH Publishers. 1993. 91 70. Cullity, B. D. Elements of X-ray Diffraction. 2nd ed. New York: AdisonWesley Publishing Co. 84-87; 1956. 71. Fierro, J. L. G. Characterization of Heterogeneous Catalysts. Part A: Method of Surface Analysis. New York: Elsevier. Vol. 57. A39. 1990. 72. Niemantsverdriet, J. W. Spectroscopy in Catalysis. New York: VCH Publishers. 1993. 73. Hair, M. L. Infrared Spectroscopy in Surface Chemistry. New York: Dekker. 1967. 74. Little, L. Infrared Spectroscopy of Adsorbed Species. New York: Academic Press. 1966. 75. Fadini, A. and Schnepel F. M. Vibrational Spectroscopy. Chichester: Ellis Horwood Ltd. 1989. 76. George, W. O. and McIntyre, P. S. Infrared Spectroscopy. Chichester: Wiley. 1987. 77. Knozinger, H. and Ratnasamy, P. Catalytic Aluminas: Surface Models and Characterization of Surface Sites. Catalysis Reviews - Science and Engineering. 1978. 17: 31–70. 78. Lambert, J. B., Shurvell, H. F., Lighter, D. A. and Cooks, R. G. Organic Structural Spectroscopy. New Jersey: Prentise-Hall. 1998. 79. Roebuck, A. and Adkins, H. 1,2-Cyclohenanediol. Organic Synthesis. 1949. 3: 217. 80. Yoda, S., Ohshima, S. and Ikazaki, F. Supercritical Drying with Zeolite for the Preparation of Silica Aerogels. Journal of Non-Crystalline Solids. 1998. 231: 41-48. 81. James, J. Characterization of Silica in Rice Husk Ash. American Ceramic Society Bulletin. 1986. 65(8): 1177-1180 82. Habashi, F. A short History of Hydrometallurgy. Hydrometallurgy. 2005. 79(1-2): 15-22. 83. Maschmeyer, T., Rey, F., Sankar, G. and Thomas, J. M. Heterogeneous Catalyst Obtained by Grafting Metallocene Complexes Onto Mesoporous Silica. Nature. 1995. 378: 159-162. 84. Lamberti, C., Bordiga, S., Arduino, D., Zecchina, A., Geobaldo, F., Spano, G., Genoni, F., Petrini, G., Carati, A., Villain, F. and Vlaic, G. Evidence of the Presence of Two Different Framework Ti(IV) Species in Ti-Silicalite-1 92 in Vacuo Conditions: An EXAFS and a Photoluminescence Study. The Journal of Physical Chemistry B. 1998. 102: 6382-6390. 85. Kuznicki, S. M. Large-Pored Crystalline Titanium Molecular Sieve Zeolites. U.S. Patent. 4,853,202. 1989. 86. Yilmaz, B., Miraglia, P. Q., Warzywoda, J. and Albert S. J. Synthesis of Titanosilicate ETS-4 with Controlled Morphology and Investigation of Its Crystallization Kinetics. Microporous and Mesoporous Materials. 2004. 71: 167–175. 87. Pena, M. L., Dellarocca, V., Rey, F., Corma, A., Coluccia, S. and Marchese, L. Elucidating the Local Environment of Ti(IV) Active Sites in Ti-MCM-41: A Comparison Between Silylated and Calcined Catalysts. Microporous and Mesoporous Materials. 2001. 41-45: 345-356. 88. Guidotti, M., Ravasio, N., Psaro, R., Ferraris, G. and Moretti, G. Epoxidation on Titanium-Containing Silicates: do Structural Features Really Affect the Catalytic Performance? Journal of Catalysis. 2003. 214: 242–250. 89. Chen, L. Y., Jaenicke, S. and Chuah, G. K. Thermal and Hydrothermal Stability of Framework-Substituted MCM-41 Mesoporous Materials. Microporous Materials. 1997. 12: 323-330. 90. Sabia, R., and Ukrainczyk, L. Surface Chemistry of SiO2 and TiO2–SiO2 Glasses as Determined by Titration of Soot Particles. Journal of NonCrystalline Solids. 2000. 277(1): 1-9. 91. Sugimoto, T., Zhou, X. P. and Muramatsu, A. Synthesis of Uniform Anatase TiO2 Nanoparticles by Gel–Sol Method: 1. Solution Chemistry of Ti(OH)n(4−n)+ Complexes. Journal of Colloid and Interface Science. 2002. 252(2): 339-346. 92. Park, G. A. Control of Pore Size and Condensation Rate of Cubic Mesoporous Silica Thin Films Using A Swelling Agent. Microporous and Mesoporous Materials. 2005. 78: 245–253. 93. Arnold, U., Cruz, R. S. and Mandelli, D. U. S. Activity, Selectivity and Stability of Metallosilicates Containing Molybdenum for the Epoxidation of Alkenes. Journal of Molecular Catalysis A: Chemical. 2001. 165: 149–158. 93 94. Beck, C., Mallat, T., Burgi, T. and Baiker, A. Nature of Active Sites in SolGel TiO2-SiO2 Epoxidation Catalysts. Journal of Catalysis. 2001. 204: 428–439. 95. Jorda, E., Tuel, A., Teissier, R. and Kervennal, J. Synthesis, Characterization, and Activity in the Epoxidation of Cyclohexene with Aqueous H2O2 of Catalysts Prepared by Reaction of TiF4 with Silica. Journal of Catalysis. 1998. 175: 93–107. 96. Fraile, J. M., Garcia, J. I., Mayoral, J. A. and Vispe, E. Effect of the Reaction Conditions on the Epoxidation of Alkenes with Hydrogen Peroxide Catalyzed by Silica-Supported Titanium Derivatives. Journal of Catalysis. 2001. 204: 146–156. 97. Murphy, E. F., Malat, T. and Baiker, A. Allylic Oxofuntionalization of Cyclic Olefins with Homogeneous and Heterogeneous Catalysts. Catalysis Today. 2000. 57: 115-126. 98. Lei, Z. Q. Selective Oxidation of Cyclohexene Catalyzed by PolymerBound Ruthenium–2,29-Bipyridine Complexes. Elsevier Reactive & Functional Polymers. 2000. 43: 139–143. 99. Fraile, J. M., Garcia, J. L, Mayoral, J. A. and Vispe, E. Silica-Supported Titanium Derivatives as Catalysts for the Epoxidation of Alkenes with Hydrogen Peroxide: A New Way to Tuneable Catalytic Activity through Ligand Exchange. Journal of Catalysis. 2000. 189: 40–51. 100. Davis, R. J., Crumbaugh, G. M. and Liu, Z. F. Effect of Structure and Composition on Epoxidation of Hexene Catalyzed by Microporous and Mesoporous Ti-Si Mixed Oxides. Journal of Catalysis. 1996. 159: 83–89. 101. Hutter, R., Mallat, T. and Baiker, A. Titania-Silica Mixed Oxides : III. Epoxidation of -Isophorone with Hydroperoxides. Journal of Catalysis. 1995. 157(2): 665-675. 102. Tuel, A. Synthesis, Characterization, and Catalytic Properties of the New TiZSM-12 Zeolite. Zeolites. 1995. 15: 236-242. 103. Jappar, N., Xia, Q. H. and Tatsumi, T. Oxidation Activity of Ti-Beta Synthesized by a Dry-Gel Conversion Method. Journal of Catalysis. 1998. 180: 132–141. 94 104. Clerici, M. G. and Ingallina, P. Epoxidation of Lower Olefins with Hydrogen Peroxide and Titanium Silicalite. Journal of Catalysis. 1993. 140(1): 71-83. 105. Bhaumik, A., Kumar, R. and Mukherjee, P. Triphase Catalysis over Titanium–Silicate Molecular Sieves under Solvent-Free Conditions: I. Direct Hydroxylation of Benzene. Journal of Catalysis. 1998. 178: 101– 107. 106. Corma, A., Esteve, P. and Martinez, A. Solvent Effects during the Oxidation of Olefins and Alcohols with Hydrogen Peroxide on Ti-Beta Catalyst: The Influence of the Hydrophilicity–Hydrophobicity of the Zeolite. Journal of Catalysis. 1996. 161: 11-19. 107. Reichardt, C. Solvent and Solvent Effects in Organic Chemistry. 3rd ed. Germany: Wiley-VCH. 493; 2003. 108. Xia, Q. H., Chen, X. and Tatsumi, T. Epoxidation of Cyclic Alkenes with Hydrogen Peroxide and tert-Butyl Hydroperoxide on Na- Containing TiZeolites. Elsevier Science. Journal of Molecular Catalysis A: Chemical. 2001. 176: 179–193. 109. Schindler, G. P., Bartl, P. and Hoelderich, W. F. Oxidative Cleavage of Cyclohexane Derivatives over Titanium-Containing Y Zeolites. Applied Catalysis A: General. 1998. 166: 267-279. 110. Schuchardt, U., Cardoso, D., Sercheli,R., Pereira, R., Cruz d, R. S., Guerreiro, M. C., Mandelli, D., Spinacé, E. V. and Pires, E.L. Cyclohexane Oxidation Continues to be a Challenge. Applied Catalysis A: General. 2001. 211: 1–17. APPENDICES Retention Time (min) Component Name 2.68 Acetone 3.45 Ethyl Acetate 4.20 Cyclohexane 4.43 Cyclohexene 5.75 Toluene 7.35 Cyclohexane Oxide 7.90 2-Cyclohexen-1-ol 8.50 2-Cyclohexen-1-one Appendix 1: Component table for GC-FID peaks identification. Appendix 2: Chromatogram of the reaction mixture analysed using gas chromatography. 96 Cyclohexene Calibration Curve 1.8 1.6 mM Cyclohexene 1.4 1.2 1 0.8 y = 0.0002x 2 R = 0.9999 0.6 0.4 0.2 0 0 2000 4000 6000 8000 10000 12000 A(cyclohexene)/A(toluene) Appendix 3: Calibration curve for quantify the concentration of cyclohexene. 97 Cyclohexene Oxide Calibration Curve 0.16 0.14 mM Cyclohexene Oxide 0.12 0.1 0.08 0.06 y = 0.0001x R2 = 1 0.04 0.02 0 0 200 400 600 800 1000 1200 A(cyclohexene oxide)/A(toluene) Appendix 4: Calibration curve for quantify the concentration of cyclohexene oxide. 98 2-Cyclohexen-1-ol Calibration Curve 0.35 mM 2-Cyclohexen-1-ol 0.3 0.25 0.2 0.15 y = 0.0001x R2 = 0.9994 0.1 0.05 0 0 500 1000 1500 2000 2500 A(2-cyclohexen-1-ol)/A(toluene) Appendix 5: Calibration curve for quantify the concentration of 2-cyclohexen-1-ol. 99 2-Cyclohexen-1-one Calibration Curve 0.16 0.14 mM 2-Cyclohexen-1-one 0.12 0.1 0.08 0.06 y = 0.0001x R2 = 0.9995 0.04 0.02 0 0 200 400 600 800 1000 1200 A(2-cyclohexen-1-one)/A(toluene) Appendix 6: Calibration curve for quantify the concentration of 2-cyclohexen-1-one. 100 1,2-Cyclohexanediol Calibration Curve 0.03 mM 1,2-Cyclohexanediol 0.025 0.02 0.015 0.01 y = 0.0001x 2 R = 0.9992 0.005 0 0 50 100 150 200 250 A(1,2-cyclohexanediol)/A(toluene) Appendix 7: Calibration curve for quantify the concentration of 1,2-cyclohexenediol. 101 68.0 67 66 65 930 64 1602 63 856 %T 61 1281 2869 62 1453 3319 2931 669 1364 60 1067 59 58.0 4000 3000 2000 cm-1 1500 1000 102 Appendix 8: FTIR spectrum of 1,2-cyclohexanediol that has been synthesized as standard. 450.0 70 Abundance 75000 70000 65000 60000 55000 57 50000 45000 40000 35000 83 30000 98 25000 20000 15000 116 10000 220 5000 m/z--> 0 50 60 70 80 90 100 110 120 130 140 150 160 170 180 190 200 210 220 252 230 240 250 Appendix 9: Mass spectrum of 1,2-cyclohexanediol that has been synthesized as standard. 103 104 O H2O + OH H2O O2 OH OO OOH OH O2 OO O O O OH OH Appendix 10: Reaction mechanisms involving hydroxy radical and cyclohexene [95, 96].