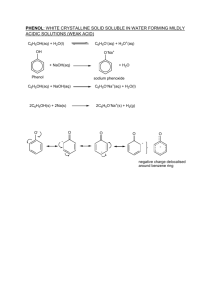
Document 14650859
advertisement

iii Specially Dedicated To my Beloved Mother To my Husband, Ervan Latuhari To my parents in law, To my Family, Brothers and Sisters iv ACKNOWLEDGEMENT First of all, I thank Almighty Allah, The Creator, for Mercy and Guidance in my whole life and giving me the ability to complete this research. I would like to express my sincere appreciation and deepest gratitude to my supervisor, Assoc. Prof. Dr. Salasiah Endud, Dr. Hadi Nur and Assoc. Prof. Dr. Zainab Ramli for their supervision, guidance, encouragement, advice, critics, motivation, and patience. Without their support and assistance, this thesis would not have been the same as presented here. It has been truly memorable and educative being a researcher under their supervision. I wish to express special appreciation to Prof. Dr. Halimaton Hamdan, Didik Prasetyoko, M.Sc. and Zeolite and Porous Material Group (ZPMG) members for their help support and valuable hints. I am also indebted to Ministry of Science, Technology and Innovation (MOSTI) for its support in providing the research grant for the IRPA funding 09-02-06-0057SR0005/09-04. My study would not have been possible without this funding. I must give my special thanks to Ibnu Sina Institute for Fundamental Science Studies, Department of Chemistry, Faculty of Science, Universiti Teknologi Malaysia and also my research friends, thank you for your help and support. My sincere appreciation also extends to all of my dearest family in www.S36chantique.com and all my sisters in H21 who have given me a beautiful friendship. I thank all of them, who are too numerous to mention each of them individually. And last, I would like to give greatest thank to my beloved mother, Andi Djuaeda, my parents in law, my dearest sisters and brothers, and all of my lovely big family, for their support, encouragement, care and love. And especially grateful to someone who is very patience, love, and supportive to me, my husband Ervan Latuhari, thank you so much. v ABSTRACT Mesoporous molecular sieve Al-MCM-41 with Si/Al=20 and polymethacrylic acid (PMAA) were used as supports for the immobilization of bulky iron(III)5,10,15,20-tetra-(4-pyridyl) porphyrin (Fe-TPyP). Metalloporphyrin of Fe(III) was encapsulated inside the mesopores of the ordered structure of Al-MCM-41 by sequential synthesis of Fe-TPyP via treatment of FeCl3 with 5,10,15,20-tetra-(4pyridyl) porphyrin (TPyP), followed by encapsulation of Fe-TPyP. Fe-TPyP complexes were also successfully encapsulated into PMAA by polymerizing methacrylic acid (MAA) with a cross-linker around the Fe-TPyP complexes. The materials obtained were characterized by X-ray Diffraction (XRD), Fourier Transform Infrared (FTIR), Ultraviolet Visible Diffuse Reflectance (UV-Vis DR), Electron Spin Resonance (ESR), Luminescence and 13C CP/MAS NMR spectroscopies, Thermogravimetric Analysis (TGA) and elemental analysis. The powder XRD data confirmed that the ordered structure of mesoporous Al-MCM-41 remained intact after encapsulation process. Characterization of Fe-TPyP composite with Al-MCM-41 and PMAA using FTIR, UV-Vis DR and ESR confirmed that the structure of Fe-TPyP in inorganic and polymer supports is similar with bare Fe-TPyP. The specific interaction of Fe-TPyP in Al-MCM-41 and/or PMAA was studied by ESR, 13C CP/MAS NMR and Luminescence spectroscopies. The ESR spectra of Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA composites showed that there is a shift towards a higher g-value confirming the interaction between Fe-TPyP and supports is occurred. By quenching of the luminescence spectra of Fe-TPyP/PMAA with various concentration of Fe-TPyP, it is evidenced that there is some interaction between Fe-TPyP and PMAA. Further evidence of interaction was corroborated by 13 C CP/MAS NMR spectra with show that the peak of carboxyl of PMAA is shifted to higher magnetic field. Single-point BET surface area analysis was used to determine specific surface area of the composites. It is revealed that the surface area of Fe-TPyP/Al-MCM-41 composites is decreased with an increase in Fe-TPyP, suggesting the encapsulation of the complex in the pores of Al-MCM-41 has been achieved. With mesoporous molecular sieve (Al-MCM-41) and the polymer (PMAA) as supports, the immobilized iron-porphyrin system has demonstrated excellent activity for the single-step synthesis of phenol from benzene under mild reaction conditions. The effect of reaction time, solvent, amount of Fe-TPyP loading, temperature and the performance of the recovered catalysts have been studied. The immobilized iron-porphyrin in PMAA (Fe-TPyP/PMAA) gives a higher activity compared to Fe-TPyP supported on Al-MCM-41 (Fe-TPyP/Al-MCM-41). However, the product selectivity of Fe-TPyP/PMAA is not as good as that of Fe-TPyP/ Al-MCM-41. Thus, it is reasonable to assume that the hydrophobic nature of Fe-TPyP/PMAA would account for the high activity, while the rigid, ordered structure of Fe-TPyP/Al-MCM-41 would contribute towards the high selectivity in the single-step synthesis of phenol from benzene in the present study. vi ABSTRAK Penapis molekul mesoliang Al-MCM-41 dengan nisbah Si/Al = 20 dan asid polimetakrilik (PMAA) telah digunakan sebagai penyokong untuk pemegunan kompleks ferum(III)-5, 10, 15, 20-tetra-(4-piridil) porfirin (Fe-TyP). Ferum-porfirin telah dikapsulkan di dalam mesoliang Al-MCM-41 secara sintesis berturutan Fe-TPyP melalui tindak balas FeCl3 dengan 5, 10, 15, 20-tetra-(4-piridil) porfirin (TPyP), dan diikuti pengkapsulan Fe-TPyP. Kompleks Fe-TPyP juga telah berjaya dikapsulkan ke dalam PMAA melalui proses pempolimeran asid metakrilik (MAA) dengan perangkai silang di sekitar kompleks. Sampel yang terhasil dicirikan dengan menggunakan kaedah XRD, spektroskopi FTIR, UV-Vis DR, ESR, pendarcahaya dan 13C CP/MAS NMR, TGA dan analisis unsur. Data XRD menunjukkan bahawa struktur mesoliang Al-MCM-41 yang teratur masih wujud setelah proses pengkapsulan. Pencirian komposit Fe-TPyP dengan Al-MCM-41 dan PMAA dengan kaedah FTIR, UV-Vis DR dan ESR, menunjukkan bahawa struktur Fe-TPyP di dalam penyokong tak organik dan polimer adalah serupa dengan kompleks asal Fe-TPyP. Interaksi spesifik Fe-TPyP dalam Al-MCM-41 dan/atau PMAA dikaji dengan kaedah spektroskopi ESR, pendarcahaya dan 13C CP/MAS NMR. Spektrum ESR bagi komposit Fe-TPyP/Al-MCM-41 dan Fe-TPyP/PMAA memperlihatkan anjakan ke arah nilai-g yang lebih tinggi, menunjukkan adanya interaksi antara Fe-TPyP dan penyokong. Pelindapan spektrum pendarcahaya bagi Fe-TPyP/PMAA dengan pelbagai kepekatan Fe-TPyP membuktikan terjadinya interaksi antara Fe-TPyP dan PMAA. Bukti interaksi tersebut juga turut disokong dengan spektrum 13 C CP/MAS NMR yang menunjukkan anjakan puncak karboksil bagi PMAA ke medan magnet yang lebih tinggi. Analisis luas permukaan BET titik tunggal telah digunakan untuk penentuan luas permukaan spesifik komposit. Luas permukaan komposit Fe-TPyP/Al-MCM-41 didapati menurun dengan pertambahan kandungan Fe-TPyP, menunjukkan bahawa Fe-TPyP telah terkapsulkan di dalam liang Al-MCM-41. Sampel penapis molekul mesoliang (Al-MCM-41) dan polimer (PMAA) sebagai penyokong, sistem ferum-porfirin yang dikapsulkan dalam penyokong telah digunakan untuk sintesis langkah tunggal fenol dari benzena pada keadaan tindak balas yang sederhana. Pengaruh masa tindak balas, pelarut, jumlah kandungan Fe-TPyP, suhu dan penjanaan semula mangkin bagi tindak balas tersebut juga telah dikaji. Ferum-porfirin yang terkapsulkan di dalam PMAA (Fe-TPyP/ PMAA) menunjukkan keaktifan yang lebih tinggi berbanding Fe-TPyP/Al-MCM-41. Manakala kepilihan hasil tindak balas menggunakan mangkin Fe-TPyP/PMAA adalah tidak sebaik dengan Fe-TPyP/Al-MCM-41. Maka, adalah dianggapkan bahawa sifat kehidrofobik Fe-TPyP/PMAA mungkin berperanan meningkatkan keaktifan mangkin, manakala struktur tegar dan teratur Fe-TPyP/Al-MCM-41 pula menghasilkan kepilihan yang tinggi dalam sintesis langkah tunggal fenol dari benzena dalam kajian ini. vii TABLE OF CONTENTS CHAPTER TITLE TITLE i DECLARATION ii DEDICATION iii ACKNOWLEDGEMENTS iv ABSTRACT v ABSTRAK vi TABLE OF CONTENTS vii LIST OF SCHEMES x LIST OF TABLES xi LIST OF FIGURES xii ABBREVIATIONS xv LIST OF APPENDICES 1 2 PAGE xvii INTRODUCTION 1.1 Research Background and Problem Statement 1 1.2 Research Objectives 8 1.3 Scope of Study 9 1.4 Outline of Research 10 1.5 Outline of Thesis 11 LITERATURE REVIEW 2.1 Introduce to Metalloporphyrins Complexes 12 2.2 Heterogenization of Metalloporphyrins 14 viii 2.2.1 2.2.2 16 Metalloporphyrins Supported on Polymer Matrix 21 2.3 Oxidation of Benzene to Phenol 23 2.4 Characterization Techniques 25 2.4.1 X-ray Powder Diffraction (XRD) 26 2.4.2 Fourier Transform Infrared Spectroscopy (FTIR) 27 UV-Vis Diffuse Reflectance Spectroscopy (UV-Vis DR) 29 2.4.4 Electron Spin Resonance (ESR) 30 2.4.5 Atomic Absorption Spectroscopy (AAS) 32 2.4.6 Single-Point BET Surface Area Analysis 33 2.4.7 Thermogravimetry Analysis (TGA) 34 2.4.8 Scanning Electron Microscopy (SEM) 35 2.4.9 Luminescence Spectroscopy 36 2.4.3 2.4.10 3 Metalloporphyrins Supported on Molecular Sieves 13 C CP Magic-Angle-Spinning NMR Spectroscopy (13C CP/MAS NMR) 37 ENCAPSULATION OF IRON(III)-PORPHYRIN WITHIN ORDERED MESOPOROUS Al-MCM-41 3.1 Direct Synthesis of Mesoporous Molecular Sieve AlMCM-41 39 Preparation of Iron(III)-Tetra (4-Pyridyl) Porphyrin (Fe-TPyP) 40 3.3 Preparation of Fe-TPyP/Al-MCM-41 40 3.4 Results and Discussion 42 3.4.1 42 3.2 Characterization of Al-MCM-41 3.4.2 Characterization of Iron(III)-Tetra (4-Pyridyl) Porphyrin 47 3.4.3 Characterization of Fe-TPyP/Al-MCM-41 50 ix 4 IMMOBILIZATION OF IRON(III)-PORPHYRIN IN POLYMETHACRYLIC ACID 4.1 Polymethacrylic acid as Organic Support 64 4.2 Synthesis of Polymethacrylic acid (PMAA) 65 4.3 Synthesis of Fe-TPyP/PMAA 65 4.4 Results and Discussion 66 4.4.1 Characterization (PMAA) of Polymethacrylic acid 4.4.2 Characterization of Fe-TPyP/PMAA 5 70 SINGLE-STEP SYNTHESIS OF PHENOL FROM BENZENE OVER Fe-TPyP/Al-MCM-41 AND Fe-TPyP/ PMAA CATALYSTS 5.1 Reaction Mechanism of Benzene Oxidation to Phenol 82 5.2 The Single-Step Synthesis of Phenol from Benzene 83 5.3 Analysis of Reaction Products 85 5.3.1 Gas Chromatography (GC) 85 5.3.2 Gas Chromatography – Mass Spectrometry Analysis (GC-MS) 86 High Performance Liquid Chromatography (HPLC) 87 5.3.3 5.4 6 66 Results and Discussion 89 5.4.1 Catalytic Activity 89 5.4.2 The Selectivity of Products 91 5.4.3 Regenerability of Catalysts 92 5.4.4 Optimization of Reaction Condition 94 CONCLUSION AND RECOMMENDATION 98 REFERENCES 101 APPENDICES 1090 x LIST OF SCHEMES SCHEME NO 1.1 5.1 5.2 TITLE Basic features of the cytochrome P-450 oxidation mechanism PAGE 2 The probable products of benzene oxidation (phenol, hydroquinone, catechol, resorcinol and benzoquinone) 83 Proposed reaction path for the oxidation of benzene 84 xi LIST OF TABLES TABLE NO. TITLE PAGE 3.1 XRD data and lattice parameter of the Al-MCM-41 samples 46 3.2 Assignment of FTIR bands of TPyP and Fe-TPyP complexes 49 3.3 XRD data of iron-containing Al-MCM-41 catalysts 55 3.4 Iron content (%Fe) of Fe-TPyP/Al-MCM-41 with different amount of Fe-TPyP loading 60 Surface properties of Al-MCM-41-supported Fe-TPyP with different amount of Fe-TPyP loading 60 Assignment of 13C CP/MAS NMR spectrum of PMAA 69 3.5 4.1 4.2 13 Assignment of chemical shift of C CP/MAS NMR spectra of as-synthesized PMAA and Fe-TPyP/PMAA with various of amount of Fe-TPyP loading 79 Iron content (%Fe) of Fe-TPyP/PMAA with different amount of Fe-TPyP loading determined by AAS 79 Surface properties of PMAA-supported Fe-TPyP with different amount of Fe-TPyP loading 81 5.1 GC-FID oven-programmed setup for identifying phenol 86 5.2 Catalytic activity of single-step synthesis of phenol from benzene 89 The catalytic activity of Fe-TPyP supported in Al-MCM-41 and polymethacrylic acid (PMAA) during the recycling in single-step synthesis of phenol from benzene 93 4.3 4.4 5.3 xii LIST OF FIGURES FIGURE NO 1.1. TITLE PAGE Commercial routes to synthesize phenol from benzene (with cumene as an intermediate) 6 1.2. Oxidation reaction of benzene to phenol with dioxygen 8 2.1. Structure of iron(III) tetra-(4-pyridyl)-porphyrin (Fe-TPyP) 14 2.2. Structure of zeolite NaX (I) and iron(III) porphyrins (FeP) 17 2.3. M41S family of mesoporous materials: (a) hexagonal MCM-41; (b) cubic MCM-48; (c) lamellar MCM-50 19 Horväth-Kawazoe pore size distribution for MCM-41, zeolite Y and amorphous silica 20 Schematic of the structure of the mesoporous material MCM-41 with cylindrical mesopores packed in a hexagonal array and amorphous siliceous pore walls 20 Polymer immobilized rhodium catalyst, PRH. PRH was prepared from the copolymerization of [(idppe)Rh(nbd)]BF4 (2 mol%) into an ethylene dimethacrylate-based polymer (98 %) 23 One-step benzene to phenol conversion using N2O in the gas phase 25 2.8 Bragg reflection diagram 26 2.9 Optical layout of the Michelson interferometer (S = IR source, D = detector) 28 2.10 The IUPAC classification of adsorption isotherms 33 3.1 Reaction of TPyP with FeCl3 in ethanol in the synthesis of iron-porphyrin complexes 41 Theoretical encapsulation of Fe-TPyP within ordered mesoporous Al-MCM-41 42 FTIR spectra of (a) as-synthesized and (b) calcined samples of Al-MCM-41 43 2.4. 2.5. 2.6. 2.7. 3.2 3.3 xiii 3.4 3.5 XRD patterns of the Al-MCM-41 samples as-synthesized and calcined 45 TGA thermograms of (a) as-synthesized and (b) calcined samples of Al-MCM-41 46 3.6 FTIR spectra of (a) TPyP and (b) Fe-TPyP complexes 48 3.7 UV-Vis DR spectra of (a) TPyP and (b) Fe-TPyP 50 3.8 FTIR spectra of (a) Fe-TPyP complexes, (b) Al-MCM-41 and (c) Fe-TPyP/Al-MCM-41 51 FTIR spectra of Fe-TPyP/Al-MCM-41 with various of Fe-TPyP loadings 53 XRD patterns of Al-MCM-41 and Fe-TPyP/Al-MCM-41 with various of Fe-TPyP loadings 54 UV-Vis DR spectra of (a) Fe-TPyP and (b) Fe-TPyP/ Al-MCM-41 56 UV-Vis DR spectra of Fe-TPyP/Al-MCM-41 with various of amount of Fe-TPyP loading 57 3.13 ESR spectra of (a) Fe-TPyP and (b) Fe-TPyP/Al-MCM-41 58 3.14 ESR spectra of Fe-TPyP and Fe-TPyP/Al-MCM-41 with various amount of Fe-TPyP loading 59 TGA thermograms of (a) calcined Al-MCM-41, (b) Fe-TPyP/Al-MCM-41 and (c) Fe-TPyP complexes 61 Scanning electron micrographs of (a) Al-MCM-41 and (b) Fe-TPyP/Al-MCM-41 62 Proposed mechanism of Fe-TPyP complex-Al-MCM-41 supports interaction 63 4.1 Structure of polymethacrylic acid (PMAA) 65 4.2 Schematic representation of the procedure of synthesis of composite Fe-porphyrin-polymethacrylic acid. Methacrylic acid (MAA) monomers assemble with the metalloporphyrin, followed by cross-linking polymerization 66 FTIR spectrum of as-synthesized polymethacrylic acid (PMAA) 67 TGA thermograms of as-synthesized polymethacrylic acid (PMAA) 68 4.5 The luminescence excitation and emission spectra of assynthesized polymethacrylic acid (PMAA) (Ȝex = 333 nm , Ȝem = 574 nm) 68 4.6 13 3.9 3.10 3.11 3.12 3.15 3.16 3.17 4.3 4.4 4.7 C CP/MAS NMR spectrum of as-synthesized polymethacrylic acid (PMAA) 69 FTIR spectra of (a) Fe-TPyP complexes, (b) as-synthesized PMAA and (c) Fe-TPyP/PMAA 71 xiv 4.8 UV-Vis DR spectra of (a) Fe-TPyP and (b) Fe-TPyP/ PMAA 72 UV-Vis DR spectra of Fe-TPyP/PMAA with various of amount of Fe-TPyP loading 73 ESR spectra of Fe-TPyP and Fe-TPyP/PMAA (containing 50 and 100 µmol Fe-TPyP) 74 The luminescence emission spectra of (a) as-synthesized PMAA, (b) Fe-TPyP/PMAA and (c) Fe-TPyP Ȝex = 333 nm) 75 The luminescence emission spectra of as-synthesized PMAA and Fe-TPyP/PMAA with different of amount of Fe-TPyP loading (Ȝex = 333 nm) 76 4.13 The Stern-Volmer kinetics: the dependence of the ratio of the luminescence intensities (I0/I) on the Fe-TPyP concentration 77 4.14 13 4.9 4.10 4.11 4.12 C CP/MAS spectra of Fe-TPyP/PMAA with various of amount of Fe-TPyP loading (a) 100 ȝmol, (b) 50 ȝmol, (c) 25 ȝmol, (d) 5 ȝmol and (e) as-synthesized PMAA 78 TGA thermograms of (a) Fe-TPyP complexes, (b) Fe-TPyP/PMAA and (c) as-synthesized polymethacrylic acid (PMAA) 80 5.1 Block diagram of a gas chromatograph 86 5.2 Effect of the difference catalyst on the phenol yield for 20 hours reaction 90 The product selectivity of single-step synthesis of phenol in aqueous hydrogen peroxide using Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA catalysts 91 The percentage conversion of benzene to phenol in aqueous hydrogen peroxide using Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA 92 Effect of reaction time on the phenol yield in the singlestep synthesis of phenol from benzene over Fe-TPyP/ Al-MCM-41 and Fe-TPyP/PMAA catalysts 95 Effect of different solvent on phenol yield using Fe-TPyP/ Al-MCM-41 and Fe-TPyP/PMAA as catalysts at 70 °C 95 Effect of amount of Fe-TPyP loading on the phenol yield in free-solvent at 70 °C for 20 hours reaction time over Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA catalysts 96 Effect of reaction temperature on phenol yield in freesolvent for 20 hours reaction time over Fe-TPyP/ Al-MCM-41 and Fe-TPyP/PMAA catalysts 97 4.15 5.3 5.4 5.5 5.6 5.7 5.8 xv ABBREVIATIONS λ wavelength 2θ Bragg angle AAS Atomic Absorption Spectroscopy Al-MCM-41 Aluminium Mobil Crystalline Materials-41 CuKα X-ray diffraction from copper KĮ energy levels CP/MAS NMR Cross-Polarization Magic-Angle-Spinning Nuclear magnetic Resonance UV-Vis DR Ultraviolet Visible Diffuse Reflectance Fe-TPyP iron(III)-tetra-(4-pyridyl)porphyrin FTIR Fourier Transform Infrared GC Gas Chromatography GC-MS Gas Chromatography – Mass Spectrometry h hour HPLC High Performance Liquid Chromatography IR Infrared KBr Potassium bromide MAS NMR Magic-angle-spinning nuclear magnetic resonance MCM-41 Mobil Crystalline Materials-41 Fe-TPyP/Al-MCM-41 iron(III)-porphyrin supported on Al-MCM-41 OH hydroxyl PMAA Polymethacrylic acid EGDMA ethylene glycol dimethacrylate AIBN 2.2’-azobis (2-methyl) propionitrile Fe-TPyP/PMAA iron(III)-porphyrin supported on polymethacrylic acid SEM Scanning Electron Microscopy Si/Al silicon-to-aluminium ratio xvi TGA Thermogravimetric Analysis TO4 Tetrahedral unit where T = Al or Si TPyP Tetra-(4-pyridyl)-porphyrin TS-1 Titanium silicalite XRD X-ray Diffraction technique ESR Electron Spin Resonance TON Turnover Number xvii LIST OF APPENDICES APPENDIX TITLE A Ultraviolet-Visible Diffuse Reflectance Spectroscopy (UV-Vis DR) 109 B Luminescence Spectroscopy (LS) 110 C Scanning Electron Microscopy (SEM) 111 D Gas Chromatography (GC) 113 E Gas Chromatography-Mass Spectrometry (GC-MS) 118 F High Performance Liquid Chromatography (HPLC) 124 G Reaction Path for the Oxidation of Benzene to Phenol 126 List of Publications 128 H PAGE CHAPTER 1 INTRODUCTION 1.1 Research Background and Problem Statement Selective catalytic oxidation of hydrocarbons under mild conditions is of academic interest and industrial importance [1]. In synthetic organic chemistry, oxidation represents one of the most important methods for substrate functionalization and functional group transformation. In the chemical industry, oxygenated products of petroleum namely, alcohols, aldehydes or acids, are important feedstocks for various industrial processes. Traditionally, oxidation of hydrocarbons are performed with stoichiometric amounts of inorganic oxidants such chromium chloride and potassium permanganate [2]. The use of these oxidants for oxidation reaction leads to a big environmental problem because of the generation of numerous amounts of by-products. In recent years, as a result of increasing environmental constraints, “clean” oxidants such as dioxygen (or air), hydrogen peroxide, and alkyl hydroperoxides, which are inexpensive, is becoming more important both in industry and academia, and chemical processes based on cleaner technologies are expected to increase significantly in the next few years. 2 In biological systems, nature has its unique way for doing selective O2 oxidation, which is accomplished by certain enzymatic systems. Some enzymes of the mono- and dioxygenase types incorporate one or both oxygen atoms of O2 respectively into a substrate. A well-known monooxygenase, iron porphyrin-based cytochrome P-450, has been the subject of intensive study [3] largely because of their ability to catalyze a wide variety of oxidation transformations, such as alkenes epoxidation and alkanes hydroxylation with molecular oxygen. The key steps in the catalytic cycle is reductive activation of O2, whereby one oxygen atom is reduced to H2O and the other oxygen atom becomes available to form a high-valent iron oxo species for the oxidation process (see Scheme 1.1) [4]. In the last two decades, therefore, increasing attention in catalytic oxidation has been focused on the reactivity and oxidation properties of biomimetic systems based on Fe(II), Ru(II) and Mn(II)[5-7]. SO Fe III e S II Fe FeV O O2 II H2O Fe (O2) FeIII O22- e 2H+ Scheme 1.1 Basic features of the cytochrome P-450 oxidation mechanism [4] 3 Synthetic metalloporphyrins are widely used as homogeneous catalysts for hydrocarbon oxidation, as well as model for cytochrome P-450 [8-9]. Metalloporphyrin complexes of iron [10], manganese [11] and ruthenium are known to be active catalysts for alkenes epoxidation. There are, however, several disadvantages in using metalloporphyrins as catalysts in homogeneous oxidation processes. The difficulty in separating the catalysts from the product substantially increases the cost of using homogeneous catalysis in commercial processes. Heterogeneous catalysts, on the contrary, can be easily separated from the reaction products simply by filtration. Yet most heterogeneous catalysts are less selective in complex reactions. Therefore, it is highly desirable to develop materials based on metalloporphyrin, which possess both the high selectivity of homogeneous catalysts and the convenience of heterogeneous catalysts. One approach to achieve this goal is to immobilize homogeneous catalysts on porous solid supports, which simultaneously has the advantages of tuning the liquid phase oxidation from homogeneous into heterogeneous. Microporous materials with regular arrays of internal channels and uniform pores such as zeolite [12] have been extensively studied as inorganic support. Immobilization of metalloporphyrin catalysts on microporous zeolite appears to be a good way to render these materials active for organic substrate oxidation. Zeolites have large internal surfaces and specific sites available for active metal substitution thus allowing the preparation of materials for selective processes. Furthermore, the uniform pore sizes provide both size- and shape-selectivity towards the reactant and product molecules. Based on isomorphous substitution approach a number of materials of potential industrial usage have been developed. A typical example is TS-1, a titanium modified silicalite that catalyses olefin epoxidation, alcohol oxidation and phenol hydroxylation with 30% hydrogen peroxide [13]. In addition, metalloporphyrin complex such as cis-Mn(bypy)3 [14] encapsulated in zeolite Y have been reported to be active catalysts towards cyclohexene oxidation. 4 Supporting metalloporphyrins on zeolite also provides a physical separation of active sites, thus minimizing catalyst self-destruction and dimerization of unhindered metalloporphyrins [15]. Although this approach has been demonstrated to be very successful, the main problem is the pore sizes of zeolites are very small (<13 Å) which limit their applications to reactions in which large molecules are involved [16]. In 1992, Beck et al. [17] reported the preparation and characterization of a new family of crystalline mesoporous molecular sieves, which are designated as M41S. MCM-41 is a member of this family associated with unique pores (20-100 Å) and large well-defined internal surface areas (>1000 m2 g-1). Due to the large pores of these mesoporous molecular sieves, high molecular mass organic molecules can easily gain access into the pores. Transition metal complexes and organometallic compounds can be immobilized onto the mesoporous MCM-41 supports by physical adsorption or covalent linkage. Titanocene dichloride was anchored to MCM-41 by Maschmeyer et al. [18]. Copper-salen and iron-salen complexes encapsulated in the channels of Al-MCM-41 have been reported to be an active catalyst towards polymerization of bisphenol-A at room temperature using hydrogen peroxide as oxidant and dioxane as solvent [19]. More recently, much effort was focused on the immobilization of metalloporphyrins onto the silica MCM-41 surface. Che and co-workers [20] have immobilized a ruthenium porphyrin on modified MCM-41. It was reported that the derived catalyst gives higher turnover numbers (TON) in the epoxidation of olefins than the free ruthenium porphyrin. It is interesting to note that in the oxidation of cis-stilbene with the modified MCM-41 material, the major product was trans-stibene oxide. In contrast, oxidation of cisstilbene catalyzed by free ruthenium porphyrin gave a 1:1 mixture of cis- and transstilbene oxides. The high selectivity to give trans-stilbene oxide was attributed to the steric constraint imposed by the uniform channels of the MCM-41 support. This example demonstrated the potential of mesoporous MCM-41 materials as size and shape selective catalysts. 5 Stimulated by these works, we are interested in modifying the MCM-41 materials with metalloporphyrin as catalysts for selective oxidation reactions. MCM-41 can serve as a support for the metalloporphyrin species by providing a large surface area and uniform surface for catalytic reaction. The larger pore dimensions would allow processing of bulky chemicals of interest. In this research, iron porphyrin has been immobilized within ordered mesoporous Al-MCM-41. It is well known that iron porphyrin complexes is effective catalyst for the conversion of olefins into trans-diols or trans-diol monoethers by using H2O2 [21]. In order to tune the activity of the supported catalysts the knowledge on the microenvironment of the immobilized complexes is essential. However, there are few reports on correlation between the structure of the immobilized catalysts and the catalytic activities. It is anticipated that immobilization of the metalloporphyrins in inorganic or organic support will stabilize and/or modify the catalytic performance by influencing the chemoselectivity, regioselectivity and shape selectivity of the reaction. Supported catalysts are also often plagued by leaching of the metal into solution. Our approach to this problem is to radically change the nature of the support. The even distribution of large, regular pores and extremely high surface area that characterizes mesoporous molecular sieve MCM-41 makes them ideal supports. This support has the added benefit that the silica structure has stability to chemical reagents. Also, easy separation of the products from the separation medium, along with the recovery and reuse of the expensive catalyst provide an attractive advantage over homogeneous catalysts. The key feature of the MCM-41, which separates it from currently used zeolite support, is its extreme porosity. However, the MCM-41, an inorganic material, is hydrophilic and rigid. In this research, we also propose a procedure to immobilize iron porphyrin on the polymer support, namely polymethacrylic acid (PMAA). One expects that the flexibility and hydrophobicity of the polymer as support give certain advantages in oxidation of organic compounds. The production of porous polymers containing large aromatic moieties or transition metal complexes such as the iron porphyrin complexes is considered to be useful, since they are in 6 high demand for a variety of applications ranging from catalysis, chromatography, diagnostics and sensors [1]. To the best of our knowledge, iron porphyrin complexes supported on PMAA has not yet been reported. Phenol is produced globally on the scale of 17 billion pounds/year [22] due to demand for bisphenol A (polycarbonate resins), phenolic resins, coprolactam (nylon 6.1), xylenols, aniline, alkylphenols and others. It is used in the manufacture of plywood, construction, automotive and appliance industry. It is also used as a raw material in the production of nylon and epoxy resins, disinfectant and slime-killing agent. Phenol has been mainly manufactured using the cumene method by which the selectivity for the phenol is high. However, this cumene process consists of three steps and produces acetone as a byproduct (Figure 1.1) [23]. The efficiency of the three-step cumene process strongly depends on the price of the by-product acetone, which is considerably varying. Phosporic acid + + O2 AlCl3 Benzene O Propene Cumene Air H O OH O Acid + H2SO4 Cumene hydroperoxide Figure 1.1 Phenol Acetone Commercial routes to synthesize phenol from benzene (with cumene as an intermediate) [23] 7 The cumene method has several significant shortcomings: it is a multistage synthesis; the intermediate cumene hydroperoxide is explosive; there are ecological problems and the production rate of the co-product acetone exceeds market demand. Therefore, both industry and academia are intensively searching for new routes to phenol based on direct benzene oxidation. The single-step synthesis of phenol from benzene would be an alternative. The single-step production of phenol by direct insertion of oxygen into the benzene ring is an attractive and challenging method, not only from a practical point of view but also from a synthetic chemical point of view, because the direct oxygenation of the energetically stable benzene to produce phenol has been one of the most difficult oxidation reactions [24]. The gas-phase oxidation of benzene to phenol by nitrous oxide has been widely studied over Fe-ZSM-5 [25]. In the presence of Fe-ZSM-5, the selectivity of benzene and N2O for phenol exceeded 98 and 95%, respectively, but the conversion of benzene to phenol is very low. The oxidation of benzene to phenol over H6PMo 9V3O40 and palladium acetate in VPI-5 and MCM-41 has been reported in the presence of molecular oxygen [26]. Over H6PMo9V3O40, after 4 hours at 130 oC the benzene conversion is 15% and the selectivity for phenol is above 70%. Phenol synthesis by liquid-phase oxidation of benzene with hydrogen peroxide has been also studied using iron-heteropoly acid [27]. Furthermore, Miyahara et al. has studied the liquid-phase oxidation of benzene to phenol catalyzed by Cu catalysts supported on zeolites [28], and MCM-41 [29], and also supported CuO catalysts (CuO-Al2O3) [24]. In the presence CuO-Al2O3, the phenol yield is very low (< 1%) and the leaching of Cu is less than 10%. An attractive alternative route is the direct oxidation of benzene to phenol using molecular oxygen and a suitable catalyst. A one-step process such as this would require less energy and generate zero waste, while producing only phenol. This reaction model of hydroxylation of benzene with oxygen is presented in Figure 1.2. 8 OH O2 / H2O2 Catalyst Figure 1.2 Oxidations reaction of benzene to phenol with dioxygen Recently, the best catalyst for benzene to phenol oxidation by nitrous oxide is Fe-ZSM-5 zeolite, which provides nearly 100% benzene selectivity, but low conversion of benzene [25]. The remarkable catalytic performance of this zeolite was show to be related to the presence of iron and upon high temperature treatment. In these systems, the reaction only occurs in the gas phase (ca. 300º) and there is no report on single-step liquid phase oxidation of benzene to phenol in the literature. For these reasons, in this research, we will study the single-step liquid phase oxidation of benzene to phenol using iron(III)-porphyrin supported on Al-MCM-41 and polymethacrylic acid (PMAA). 1.2 Research Objectives The main objectives of the research are: i. To synthesize Al-MCM-41 and polymethacrylic acid (PMAA). ii. To synthesize complexes iron(III) supported tetra-(4-pyridyl)-porphyrin on mesoporous (Fe-TPyP) Al-MCM-41 and polymethacrylic acid (PMAA) matrix. iii. To investigate the physicochemical properties of Fe-TPyP encapsulated in Al-MCM-41 and Fe-TPyP supported on polymer matrix. iv. To compare the performance of the hybrid catalysts of Fe-TPyP supported on mesoporous Al-MCM-41 and polymethacrylic acid (PMAA) in the single-step synthesis of phenol from benzene. 9 1.3 Scope of Study The scope of this research is to synthesize iron(III)-porphyrin encapsulated Al-MCM-41 and iron(III)-porphyrin supported on polymethacrylic acid (PMAA), to characterize these catalyst by XRD, FTIR, UV-Vis DR, ESR, Luminescence, and 13C CP/MAS NMR spectroscopies along with Single-point BET surface area analysis, AAS, TGA and SEM, to test the performance of these catalysts for the liquid phase single-step oxidation of benzene to phenol and finally, to analyze the reaction products using GC, GC-MS and HPLC techniques. 10 1.4 Outline of Research • Synthesis of iron(III) tetra-(4-pyridyl)-porphyrin (Fe-TPyP) Characterization of catalysts using FTIR and UV-Vis DR spectroscopies. No • Yes Synthesis of iron(III)-porphyrin supported on mesoporous Al-MCM-41 (Fe-TPyP/Al-MCM-41) • Synthesis of iron(III)-porphyrin supported on polymethacrylic acid (Fe-TPyP/PMAA) Characterization of catalysts by FTIR, XRD, UV-Vis DR, ESR, Luminescence and 13C CP/MAS NMR spectroscopies along with AAS Single-point BET Single-step synthesis of phenol from benzene Analysis of reaction product by GC, GC-MS, HPLC 11 1.5 Outline of Thesis This thesis focuses on the development of hybrid catalyst systems with the main aim at the preparation, characterization and catalytic application of iron(III)porphyrin (Fe-TPyP) supported on mesoporous molecular sieve Al-MCM-41 and polymethacrylic acid (PMAA). This thesis is also organized into six chapters. Chapter 1 describes the research background and problem statement, research objectives, scope of the research, outline of research and outline of the thesis. Chapter 2 presents some literature review on the chemistry of metalloporphyrin, mesoporous molecular sieve MCM-41, the polymer support, and the liquid-phase oxidation of benzene to phenol. Chapter 3 demonstrates that iron(III) tetra-(4-pyridyl)-porphyrin (Fe-TPyP) may be encapsulated into the pores and channels of the mesoporous material Al-MCM-41 by impregnation method, while Chapter 4 presents the preparation of iron-porphyrin supported into polymethacrylic acid (PMAA) by direct polymerization of iron(III) tetra-(4-pyridyl)-porphyrin (Fe-TPyP) with the monomer, methacrylic acid (MAA). Chapter 5 discusses the catalytic activity of these materials in the single-step synthesis of phenol from benzene. Finally, Chapter 6 presents the conclusion of the results obtained and provides recommendations for future research. 12 CHAPTER 2 LITERATURE REVIEW 2.1 Introduction to Metalloporphyrin Complexes In the 1970s several groups, e.g., those of Collman, Baldwin, Traylor, and Momenteau, carried out elegant studies on model metalloporphyrins with the goal of emulating the reversible oxygen binding of hemoglobin and myoglobin. The aim of these studies was to prevent further reaction of the iron-dioxygen complex by hindering the irreversible formation of the µ-oxo complex via reaction with a second molecule of the iron(II) porphyrin (Reaction 2.1). In the hemoprotein the approach of a second molecule of iron(II) porphyrin is prevented by the steric bulk of the protein ligand [1]. PFeII PFeIII-O-O PFeIII-O-O-FeIIIP µ-peroxo PFeII 2PFeIV=O PFeIII-O-FeIIIP (Reaction 2.1) µ-oxo Groves et al. [4] were the first to show that an iron(III) porphyrin and a chemical oxidant, such as iodosylbenzene, would mimic many of the oxidation of cytochrome-P450 monooxygenases. A significant difference between these models 13 and the enzymes is the presence of the protein matrix in the latter systems. The protein binds and site-isolates the iron(III) protoporphyrin IX prosthetic group, controls the reactivity of the active oxidant, and prevent inactivation of the enzyme through aggregation or bimolecular self-oxidation of the iron(III) porphyrin. The protein also controls the access of the substrate to the active oxidant and hence the selectivity of the oxidation and it provides the hydrophobic environment for substrate binding. The deficiencies of the model systems have been partly overcome through structural modification of the porphyrin rings. Thus, bulky and electron-withdrawing substituents help to prevent deactivation of the catalyst from aggregation and self-oxidation. Further, picket-fenced, strapped, and capped porphyrins have been developed to control the access of the substrate to the active oxidant and thereby to introduce selectivity into the oxidation. Recently, a number of synthetic metalloporphyrins have been intensively developed to mimic the activity of enzymatic monooxygenases [7]. Development in this area is based on different strategies with the aim of designing selectivity, stability and high turnover number catalytic systems. These strategies involve synthesis of structured metalloporphyrins, use of efficient and clean oxidants and the search for methods to reproduce the enzyme environment responsible for the high rates and selectivities of the natural systems [10]. In the homogeneous medium, relatively high turnover numbers can be obtained initially for the oxygenation reactions catalyzed by synthetic metalloporphyrin. All the different synthetic metalloporphyrin complexes used in these catalyzed oxidation reactions can be classified in three categories. The metal derivatives of meso-tetraphenylporphyrin, H2TPP, constitute the first-generation of metalloporphyrin catalysts used in these oxidations. The second-generation of tetraarylporphyrins is represented by meso-tetrakis(pentafluorophenyl) porphyrin, H2TDCPP, and meso-tetrakis(2,6-dichlorophenyl) porphyrin, H2TDCPP,and related ligands where huge substituents have been introduced meso-positions. The thirdgeneration is an extension of the previous idea by having bromine, chlorine or fluorine atoms at the ȕ-positions of pyrrole ring such as meso-tetrakis-(2,6dichlorophenyl)-ȕ-octabromoporphyrin, H2Br8TDCP, meso-tetrakis-(2,4,6-trimethyl- 14 H2 Cl8TMP, 3-chlorophenyl)-ȕ-octachloromoporphyrin, and meso-tetrakis- (pentafluorophenyl)- ȕ-octafluoromoporphyrin, H2F8TPFPP [30]. Figure 2.1 shows a unique metalloporphyrin structure containing alternately perpendicular porphyrin molecules that give rise to an unprecedented twodimensional paddle-wheel-like pattern (44 topology). It was generally accepted that the properties of metalloporphyrin mimic the cytochrome P-450 that is known as catalyst for selective hydroxylation of inactive alkanes. As biomimetic catalysts in the oxygenation of hydrocarbons, metalloporphyrin complexes have been largely employed. The high efficiency of some of these catalysts makes them potentially useful for large-scale oxidation. N (Z) N N N Fe N N N (Z) N Figure 2.1 2.2 Structure of iron(III) tetra-(4-pyridyl)-porphyrin (Fe-TPyP) Heterogenization of Metalloporphyrins Metalloporphyrins are well-known for their high selectivity, operating at mild temperatures in which they are able to catalyze selective transformation of hydrocarbons, but their use in an industrial environment is expensive and their handling and manipulation are rather difficult [31]. On the other hand, the traditional heterogeneous catalysts are rather robust, can operate under more severe conditions, 15 have generally good stability and are manufactured at relatively low costs. But in comparison with the homogeneous catalysts, their selectivity in most cases is significantly lower. It is therefore, obvious to expect that immobilizing the active site of metalloporphyrin on the surface of heterogeneous supports may be an efficient strategy to develop catalysts with advantages of both metalloporphyrin and heterogeneous catalysts. The immobilization of metalloporphyrin on different supports seems to be a good method to satisfy environmental demands and to obtain catalysts which preserve the properties of homogeneous systems, but are more stable and can be easily recovered. Metalloporphyrin as the active site can be adsorbed physically and attached chemically to active groups at the surface of supporters [1]. Metalloporphyrins heterogenized on high-surface oxides or porous polymers in principle have the following advantages over their homogeneous analogues: (a) easy separation between catalyst and product mixture; (b) possibility to operate in a continuous way; (c) no solubility limitation of the porphyrins; (d) possibility of interaction between the complex and the supporter; (e) decreased formation of porphyrin cluster, µ-oxo-dimers; and (f) decreased ability for auto-oxidation. Two broad classes of supported complex catalyst have been developed. In the first class, the metal complex is linked to the support through attachment to one of its ligands. In the second class, reaction of a metal complex with the support results in displacement of ligands attached to the metal and their substitution by groups that form an essential part of the support. In both classes two broad types of support are used namely organic polymers and inorganic oxides [32]. Supporting the metalloporphyrin could also have added benefits arising from the structure of the support since the support provides the local environment of the catalyst [1]. For general application the supported metalloporphyrin catalyst should be: 16 (a) oxidatively stable (b) tough and resistant to physical abrasion (c) reusable (d) resistant to metalloporphyrin leaching or removal (e) suitable for batch or continuous flow system (f) suitable for use in a wide range of solvents and conditions, and (g) capable of being “tailor-made” for selective oxidation. A generally significant and sometimes critical variable is the choice of support. For a specific application, a large number of supports of widely different natures may be compared or the selection may be as narrow as different version of the same material. Changing a support may completely alter the course of reaction or prevent catalysis. Several types of support are commercially available which can be categorized based on their internal architecture: (a) Amorphous supports: metal oxides (alumina, silica), carbon (b) Layered supports: Clays (c) Microporous supports: zeolites (molecular sieves), polymers (crosslinked, functionalized). 2.2.1 Metalloporphyrins Supported on Molecular Sieves Inorganic compounds such as silica gel, clays and zeolites have been extensively investigated as support for metalloporphyrins by the encapsulation or immobilization method [14-15] and by direct synthesis [33-35]. The encapsulation or immobilization of metalloporphyrin complex on insoluble solid support appears to be a good way of heterogenizing homogeneous catalysis. Such types of heterogenizedhomogeneous catalytic systems not only offer the combined advantages of homogeneous (mild condition) and heterogeneous (easy separation) systems, but also impose extreme shape selectivity in catalytic process [11]. 17 The catalytic properties of transition metal complexes encapsulated inside the zeolite matrix have received considerable attention in recent times. The well defined and ordered structure of zeolites provides an ideal environment to entrap active metal complexes or metal cluster. Inorganic complexes encapsulated in such porous systems can even mimic natural enzymes and can therefore be termed as zeozymes. Rosa et al. [33] and Khan et al. [34] have described the catalytic activity of iron(III) porphyrin encapsulated in zeolite X (Figure 2.2). In another report, Skrobot et al. [14] have used the zeolite synthesis method to synthesize zeolite Y around manganese(III) tetra-(4-N-benzylpyridyl) porphyrin complexes. I Figure 2.2 FeP Zeolite NaX (I) and iron(III) porphyrins (FeP) [34] Among the various solid supports, the mesoporous molecular sieve MCM-41 recently synthesized by researchers in Mobil Corporation is of particular interest. Several reports on the immobilization of porphyrin molecules onto MCM-41-type mesoporous solids have appeared recently. Li, et al. [15] have described how [mesotetrakis(1-methyl-4-pyridinio)-porphyrinato]manganese(III)-penta-acetate complexes were encapsulated within the channels of DMY and MCM-41 and the activity and selectivity of these materials was reported to be high for alkene epoxidation. The encapsulation of metal complexes inside molecular sieve MCM-41 have been studied for oxidation of benzyl alcohol over (Fe-phen) complexes in MCM-41 [36] and liquid-phase oxidation of n-hexane and cyclohexane over C8RuPcMCM-41 [37]. 18 (a) Mesoporous Materials MCM-41 as the Inorganic Host Porous materials are technically used as absorbents, catalysts and catalyst supports because of their high surface area [38]. IUPAC distinguishes between three groups of porous materials that are classified as (i) microporous (pore size < 2 nm), (ii) mesoporous (pore size 2-50 nm), and (iii) macroporous (pore size > 50 nm). Zeolites are well-known and widely used microporous materials [39]. Although zeolites exhibit excellent catalytic properties owing to their crystalline aluminosilicate network, their applications are limited by the small pore size. Enlargement of the pore size was therefore a major objective in zeolite research. The class of materials containing larger mesopores encompassed porous glasses and gels such as aerosols and xerogels, and pillared, layered structures like clays, but the drawback of these materials was their disordered pore system with broad pore size distributions. Inorganic porous materials such as zeolites, aluminas, silica gels and mesoporous MCM-41 molecular sieves provide new classes of heterogeneous hosts for catalysis processes [40]. In 1992, scientists at Mobil Research and Development Corporation discovered the M41S family of mesoporous molecular sieve [17]. The mesoporous M41S molecular sieves include MCM-41 with a hexagonal arrangement of tubular pores, MCM-48 with a cubic structure, and MCM-50 with a lamellar structure. Illustrations of the mesostructures of these materials are shown in Figure 2.3. These mesoporous materials bridge the gap between crystalline zeolites with micropores (< 13 Å) and amorphous silica gels with macropores [41]. For the first time, mesoporous materials possessing both a regularly ordered arrangement of pores and a narrow pore size distribution were prepared. M41S-type molecular sieves have channel structures and have controllable pore sizes of 1.5-10 nm in conjunction with extremely large surface areas > 1000 m2 g-1. With the discovery of the M41S family of molecular sieves featuring highly desirable properties, a great deal of research interest was initiated and has ever since devoted to mesoporous molecular sieves. Recent review articles provide comprehensive overviews on this subject [42]. 19 (a) (b) (c) Figure 2.3 M41S family of mesoporous materials: (a) hexagonal MCM-41; (b) cubic MCM-48; (c) lamellar MCM-50 MCM-41 is a novel mesoporous silicate material, possessing one-dimensional uniform pore system consisting of hexagonally arranged channels. The pore diameters vary from 15 to 100 ǖ. Unlike zeolites, the pore walls Al-MCM-41 are relatively thicker. The surface area of the MCM-41 materials is very high (up to 1000 m2/g), and the pore size distribution is nearly as sharp as that of conventional zeolites as shown in Figure 2.4. MCM-41 has attracted the attention of scientists due to its elevated specific high thermal and hydrothermal stability, possibility of controlling its pore size and its hydrophobicity and acidity [42]. 20 Sorption (Arbitrary Units) Zeolite Y 7.8 ǖ MCM-41 40 ǖ Amorphous Silica Pore diameter (ǖ) Figure 2.4 Horväth-Kawazoe pore size distribution for MCM-41, zeolite Y and amorphous silica [42] The structural model of MCM-41 is shown in Figure 2.5. Removal of the template via exposure to air at higher temperatures (calcination), leaves long-range, uniform, parallel, cylindrical mesoporous channels in a 2-D hexagonal array (space group p6mm) [43]. Amorphous silica Wall thickness ~10 ǖ Figure 2.5 Pore diameter ~35 ǖ Schematic of the structure of the mesoporous material MCM-41 with cylindrical mesopores packed in a hexagonal array and amorphous siliceous pore walls 21 The presence of hydrothermal stability and larger pore size dimensions than zeolite has made MCM-41 as a potential catalyst for producing fine chemicals within characteristic of larger molecules. Besides their potential for the catalytic conversion or adsorptive separation of bulky molecules, these mesoporous molecular sieves can also be used as host materials for spacious molecules like porphyrins or other transition metal complexes. 2.2.2 Metalloporphyrins Supported on Polymer Matrix The utility of polymer supported catalysts is now well-recognized because of their ease of workup of a separation of products and catalysts, from the economical point of view, and in application to industrial processes. Kobayashi et al. [44] has developed polystyrene-supported Sc(OTf)3 catalyst . This new technique for binding monomeric compounds to polymers will be applicable to the preparation of many other polymer-supported catalysts and reagents. In general, metal-complex catalysts are immobilized on polymers via coordinated or covalent bonds. For the purpose of this research organic polymers can be divided into two groups, linear and cross-linked [32]: (a) Linear organic polymers are prepared by one of two methods: a polycondensation or addition polymerization. (b) Cross-linked polymers may remove some of the uncertainty of what occurs structurally in a polymer-solvent system; however, new factors must now be considered. Synthetic polymers can be designed with pores and molecules complementing each other, with highly specific recognition capabilities, similar to those found in biological systems (e.g. enzymatic catalysis). These materials known as porous polymers can be used as support for transition metal complexes. A variety of linear and cross-linked organic polymer ligands have been also employed to 22 support metalloporphyrins. The extent of cross-linking of these materials affects the flexibility of the support, which in turn affects the activity of the catalyst [1]. Tong et al. [45] have used zinc(II)-protoporphyrin as a functional monomer in molecular imprinting-based fluorescent chemosensor for histamine. In another study, Haber et al. [46] have described the co-oxidation of styrene and iso-butyraldehyde in the presence of polyaniline-supported Co-, Fe-, and Mn-tetrakis (p-SO3H)PP porphyrins in the liquid phase. (b) Polymer as the Organic Host Organic polymers that have been used as supports include polystyrene, polypropylene, polyacrylates and polyvinyl chloride. Polymers offer several advantages over other supports [32]: (a) They are easily functionalized; this is particularly true of polymers containing aryl groups. (b) Unlike metal oxide surfaces, most hydrocarbon polymers are chemically inert. As a result, the support does not interfere with the catalytic group. (c) Polymers, particularly poly(styrene-divinylbenzene) can be prepared with a wide range of physical properties. As a result their porosity, surface area and solution characteristics can be altered by varying the degree of cross-linking. The principal disadvantage of polymers are their poor heat transfer ability and in many cases their poor mechanical properties which prevent them from being used in stirred reactors in which they are pulverized. It is important in developing polymer supported catalysts to have as well-defined and pure a support as possible. Polymer supported catalyst has been demonstrated to be effective in many useful carboncarbon bond-forming reactions [32]. In all cases, the catalyst was recovered quantitatively by simple filtration and reused without loss of the activity. 23 Unfortunately, heterogeneous systems involving polymers are often difficult to synthesize and optimize and characterization is often incomplete. Many different systems have been examined which successfully heterogenized a typically homogeneous catalysts by copolymerizing it into the matrix of a cross-linked organic polymer. The Gagne laboratory recently reported on a heterogeneous rhodium catalyst (PRH), [(idppe)Rh(nbd)]BF4 immobilized within the matrix of highly crosslinked, macroporous, ethylene dimethacrylate (EDMA) polymer (Figure 2.6) [47]. P P Rh Rh P BF4 - P PRh Figure 2.6 Polymer immobilized rhodium catalyst, PRH. PRH was prepared from the copolymerization of [(idppe)Rh(nbd)]BF4 (2 mol%) into an ethylene dimethacrylate-based polymer (98 %) [47] 2.3 Oxidation of Benzene to Phenol The oxidative hydroxylation of aromatics, in particular the direct oxidation of benzene to phenol, is one of the most difficult problems in the field of organic synthesis [25]. Phenol and its derivatives are industrially important in the synthesis of many dyes, insecticides and compound for pharmaceuticals [48]. The existing commercial process for the synthesis of phenol involves benzene as the starting material. The process comprises multiple steps with cumene as an intermediate More than 90% of the industrial production of phenol is done using the cumene process, which consists of three steps and produces acetone as a by-product [49]. In the first step, benzene is alkylated with propylene using phosphoric acid and AlCl3 as 24 catalyst to give rise to cumene. Cumene is then oxidized using air to give cumene hydroperoxide which is then hydrolyzed using sulfuric acid to yield phenol. Acetone is formed as by-product in the final step. There are several disadvantages of this process: conversions at each step must be kept low to attain high selectivity and corrosive and hazardous catalysts such as H2SO4 are used. In addition, since acetone is formed as by-product, the economics of the process is closely related to the market demand for acetone. Thus, there is a need to replace the current commercial process by a one-step process that synthesizes phenol from benzene. Selective insertion of an oxygen atom into hydrocarbons, such as in the conversion of benzene to phenol, represents a challenge with respect to the one-step production of phenol [50]. Although a direct oxidation process of benzene to phenol would be the most economical route, until now only the indirect manufacturing processes have been operated. Recently, many researchers have chosen the one-step hydroxylation of benzene to phenol [50-52] as an alternative. However, phenol is oxidized more easily than benzene and thus, many by-products like hydroquinone and benzoquinone are obtained. There are three main pathways for the direct oxidation of benzene to phenol [51]. Hydroxylation of benzene by nitrous oxide can be carried out in the gas phase over ZSM-5 zeolites at 623–673 K with >90% phenol selectivity at 20-30% benzene conversion [50, 52]. In these embodiments, benzene is reacted with N2O in the gas phase at elevated temperatures to give phenol (Figure 2.7). Main problems of an industrial application are deactivation of the catalyst by heavy coke formation and the low phenol selectivity of nitrous oxide. Another pathway for direct synthesis of phenol is the catalytic liquid-phase hydroxylation of benzene using hydrogen peroxide as an oxidant. The reaction has been carried out in acetone, methanol or acetonitrile as solvents using vanadium- or titanium-containing heterogeneous catalysts like Ti/MCM-41 [53] or VOx/MCM-41. 25 The benzene hydroxylation by hydrogen peroxide in the presence of a VOx/MCM-41 catalyst resulted in a benzene conversion of 10% and the phenol selectivity amounted to 38% [54]. Other attempts for the catalytic benzene oxidation have been undertaken employing molecular oxygen both in the gas and liquid-phase. Conventional methods of partial oxidation lead to the destruction of the aromatic ring and thus, to low phenol selectivity [55]. OH o 300-500 C + Figure 2.7 N2O + H-ZSM-5 N2 One-step transformation of benzene to phenol using N2O in the gas phase 2.4 Characterization Techniques Characterization of the catalysts were carried out by means of several methods: X-ray diffraction (XRD), Fourier transform infrared (FTIR), ultravioletvisible diffuse reflectance (UV-Vis DR), electron spin resonance (ESR), atomic absorption spectroscopy (AAS), single-point BET surface area analysis, thermogravimetric analysis (TGA) and scanning electron microscopy (SEM). Luminescence and 13 C CP/MAS NMR spectroscopy techniques are used to investigate the physicochemical properties of the polymer supported catalysts. 26 2.4.1 X-ray Powder Diffraction (XRD) X-ray powder diffraction is an extremely useful technique in material characterization. A lattice array of atoms can be regarded as an infinite stack of parallel, equally space planes. Any rational plane (hkl) of the lattice array can be chosen as the plane in question, and the whole array can be thought of as a stack of planes parallel to this one. The principal equation used in the analysis of an X-ray powder pattern is the Bragg’s Law, which states that: n.λ = 2 dhkl sinθ; n = 1, 2,….. (Equation 2.1) The path difference between the incoming wave and the scattered wave must be an integral number of wavelengths, nλ, when n is an integer; dhkl is the interplanar spacing of the lattice and θ is single of incidence (Figure 2.8) [38] X-ray Source θ θ dhkl d sin θ Figure 2.8 Bragg reflection diagram [38] The presence of distinct hk0 reflection of ordered MCM-41 mesoporous materials in X-ray diffraction data suggests a framework with long range regularity. The hk0 reflections can be indexed to a hexagonal lattice in MCM-41 mesoporous molecular sieves. 27 Experimental procedure: Powder X-ray diffraction (XRD) patterns were acquired on a Bruker D8 Advanced powder diffractometer Cu Kα radiation (λ = 1.5418 A, kV = 40, mA = 40). Approximately 1 g of sample was carefully ground to a fine powder and then lightly pressed between two glass slides to get a thin layer. After locating and locking sample holder in a proper place of the analyzer, samples were measured in the 2θ scale over 1.5 to 10o. A scanning speed of 2o and 1s step time were used. The unit cell parameter, a0 of the hexagonal structure of MCM-41 was calculated from the formula: a0 = 2d100¥3 2.4.2 (Equation 2.2) Fourier Transform Infrared Spectroscopy (FTIR) FTIR is an important method of structure characterization giving information on short range and long range bond order caused by lattice coupling, electrostatic and other effects [42]. For most spectrometric measurements made using a Michelson interferometer [56], the sample is held between the interferometer and the detector. Therefore, both partial waves from the interferometer experience the same attenuation and phase shift when they interact with the sample. Because the phases of both waves are shifted by the same amount, this shift has no effect on the resultant phase of the measured interferogram. The optical layout is shown in Figure 2.9. A beam of radiation from the source, S, is focused on a beam splitter which is constructed of materials such that about half the beam is transmitted to a moving mirror which reflects the beam back to the beam splitter which then reflects part of this beam through a sample to a detector, D. The other half of the beam from the source is reflected from the beam splitter to a fixed mirror which reflects the beam through the beam splitter to the detector, D, via the sample. A suitable material with the necessary optical properties for beam splitting in the mid-infrared is KBr coated with germanium. 28 Fixed mirror Beam splitter Moving mirror S į/2 Sample D Figure 2.9 Detector Optical layout of the Michelson interferometer (S = IR source, D = detector) Experimental procedure: Infrared spectra were recorded on Shimadzu Fourier-Transformed Infrared (FTIR) 8300 spectrometer. The technique of KBr wafer was used by mixing about 0.25 mg sample with 300 mg KBr powder and then pressed under vacuum at ca. 10 tonnes. The pellet was then put in a sample holder to determine its characteristic peaks. IR spectra were set and detected in % transmittance rather than absorbance unit. Twenty scans over the range of 4000 – 400 cm-1 were carried out for each sample. 29 2.4.3 UV-Vis Diffuse Reflectance Spectroscopy (UV-Vis DR) UV-Vis diffuse reflectance spectroscopy (UV-Vis DR) is a powerful technique for the qualitative and quantitative determination of the reflectan spectra of solid samples or molecules embedded on the solid surface [57]. The UV-Vis DR can reveal the chemical valence of incorporated transition metal ion [58]. UV-Vis diffuse reflectance spectroscopy measures the amount of light reflected from the sample surface with an integrating sphere. The data are reported as a percent of reflectance (% R) read on the transmittance scale of the instrument and correspond to R = I/Io where Io is the intensity of the incident light and I is the intensity of light reflected from the sample. Kubelka and Munk have developed a theory concerning diffuse reflectance of light-absorbing layers on surfaces. The Kubelka-Munk equation is expressed as R∞ = R’∞ (sample)/R’∞ (standard) F(R∞) = (1 - R∞)2 /2R∞ = 2.303εc/s (Equation 2.3) (Equation 2.4) where R’∞ is the absolute reflectance, ε is the molar absorption coefficient, c is the molar concentration (M), and s is the scattering coefficient. R∞ is the relative reflectance, which is measured against a standard material such as MgO or BaSO4. Converting the Kubelka-Munk equation into logarithmic form is one convenient way to display reflectance spectra as shown in equation (Equation 2.5): log F(R∞) = log ε + log (2.303 c/s) (Equation 2.5) Since the scattering coefficient s is practically independent of wavelength (λ), a plot of log F(R∞) versus λ of the reflectance spectrum should be equal to the real absorption spectrum (a plot of log ε versus λ) obtained by transmission measurement, except for a displacement by –log 2.393c/s in the direction of the ordinate. 30 A plot of log F(R∞) versus λ is used to portray reflectance data, and this plot corresponds to the true adsorption spectrum because the quantity F(R∞) is linearly proportional to the molar absorption coefficient ε as shown in Equation 2.4. More frequently, however, reflectance spectra are often presented in the form of log (1/R∞) or % R∞ versus λ although the quantity logs (1/R∞) in reflectance spectra is not linearly proportional to ε. This corresponds to absorbance or percent transmittance spectra in regular absorption spectroscopy. The quantity log (1/R∞) is often referred to as the apparent absorbance. The diffuse reflectance spectra of the molecules adsorbed on adsorbents such as inorganic oxides are usually broadened compared with the adsorption spectra obtained in solution. The absorption maxima and the molar absorption coefficients also change depending on the interaction between the adsorbed molecule and the adsorbent. Experimental procedure: UV-Vis diffuse reflectance (UV-Vis DR) spectra were recorded on Perkin Elmer Lambda 900 spectrometer. About 0.03 g of the sample was placed on a sample holder. After locating and locking sample holder in a proper place in the analyzer, samples were measured in the λ (wavelength) scale of 300 - 800 nm. 2.4.4 Electron Spin Resonance (ESR) Electron spin resonance (ESR) spectroscopy provides detailed structural information on a variety of paramagnetic organic and inorganic samples. The ESR technique has been frequently used to investigate the nature of the catalytic site and its coordination number [59]. 31 The application of ESR in heterogeneous catalysts studies, including determination of oxidation states, formation of ion pairs, and monitoring of ion migration [59]. This is important when heterogeneous systems are used to facilitate the electron transfer process such as organized molecular assemblies and oxide surfaces. In this research, ESR is used to detect porphyrin π-cation radicals which are generated by photo induced electron transfer in vesicles and mesoporous MCM-41 molecular sieves and polymer. The photoyields of porphyrin π-cation radicals can also be clearly measured by ESR. ESR spectroscopy is uniquely suited to the study of radiation effects. To appreciate this uniqueness, one must realize that must molecules, especially organic molecules, contain an even number of electron is zero in a diamagnetic. A molecule having an unpaired electron is called a free radical. Free radicals are necessarily paramagnetic. ESR spectroscopy can be used to detect and, in favorable circumstances, to identify free radicals [60]. Experimental procedure: The coordination environment of Fe-complexes in supports samples was further confirmed by electron spin resonance (ESR) analysis recorded on a JEOL ESR spectrometer (JES-FA100) operating in the X-band region. The microwave power employed was 0.99800 mW, and the amplitude of magnetic field modulation at 100 kHz was 0.2 mT. All observations were made at room temperature. Forty milligrams of the sample taken in a quartz tube with 4mm outer diameter, evacuated to ~10-3 Torr. The tube was sealed under vacuum and then set in the quartz Dewar vessel fitted in the ESR cavity. Manganese (g = 2.0000) was used as a reference to mark the g-value. 32 2.4.5 Atomic Absorption Spectroscopy (AAS) Absorption spectroscopy is used for the qualitative and quantitative determination of total atom in solutions. The absorbance of molecules dissolved in a suitable solvent is then converted to atomic state measured by transmitting monochromatic light through the solution. As a consequence of interaction between the photons and absorbing species free atom in the flame, only a fraction of incident beam is transmitted through the solution. The fraction of incident light absorbed by the solution is proportional to the concentration of an absorbing species. The absorbance in solution is defined by Beer’s law [61], A = εbc (Equation 2.6) Where A is the absorbance, ε is the molar absorption coefficient (M-1 cm-1), b is the path length (cm) through solution, and c is the molar concentration (M) of an absorbing species. Since the energy of the absorbed photon is characteristic of each atom, this property is exploited for quantitative analysis. This technique results extremely useful in catalysis laboratories for determining the atomic composition of the catalysts [59]. Experimental procedure: Atomic absorption spectroscopy technique was used to calculate the percentage of iron coordinated to the ligands (TPyP). 50 mg of the sample under test, placed in a platinum crucible, was heated at 900 oC for 1 hour using a muffled furnace. After cooling, the sample was dissolved in 1 mL of 40 % v/v HF and evaporated. The solid obtained was dissolved using 1 mL of 1M HCl and then diluted with water until 25 mL. Percentage of the iron in this solution was calculated by comparing with absorption of series of standard solution of Fe(NO3)3 using atomic absorption spectroscopy (AAS) technique. 33 2.4.6 Single-Point BET Surface Area Analysis The physisorption of N2 has been used to characterize the porosity of Al-MCM-41 materials. Adsorption isotherms are classified by the IUPAC according to the so-called BDDT scheme (Figure 2.10). In the following work, only Type II or IV isotherms are used in the determination of surface areas (the surface of mesopores walls) and pore size distribution of mesopores solids. For determination of surface area are most easily studied by means of the BET method [62]. I II a III IV a V Figure 2.10 VI The IUPAC classification of adsorption isotherms [62] 34 Experimental procedure: Surface area of the catalysts were analyzed using nitrogen adsorption technique, whereas for all materials were analyzed with Surface Area Analyzer instrument (Thermo Finnigen Qsurf Series) was used to investigated only the surface areas using single-point BET Technique. The single point equation assumes the intercept is zero since the slope is always so much larger than the intercept. Therefore, the utilization allows the use of one gas mixture, typically 30 % nitrogen balance helium and results in a very determination of surface area. 2.4.7 Thermogravimetric Analysis (TGA) Thermogravimetric analysis (TGA) was used to determine the amount of organic occluded in the as-synthesized materials. In this research, it is relevant to determine the amount of the organic guests molecules incorporated in the materials. Thermogravimetric analysis, measures the change in weight of the sample, as a function of temperature. This technique is used to determine the framework weight of MCM-41 samples. As-synthesized MCM-41 samples typically contain remnants of organic templates and of course of bond water. Thermogravimetric analysis allows the analysis of volatile material given-off from the MCM-41 when the sample undergoes a temperature ramp. The temperature range is usually between 25 and 800 oC. A typical heating rate is degree Celsius per minute using air as the carrier gas. Since the MCM-41 materials are usually activated by calcination in air and stabilized under nitrogen at elevated temperatures, it is essential to have an understanding of the effects of thermal treatment on their chemistry and structure [63]. Calcination of as-synthesized MCM-41 materials is required to remove the hydrocarbon templates and residues. In this experiment the weight of the samples is measured while the temperature is increased. Commonly, two regions are observed in which, first, water is lost, and then the organic template is burnt off. TGA may 35 also be used to measure the degree of water content. In the present study, TGA was used as a qualitative analysis technique only. Experimental procedure: The thermogravimetric analysis was performed in a flow of N2 by using a Perkin Elmer Diamond Pyris Thermal analyzer. Approximately 10 mg of the samples under test, placed in a platinum crucible, was heated from 40 oC to 900 oC at a scan rate of 10 oC min –1. The flow rate of nitrogen over the sample was 20 mL min-1. 2.4.8 Scanning Electron Microscopy (SEM) The scanning electron microscope (SEM) has unique capabilities for analyzing surfaces. SEM was used to study the surface morphologies of the bulk particles of the materials and models information on the submicron (10 -9 – 10-6 m) to micron (~10 -6 m) length scales. This form of imaging is based upon the low energy (< 50 eV) secondary electron emitted from the surface of the specimen. The beam can be concentrated to a small probe that may be deflected across the specimen using scanning coils. The secondary electrons emitted from different parts of the specimen are obtained. Scanning electron microscopy (SEM) provides information on the surface topography, texture, and morphology of the specimen. It offers a great depth of focus which facilities three-dimensional visualization of specimen surfaces [63]. Experimental procedure: Scanning electron microscopy was performed on electron microscope Philips XL40 under vacuum condition at 5 bar pressure. This measurement was carried out in order to determine the morphology of the sample and the crystal size. The 36 materials samples were mounted over sample holder (stubs) using double sided tape. The samples were coated with gold (Au) using Bio Rad Coating system at 10 -1 mbar with current flow 30 mA for 75 seconds. Sample was then placed into SEM instruments for scanning. Tungsten filament was used as electron sources and SEM micrograph was recorded with 10 kV resolutions to obtain 5000x enlargement. 2.4.9 Luminescence Spectroscopy Luminescence is common to an extremely wide range of objects of inorganic and organic nature, and synthetic materials and, for this reason, the mechanism of processes producing it is distinguished by great variety. A feature common to them is luminescence glow resulting from an emission transition of anion, molecule, or a crystal from an excited electronic state to a ground or other state with lesser energy. The emission transition is one of two possible ways of deactivating excited electronic states; the other being radiation less transitions resulting from interaction with the lattice or a transfer of energy to other ions. A unique property of luminescence that determines its applications is transformation of diverse kinds of electromagnetic and corpuscular emission, as well as of the electric, mechanical, and chemical energy into visible light [65]. In order to measure the binding ability of Fe-complexes in polymer support, the luminescence was employed to determine it. The efficiency of the energy transferred from the support to Fe-complex (Ȥ) was calculated using Equation 2.7 [66] and the experiment data: Ȥ = 1 – (I/I0) (Equation 2.7) Where: I and I0 are the intensity of luminescence of support in the presence and absence of quencher (i.e. Fe-complex), respectively. 37 Measurement of the luminescence intensities of support in the absence and in the presence of quencher (I/I0) have confirmed the occurrence of quenching of support in their electronically excited single state by the quencher. The quenching process can be described by the Stern-Volmer kinetic equation expressed as a dependence of the ratio of the luminescence intensities (I0/I) of support on the quencher concentration [66]. Experimental Procedure: Luminescence spectra were recorded on Perkin Elmer LS 55 spectrometer. About 0.04 g of the sample was placed on a sample holder. After locating and locking the sample holder in a proper place in the analyzer, samples were measured in the emission λ (wavelength) scale of 200 to 900 nm at excitation λ = 333 nm (this value was determined from pre-scanning of the polymethacrylic acid (PMAA) synthestic. 2.4.10 13 C CP Magic-Angle-Spinning NMR Spectroscopy (13C CP/MAS NMR) The most relevant prospects of the various NMR techniques as applied to adsorption studies as swell as to the identification of surface sites have been reviewed by several authors [65]. The principal characteristic of the NMR technique that makes it so useful for chemical analysis of liquids and solutions is the high resolution that allows one to observe very small atomic interactions. It is of interest to obtain NMR spectra of the solid state for several reasons [59]. Nuclear magnetic resonance spectroscopy investigates atomic nuclei possessing a mechanical spin, the nuclear spin p, such as proton 1H or the stable isotope of carbon, 13 C. These nuclei have a magnetic moment µ because of their mechanical spin and nuclear charge. Its value is γ times the nuclear spin p, where γ is a nuclear constant called the gyromagnetic ration: 38 µ = γp (Equation 2.8) For nuclei that have long spin lattice relaxation times there are two main difficulties which limit the observation of high resolution NMR spectra of solids. One of these is that normally the resonance lines are broadened by anisotropic dipole-dipole interactions and quadrupole filed gradient interactions, giving rise to line widths in the kHz range. The second problem is chemical shift anisotropy. These anisotropic interactions are also present in liquids but are averaged to zero by rapid Brownian motion. For solids, a similar averaging may be realized by magic-angle-spinning (MAS), which can eliminate dipolar and quadrupole field interaction as well as chemical shift interaction. MAS may be also combined with cross polarization (CP) to increase sensitivity of rare spins and long relaxation times. The importance of nuclear magnetic resonance spectroscopy as a tool for elucidating structures is based on the fact that the precession frequency of a spinning nucleus bonded in a molecule depends on its molecular environment. This is called the chemical shift of the Larmor frequencies. The spectrum of 1H or 13C Larmor frequencies of a compound is called its 1H or 13C nuclear magnetic resonance spectrum (NMR spectrum) [67]. Experimental procedure: MAS NMR experiments were performed using Bruker Avance 400 MHz spectrometer. The 13 C/CP MAS NMR spectra were recorded with a recycle delay of 5.0 s, number of transient of 2000 and spinning rate of 7 kHz. The chemical shifts of 13 C were referenced to TMS. 39 CHAPTER 3 ENCAPSULATION OF IRON(III)-PORPHYRIN WITHIN ORDERED MESOPOROUS Al-MCM-41 3.1 Direct Synthesis of Mesoporous Molecular Sieve Al-MCM-41 The basic molar composition of Al-MCM-41 described in this chapter was 6SiO2 : 0.3NaAlO2 : CTABr : 1.5 Na2O : 0.15 (NH4)2O : 250H2O. In this work, AlMCM-41 sample with the SiO2 to Al2O3 ratio of 20 was prepared according to the following procedure. First, a clear solution of sodium silicate was prepared by combining 2.595 g of 1.00 M aqueous NaOH solution (pellet from MERCK) with 10.015 g rice husk ash (90 wt% SiO2) and the resulting solution (mixture A) was then heated under stirring for 2 hours at 80 °C. A mixture of 1.05 g of 25 wt% aqueous NH3 solution (MERCK), 9.1115 g of cetyl N, N, N, -trimethyl ammonium bromide (CTABr) (Fluka) and 1.417 g of NaAlO2 (54 wt% Al2O3, Riedel-de Haen were put in a polypropylene bottle and the mixture (mixture B) was then heated with stirring for 1 hour at 80 °C. Subsequently, mixture B was added dropwise to a polypropylene bottle containing mixture A with vigorous stirring at room temperature. After stirring for 1 hour at 90 ºC, the gel mixture in the bottle was heated to 97 °C for 24 hours. The CTA-aluminosilicate gel was then suddently cooled to room temperature. The pH of the reaction mixture was then adjusted to 10.2 by adding 25 wt% acetic acid (CH3COOH) (MERCK). Repeated pH adjustments following the work of Kim et al. [67], was performed in order to increase thermal stability and textural uniformity of the product. The heating and pH 40 adjustment procedures were repeated two times. The precipitated product, assynthesized Al-MCM-41 containing CTA-template was filtered, washed thoroughly with doubly-distilled water and dried in an oven at 97 °C. Al-MCM-41 was calcined in air under static conditions in a muffled furnace. The calcination temperature was increased from room temperature to 550 °C for 10 hours and maintained at 550 °C for 6 hours. The mesoporous materials (as-synthesized and calcined Al-MCM-41) obtained have been characterized by XRD, FTIR and TGA techniques, according to section 2.4. 3.2 Preparation of Iron(III)-Tetra (4-Pyridyl) Porphyrin (Fe-TPyP) Iron insertion into TPyP was conducted by refluxing at 100 °C of TPyP (1 mmol) and FeCl3 anhydrous (1 mmol) in ethanol (30 mL) in an oil bath for 1 hour (see Figure 3.1). The solution was then filtered while still hot, washed with water and dried under vacuum. The Fe-TPyP obtained was characterized by FTIR and UV-Vis DR spectroscopies techniques, according to section 2.4. 3.3 Preparation of Fe-TPyP/Al-MCM-41 Fe-TPyP/Al-MCM-41 was synthesized via a slight modification of the method of Li et al. [15]. A suspension of Al-MCM-41 (0.150 g) in methanol containing Fe-TPyP (5: 10; 25; 50; and 100 ȝmol) was stirred for 24 hours at 20 °C. The resulting materials were filtered and washed with methanol until the filtrate became colorless. The solid obtained was dried at 100 °C for 4 hours which afforded Fe-TPyP. The material was characterized by XRD, FTIR, UV-Vis DR, ESR, AAS, Single-point BET Surface area analysis, TGA and SEM, according to section 2.4. Figure 3.2 shows schematically the procedure for encapsulating iron porphyrin complexes in ordered mesoporous Al-MCM-41, through impregnation of ironporphyrin complex into Al-MCM-41 mesopores. 41 N TPyP (Z) N N H N N H N N (Z) N T = 100 °C, FeCl3, Ethanol FeCl3 Ethanol 1 hour N Fe-TPyP (Z) N N N N Fe N N (Z) N + 2H+ (aq) Figure 3.1 Reaction of TPyP with FeCl3 in ethanol in the synthesis of iron- porphyrin complexes 42 Al-MCM-41 Fe-TPyP T = 20 °C, 24 hours N (Z) N N N Fe N N N Fe-TPyP/Al-MCM-41 (Z) N Figure 3.2 Theoretical encapsulation of Fe-TPyP within ordered mesoporous Al-MCM-41 3.4 Results and Discussion 3.4.1 Characterization of Al-MCM-41 The FTIR spectra of the mid-infrared region of Al-MCM-41 samples before and after calcination are shown in Figure 3.3. The mid-infrared region from 400– 1400 cm-1 contains vibrations due to the framework structure of Al-MCM-41. 43 796 458 958 1475 1388. 1226 2923 Transmittance / a.u 3440 2869 1639 (a) 1081 3443 463 793 961 1624 1066 (b) 4000 2000 1500 1000 500 Wavenumber / cm-1 Figure 3.3 FTIR spectra of (a) as-synthesized and (b) calcined samples Al-MCM-41 of 44 It in generally known that MCM-41 materials have properties between amorphous materials and zeolites. According to Chen et al. [54], the IR spectrum indicated that MCM-41 exhibit framework vibration similar to those of amorphous materials. Before calcination, the IR spectrum of Al-MCM-41 exhibits a broad peak at around 3440 cm-1, assigned to hydroxyl stretching of physically adsorbed water. Peaks observed at 2923, 2869 and 1475 cm-1 are assigned to symmetric C-H and asymmetric CH2 vibrations of the surfactant molecules. After calcination at 550 °C, all the bands which belong to the organic groups are no longer observed in the IR spectrum proving the complete removal of the surfactant template (CTABr). The powder X-ray diffractogram patterns of the as-synthesized and calcined Al-MCM-41 samples are shown in Figure 3.4. An intense peak representing the (100) diffraction is observed on both samples and in good agreement with reported patterns from MCM-41 materials [17]. Peaks corresponding to (110), (200) and (210) reflections which are characteristic of hexagonal structure can be seen clearly, but are not well resolved in the case of as-synthesized Al-MCM-41. The four peaks observed on the calcined Al-MCM-41 sample suggest that highly ordered mesoporous structure was obtained. The unit cell parameters and dspacing values of the Al-MCM-41 samples are given in Table 3.1. The hexagonal unit cell parameter a0 was calculated from the equation a0=2d 100¥3. The presence of a single intense peak at 2.135° 2ș indicates that the as-synthesized Al-MCM-41 possesses regular mesopores [17]. For the calcined sample, the intensity of the peaks becames stronger and the ș value was reduced from ca. 2.135 to 2.202°, indicating a decreased in the lattice parameter, owing to the removal of the organic surfactant template from the channels by calcination at 550 °C, and subsequent condensation process of silanol groups in the MCM-41 walls. Comparing the diffraction peak intensity and peak width, it is obvious that the calcined Al-MCM-41 seems more highly ordered than the assynthesized Al-MCM-41, suggesting that the degree of ordering is improved by the removal of the surfactant template. 45 (100) Calcined Al-MCM-41 hkl d(nm) 100 110 200 210 4.009 2.310 2.019 1.520 Relative Intensity / a.u (110)(200) (210) (100) As-synthesized Al-MCM-41 (110)(200) 1.5 2 3 4 hkl d(nm) 100 110 200 210 4.135 2.403 2.088 1.578 (210) 5 6 7 8 9 10 ș / ° Figure 3.4 samples XRD patterns of the as-synthesized and calcined Al-MCM-41 46 Table 3.1 : XRD data and lattice parameter of the Al-MCM-41 samples ș / o d100 / nm Unit Cell Parameter, a0 /nm As-synthesized 2.135 4.135 4.780 Calcined 2.202 4.009 4.635 Sample The thermogravimetric analysis (TGA) of as-synthesized and calcined samples of Al-MCM-41 was carried out at 50 °C to 900 °C, under flowing nitrogen at the rate of 10 °C/minute. The TGA profiles of as-synthesized and calcined samples of Al-MCM-41 samples are shown in Figure 3.5. 100 90 (b) 80 (i) Weight / % 70 (ii) 60 50 (iii) 40 (a) 30 20 10 0 300 600 900 Temperature / oC Figure 3.5 Al-MCM-41 TGA thermograms of (a) as-synthesized and (b) calcined samples of 47 The thermograms of the as-synthesized sample show the regions of weight loss as depicted in Figure 3.5(a). The first region occurs at temperatures around 50 to 150 °C, which can be reasonably assigned to desorption of water. Above 150 °C, breakage, decomposition and thermal desorption of organic fragments occur. The remaining part corresponds to organic material whose mass loss is related to three exothermal stages: (i) between 150–285 °C: decomposition of the surfactant; (ii) large weight change between 285–490 °C: breaking of the hydrocarbon chain) and (iii) between 490–620 °C: combustion of the residual surfactant and water loss associated with condensation of silanol groups. Thermogravimetric analysis curve of the calcined form of Al-MCM-41 clearly lacks inflection points indicative of template weight loss (Figure 3.5 (b)). This result suggests that the cationic template has been removed almost completely from the framework of Al-MCM-41. 3.4.2 Characterization of Iron(III)-Tetra (4-Pyridyl) Porphyrin The dark-purple solid of iron(III)-tetra (4-pyridyl) porphyrin (Fe-TPyP) obtained was characterized by using FTIR and UV-Vis DR spectroscopies. The FTIR spectra of the TPyP (commercial) and Fe-TPyP are shown in Figure 3.6 and the major FTIR bands of TPyP and Fe-TPyP complexes are tabulated in Table 3.2. In the FTIR spectrum of TPyP, the broad peak at 3431 cm-1 is related to the stretching of the N-H bonds of the aromatic. Another characteristic peak is the =C-H stretching of the aromatic observed at 3100–2900 cm-1. The strong peak at wavenumber 1595 cm-1 is due to the C=N conjugation of the aromatic; the C=C and C-C stretching are assigned to the peaks at 1632 cm-1 and 1468 cm-1, respectively, and peaks at 1000–700 cm-1 show the C-H bonds of the aromatic. 48 873 721 1468 1595 Transmittance / a.u 3431 3082 1632 (a) 724 1464 1630 1591 3413 2935 878 (b) 4000 2000 1500 1000 Wavenumber / cm-1 Figure 3.6 FTIR spectra of (a) TPyP and (b) Fe-TPyP complexes 500 49 On introduction of iron in the porphyrin, a shifting of the peaks at 1595 cm-1 (C=N bond), 1632 cm-1 (C=C bond) and 1468 cm-1 (C-C bond) in IR spectrum of TPyP to lower values is observed in the IR spectrum of Fe-TPyP. This is a clear indication of the incorporation of iron in the porphyrin. Table 3.2 : Assignment of FTIR bands of TPyP and Fe-TPyP complexes Wavenumber / cm-1 Characteristic Vibration TPyP Fe-TPyP N-H bonds of aromatic 3431 3413 =C-H bonds of aromatic 3082 3083 and 2935 C=C bonds 1632 1630 1595 and 1553 1591 and 1541 C-C bonds of aromatic 1468 1464 C-H bonds of aromatic 873 and 721 878 and 724 C=N conjugate of aromatic The dark-purple colour of the samples indicated that iron(III) was present in the porphyrin, and this was confirmed by UV-Vis diffuse reflectance spectroscopy (UV-Vis DR). The UV-Vis DR spectra of TPyP and Fe-TPyP are given in Figure 3.7. The UV-Vis DR spectra of iron(III) compounds with porphyrin ligands coordinated exhibit attributed to charge transfer porphyrin ĺ iron transitions. The new peaks at 424 nm in Figure 3.7(a) reveals that iron(III)-porphyrin has been prepared. This peak is attributed to internal ʌ ĺ ʌ* transitions of the ligand porphyrin. In addition, the UV-Vis DR spectrum of Fe-TPyP presents absorption bands at 510, 585 and 644 nm. The three peaks present in the region of 500–700 nm are typical of those of high-spin Fe(III) porphyrins [3]. The three peaks in this region are also attributed to d-d transitions of the iron(III). 50 510 585 644 K-M / a. u 424 (a) (b) 400 500 600 700 800 Wavelength / nm Figure 3.7 UV-Vis DR spectra of (a) Fe-TPyP and (b) TPyP 3.4.3 Characterization of Fe-TPyP/Al-MCM-41 Al-MCM-41 encapsulated Fe-TPyP complexes have been prepared by the method of Li et al. [15]. The solid obtained has been characterized by XRD, FTIR, UV-Vis DR and ESR along with AAS, single-point BET surface area, TGA and SEM. Figure 3.8 shows the IR spectra of the free Fe-TPyP complex, the pure Al-MCM-41 and the Al-MCM-41 encapsulated Fe-TPyP complex. The major bands of Al-MCM-41 dominate the 1300–400 cm-1 region of the spectrum of Fe-TPyP/ Al-MCM-41. However, the presence of TPyP is obvious, because bands originating from Fe-TPyP were observed in the 1700–1300 cm-1 region of the spectrum. 51 C-C 724 1466 C=C 878 1650 3431 2921 (a) 3443 463 1624 961 C=N 793 Transmittance / a.u 1591 (b) C=N 798 1081 C-C 969 723 663 1464 1404 1383 1637 1595 (c) 1080 3446 463 C=C 2000 1500 1000 500 Wavenumber / cm-1 Figure 3.8 FTIR spectra of (a) Fe-TPyP complexes, (b) Al-MCM-41 and (c) Fe-TPyP/Al-MCM-41 52 The spectrum of Fe-TPyP/Al-MCM-41 exhibits bands at 1637 cm-1 (C=C bond), 1404 cm-1 (C-C bond) and 1595 cm-1 (C=N bond), corresponding to stretching vibrations of phenyl group of the porphyrin. Compared with the spectrum of the pure Al-MCM-41 sample, the observation of those bands clearly indicate that Fecomplexes have been successfully encapsulated in the channels of Al-MCM-41 [15]. The IR bands of encapsulated complexes are weak due to low concentration of Fe-TPyP in Al-MCM-41. Figure 3.9 shows the IR spectrum of Fe-TPyP/Al-MCM-41 with various amounts of Fe-TPyP loading. All the samples show identical bands in the region 1700–1300 cm-1. The bands at 1595 and 1553 cm-1 (C=N bond), 1637 cm-1 (C=C) show increasing intensity with the increase in the amount of Fe-TPyP loading. As the amount of Fe-TPyP loading increases from 10 ȝmol – 100 ȝmol, the peaks in the 1700–1300 cm-1 region due to the vibrations of C=N and C=C of the porphyrin ligand become increasingly prominent. On the other hand, for the sample with 5 ȝmol of Fe-TPyP loading, those peaks are not observed may be caused by the low level incorporation of Fe-TPyP in Al-MCM-41. X-ray powder diffraction (XRD) patterns of the encapsulated Fe-TPyP are given in Figure 3.10, which are consistent with the XRD pattern for unloaded molecular sieve Al-MCM-41 without any peaks arising from Fe-TPyP. Results of the X-ray diffraction study also indicate that the Al-MCM-41 support remains structurally unchanged and believed that the iron-porphyrin is dispersed molecularly within the mesoporous channels. A typical XRD pattern of the Al-MCM-41 encapsulated Fe-TPyP complex shows three weak peaks which can be assigned to (110), (200) and (210) indicating that after encapsulation the long range order of the inorganic host, Al-MCM-41, has diminished, but fundamentally the mesostructure of the host material is still maintained. The reduction in intensity of the (100) diffraction peak became noticeable when the Fe-TPyP loading reached 100 ȝmol, but the basic structure of Al-MCM-41 was still maintained even for the sample with 100 ȝmol of Fe-TPyP loading. 53 Fe-TPyP =100ȝmol C=C C=N C=C C=N C-C Fe-TPyP =50ȝmol C-C Transmittance / a.u Fe-TPyP =25ȝmol C=C C=N C-C Fe-TPyP =10ȝmol C=C C=N C-C Fe-TPyP =5ȝmol 4000 3000 2000 1000 500 Wavenumber / cm-1 Figure 3.9 FTIR spectra of Fe-TPyP/Al-MCM-41 with various amounts of Fe-TPyP loadings 54 (100) Fe-TPyP =100ȝmol (110) (200) (210) Fe-TPyP =50ȝmol Relative Intensity / a.u Fe-TPyP =25ȝmol Fe-TPyP =10ȝmol Fe-TPyP =5 ȝmol Fe-TPyP =0 ȝmol 1.5 2 3 4 5 6 7 8 9 10 ș / ° Figure 3.10 XRD patterns of Al-MCM-41 and Fe-TPyP/Al-MCM-41 with various amounts of Fe-TPyP loadings 55 It was also noted that the peaks shift to higher 2ș angles with a corresponding decrease in the a0 values as the amount of Fe-TPyP loading is increased (Table 3.3). The decreased unit cell parameter with respect to Al-MCM-41 can be explained by the incorporation of iron(III)-porphyrin into the mesoporous channel of Al-MCM-41. Table 3.3 : XRD data of iron-containing Al-MCM-41 catalysts Fe-TPyP Samples loading / ș / o unit cell d 100 / nm parameter, a0 / nm ȝmol Al-MCM-41 - 2.202 4.009 4.635 Fe-TPyP/Al-MCM-41-5 5 2.205 4.003 4.628 Fe-TPyP/Al-MCM-41-10 10 2.208 3.998 4.622 Fe-TPyP/Al-MCM-41-25 25 2.210 3.994 4.617 Fe-TPyP/Al-MCM-41-50 50 2.213 3.988 4.610 Fe-TPyP/Al-MCM-41-100 100 2.214 3.986 4.608 Figure 3.11 show the UV-Vis DR spectrum of Fe-TPyP and Fe-TPyP/ Al-MCM-41. The Al-MCM-41 encapsulated Fe-TPyP shows the Soret band at 414 nm, which are blue-shifted when compared to the spectrum of Fe-TPyP, and the three peaks in the range of 500–700 nm (517, 589 and 644 nm) are typical of highspin Fe(III)-porphyrins [68-69]. The occurrence of the absorption maximum at 589 nm can be assigned to the axial electrostatic interaction between the iron-porphyrin and the anionic Al-MCM-41 pore surfaces, indicating the incorporation of Fe-TPyP in the support [33]. Figure 3.12 depicts the UV-Vis DR spectra of Fe-TPyP/Al-MCM-41 with various amounts of Fe-TPyP loading. Especially interesting is that even with the FeTPyP loading as low as 5 µmol, the bands around 414–420 nm (Soret), 589 nm, and the three peaks in the 500–700 nm could still be observed in the UV-Vis DR spectra. This implies that UV-Vis DR spectroscopy is a very sensitive characterization technique and has proven to be a powerful tool for identifying the electronic structure of the iron complex formed inside the channels of Al-MCM-41 in the present study. 56 K-M / a. u 462 589 517 414 (a) 644 (b) 400 500 600 700 800 Wavelength / nm Figure 3.11 UV-Vis DR spectra of (a) Fe-TPyP and (b) Fe-TPyP/Al-MCM-41 The local structure of iron(III) species in molecular sieve materials also can be easily detected by other techniques such as ESR. Fe-TPyP displays similar ESR spectrum with that of high-spin Fe(III) signal at g = 1.984 (Figure 3.13(a)). As depicted in Figure 3.13(b), two different signals occur in the ESR spectrum of the Fe-TPyP/Al-MCM-41 sample. The broad signal at g = 4.284 is assigned to Fe(III) in distorted framework tetrahedral coordination, whereas the signal at g = 2.00067 belongs to high-spin Fe(III)-porphyrin. A radical signal at g = 2.00067 was also observed indicating the formation of Fe-complex intra mesoporous cavity [70]. Figure 3.14 shows the ESR spectra of Fe-TPyP/Al-MCM-41 with different Fe-TPyP loadings. As one can see, an increase content from 5–100 ȝmol of Fe-TPyP is accompanied by a gradual increase in intensity of the ESR line in the region of the magnetic field at g = 2.0006. 57 Fe-TPyP =100ȝmol K-M / a. u Fe-TPyP =50ȝmol Fe-TPyP =25ȝmol Fe-TPyP =10ȝmol Fe-TPyP =5ȝmol 400 500 600 700 800 Wavelength / nm Figure 3.12 UV-Vis DR spectra of Fe-TPyP/Al-MCM-41 with various of amount of Fe-TPyP loading Intensity / a. u 58 (a) g = 1.984 g = 4.284 g = 2.00067 100 200 300 400 (b) 500 B / mT Figure 3.13 ESR spectra of (a) Fe-TPyP and (b) Fe-TPyP/Al-MCM-41 59 g = 2.00067 Fe-TPyP=100ȝmol Intensity / a. u Fe-TPyP=50ȝmol Fe-TPyP=25ȝmol Fe-TPyP= 10ȝmol Fe-TPyP=5ȝmol 100 200 300 400 500 B / mT Figure 3.14 ESR spectra of Fe-TPyP and Fe-TPyP/Al-MCM-41 with various Fe-TPyP loadings 60 Table 3.4 gives the values obtained from atomic absorption measurements of the Fe content of Fe-TPyP/Al-MCM-41 samples. It is seen that the increase of iron content corresponds with the increase of FeTPyP loaded in Al-MCM-41. Table 3.4 : Iron content (%Fe) of Fe-TPyP/Al-MCM-41 with different amount of Fe-TPyP loading Sample Fe (%) Fe-TPyP/Al-MCM-41-100 (Fe-TPyP = 100ȝmol) 0.22 Fe-TPyP/Al-MCM-41-50 (Fe-TPyP = 50ȝmol) 0.17 Fe-TPyP/Al-MCM-41-25 (Fe-TPyP = 25ȝmol) 0.15 Fe-TPyP/Al-MCM-41-10 (Fe-TPyP = 10ȝmol) 0.10 Fe-TPyP/Al-MCM-41-5 (Fe-TPyP = 5ȝmol) 0.08 Table 3.5 shows the surface properties of Al-MCM-41-supported Fe-TPyP catalysts determined from surface area analysis. The BET surface area was decreased with increasing amount of iron loading up to 100 ȝmol, due to the filling of pores by Fe-TPyP. Table 3.5: Surface properties of Al-MCM-41-supported Fe-TPyP with different amount of Fe-TPyP loading Samples Surface area (m2g -1) Al-MCM-41 813 Fe-TPyP/Al-MCM-41-5 (Fe-TPyP = 5 ȝmol) 734 Fe-TPyP/Al-MCM-41-10 (Fe-TPyP = 10 ȝmol) 652 Fe-TPyP/Al-MCM-41-25 (Fe-TPyP = 25 ȝmol) 471 Fe-TPyP/Al-MCM-41-50 (Fe-TPyP = 50 ȝmol) 383 Fe-TPyP/Al-MCM-41-100(Fe-TPyP=100 ȝmol) 217 61 Figure 3.15 shows the thermogravimetric analysis curves of Al-MCM-41 and Fe-TPyP/Al-MCM-41. Exothermic weight losses were observed for all the materials at temperatures below 150 °C, which correspond to the loss of physisorbed water. The parent host material (Al-MCM-41) exhibits an additional exothermic weight loss at over 300 °C. The thermogram of Fe-TPyP/Al-MCM-41 sample shows the weight loss extends in the region 400–500 °C and 500–600 °C, attributed to the decomposition or burning of the iron-porphyrin. In the thermogravimetric curves obtained, it was possible to observe that for free Fe-TPyP, the content of organic matter (porphyrin) was around 48% and for the encapsulated Fe-TPyP/Al-MCM-41, the content was found to be much lower (around 12%), related to dry matter basis. 100 90 (a) Water loss 80 Weight / % 70 60 (b) 50 40 30 20 Decomposition of organic matter (porphyrin) 10 0 300 600 (c) 900 Temperature / oC Figure 3.15 TGA thermograms of (a) calcined Al-MCM-41, (b) Fe-TPyP/ Al-MCM-41 and (c) Fe-TPyP complexes 62 The size and morphology of the Al-MCM-41 and Fe-TPyP/Al-MCM-41 materials obtained by encapsulation of iron-porphyrin within mesoporous material Al-MCM-41 (Fe-TPyP/Al-MCM-41) were investigated by scanning electron microscopy. The SEM micrographs of these materials are presented in Figure 3.16. (a) (b) Figure 3.16 Al-MCM-41 Scanning electron micrographs of (a) Al-MCM-41 and (b) Fe-TPyP/ 63 All the samples do not have well defined hexagonal structure. Further, aggregates without regular shapes are observed in agreement with previous reports [71] for metal-complexes incorporated materials indicating a slight reduction in hexagonal symmetry of Al-MCM-41 due to metal-complex incorporation. The micrographs of Fe-TPyP/Al-MCM-41 also confirm the uniformity of particle size, but after incorporation of iron-porphyrin, the particle size has become smaller than that of the pure Al-MCM-41. This observation lends to support the fact that there was a decrease of the surface area of Al-MCM-41 after incorporation of Fe-TPyP (see Table 3.4). The proposed interaction between the negatively charged aluminium in the framework of Al-MCM-41 and the Fe-center of the porphyrin complex is presented in Figure 3.17. The strong electrostatic interaction between Fe and the support has been reported to prevent leaching of the Fe-TPyP species into solution during the oxidation reaction [19]. N (Z) N N N Fe N N N (Z) N Electrostatic Interaction H+ O O Si _ Al O O Si Al-MCM-41 Figure 3.17 interaction Proposed mechanism of Fe-TPyP complex-Al-MCM-41 support 64 CHAPTER 4 IMMOBILIZATION OF IRON(III)-PORPHYRIN IN POLYMETHACRYLIC ACID 4.1 Polymethacrylic acid as Organic Support Synthetic polymers can be designed with pores and molecules complementing each other, with highly specific recognition capabilities, similar to those found in biological systems (e.g. enzymatic catalysis). These materials known as porous polymers can be used as support for transition metal complexes [72]. Organic polymers that have been used as supports include polystyrene, polypropylene, polyacrylates and polyvinyl chloride. Methacrylic acid (MAA) is a kind of important monomer used for synthesizing functional polymer. PMAA and its copolymers are widely used as paint, adhesive and thickener. The structure of polymethacrilic acid is shown in Figure 4.1. CH3 CH2 O Figure 4.1 C CH3 CH2 OH O C CH2 OH Structure of polymethacrylic acid (PMAA) 65 Polymethacrylic acid is also used as an enteric-coating polymer in the pharmaceutical industry because of its carboxylic groups that can transform to carboxylate groups in the pH range 5–7 by salt formation with alkali or amines. PMAA contains carboxyl group which is strongly hydrogen-bonded, hence affecting the enthalpy of PMAA anhydridization [73]. 4.2 Synthesis of Polymethacrylic acid (PMAA) Polymethacrylic acid was prepared using the method of Tamayo et al. [74]. Monomer methacrylic acid (MAA) (2 mmol) and toluene (6 mL) were placed into a 25 mL glass tube and the mixture was left in contact for 10 minutes. Subsequently, Ethylene glycol dimethacrylate(EGDMA) (10 mmol) and 2. 2’-azobis (2-methyl propionitrile) (AIBN) (15 mg) were added. The glass tube was sealed and thermostated at 60 °C in an oil bath to start the polymerization process. After 24 hours, the obtained samples were air dried and weighted. The polymer obtained characterized by FTIR, TGA, Luminescence and 13C CP/MAS NMR techniques. 4.3 Synthesis of Fe-TPyP/PMAA Fe-TPyP/PMAA was prepared using the same procedure as described in Figure 4.2. Fe-TPyP (5; 10; 25; 50 and 100 µmol), toluene (6 mL) and MAA (2 mmol) were placed into a 25 mL glass tube and the mixture was left in contact for 10 minutes. Subsequently, EGDMA (10 mmol) and AIBN (15 mg) were added. The glass tube was sealed and thermostated at 60 °C in an oil bath to start the polymerization process. After 24 hours, the obtained micro-spheres were air dried and weighted. Characterization of samples was done using FTIR, UV-Vis DR, 13 C CP/MAS NMR, AAS and luminescence spectroscopies, TGA, single-point BET surface area analysis, SEM and ESR techniques. Figure 4.2 shows the general route to immobilize metalloporphyrin in polymer [75]. 66 Prearrangement Solvent MAA Fe-porphyrin Cross linker Polymerization, T = 60 °C for 24h + initiator Fe-porphyrin suported on Polymethacrylic acid (PMAA) Figure 4.2 Schematic representation of the procedure of synthesis of composite Fe-porphyrin-polymethacrylic acid. Methacrylic acid (MAA) monomers assemble with the Fe-porphyrin, followed by cross-linking polymerization [75] 4.3 Results and Discussion 4.3.2 Characterization of Polymethacrylic acid (PMAA) Polymethacrylic acid (PMAA) was prepared following the procedure of Tamayo et al.[74]. The polymer was prepared by polymerizing functional monomer, crosslinker and initiator at 60 °C for 24 hours. The white powders obtained have been characterized using FTIR, TGA, luminescence spectroscopy and NMR. 13 C CP/MAS 67 Figure 4.3 shows the IR spectrum of the as-synthesized PMAA described previously in section 4.2. Signals observed at 3441, 1724, and 1157 cm-1 are assigned to O-H bond, C=O bond and C-O bond, respectively [76]. These peaks indicate that 4000 2000 1157 1724 3441 1458 2955 Transmittance / a.u the polymethacrylic acid have been successfully prepared. 1500 1000 500 Wavenumber / cm-1 Figure 4.3 FTIR spectrum of as-synthesized polymethacrylic acid (PMAA) The results of the thermal degradation of PMAA in flowing nitrogen (Figure 4.4) shows that the first endothermic weight loss below 150 °C and in 150 – 250 °C temperature range is due to the release of adsorbed water through the formation of intra- and inter- molecular anhydride links and also to the decarboxylation of a fraction of the –COOH groups by which CO2 is formed. In the second degradation stage, the polymer decomposes with the elimination of CO and CO2 by way of abundant backbone scission and formation of a small concentration of unsaturation [77]. The luminescence emission spectrum of PMAA under excitation at is shown in Figure 4.5. The polymer exhibits an emission band at 574 nm. 330 nm 68 100 90 80 70 Weight / % 60 50 40 30 20 10 0 300 600 900 o Temperature / C Figure 4.4 TGA thermogram of as-synthesized polymethacrylic acid (PMAA) Intensity / a. u 574 333 300 400 500 600 700 Wavelength / nm Figure 4.5 The luminescence exitation and emission spectra of as-synthesized polymethacrylic acid (PMAA) (Ȝex = 333 nm, Ȝem = 574 nm) 69 C * Impurities CH3 CH2 COOH * * * 200 * 150 100 50 0 Chemical shift / ppm 13 Figure 4.6 C CP/MAS NMR spectrum of as-synthesized polymethacrylic acid (PMAA) The 13 C CP/MAS NMR spectrum of as-synthesized PMAA is shown in Figure 4.6. The spectrum shows four chemical shifts due to the presence of four different types of carbon and the assignment of each peak is given in Table 4.1. Table 4.1: Assignment of 13C CP/MAS NMR spectrum of PMAA [78] Types of C Chemical shift, į (ppm) -CH3 16.8 C 43.4 =CH2 60.8 -COOH 173.6 70 4.3.3 Characterization of Fe-TPyP/PMAA The Fe-TPyP catalysts immobilized in polymethacrylic acid were prepared following the procedure for the preparation of polymethacrylic acid, by addition of Fe-TPyP during the polymerization process. The products were characterized using FTIR, UV-Vis DR, ESR, Luminescence and 13 C CP/MAS NMR spectroscopies, AAS, single-point BET surface area analysis, TGA and SEM techniques. Figure 4.7 above shows the IR spectra of Fe-TPyP complexes, as-synthesized polymethacrylic acid and the polymethacrylic acid supported Fe-TPyP complexes. Comparison of the peaks at 1724 cm-1 (C=O), 1636 cm-1 (C=C) and 1452 cm-1 (C-C) in the spectrum of polymethacrylic acid supported Fe-TPyP complex with those of the as-synthesized polymethacrylic acid at 1728 cm-1 (C=O) and 1159 cm-1 (C-H) clearly indicate that Fe-TPyP has been successfully anchored on the polymer. Furthermore, the IR peaks observed in the spectrum of PMAA at 3441, 1724, and 1157 cm-1, assigned to O-H bond, C=O bond and C-O bond [76], respectively, were shifted to higher values in the spectrum of the polymethacrylic acid supported Fe-TPyP complex. The shifts to higher wavenumbers are consistent with the expected shift when Fe-TPyP is immobilized in the polymer. This shows some chemical interaction occurs between PMAA and Fe-TPyP. The PMAA supported Fe-TPyP has been characterized by UV-Vis DR spectroscopy and the spectra of Fe-TPyP and Fe-TPyP/PMAA are given in Figure 4.8. The highly conjugated porphyrin macrocycle shows an intense absorption at around 418 nm (the Soret Band) as well as several weaker absorptions (Q bands) at higher wavelengths (500 to 700 nm). On the other hand, Fe-TPyP exhibts a broad single band at 418 nm and three typical bands attributed to high spin Fe(III)porphyrin species in the region 500 – 700 nm (bands at 517, 590 and 644 nm). The results of the UV-Vis DR analysis have shown that the bands at 589 nm in the spectrum of Fe-TPyP/PMAA corresponds with the axial coordination of the Fe-TPyP to OH-containing PMAA [33]. 71 878 724 1464 3413 3083 1630 (a) 3441 1458 Transmittance / a.u 1591 (b) 4000 2000 1049 1159 1452 1728 3452 1636 1157 1724 (c) 1500 1000 500 Wavenumber / cm-1 Figure 4.7 FTIR spectra of (a) Fe-TPyP complexes, (b) as-synthesized PMAA and (c) Fe-TPyP/PMAA 72 510 585 644 424 K-M / a. u 418 (a) 517 400 590 500 644 600 (b) 700 Wavelength / nm Figure 4.8 UV-Vis DR spectra of (a) Fe-TPyP and (b) Fe-TPyP/ PMAA Figure 4.9 shows the UV-Vis DR spectra of Fe-TPyP/PMAA with different amounts of Fe-TPyP loading. The Soret band at around 417 nm shows an increase of the intensity with the increase in the amount of Fe-TPyP loading. Figure 4.10 shows the ESR spectra of Fe-TPyP and Fe-TPyP/PMAA (containing 50 and 100 µmol Fe-TPyP). The signal at g = 2.00056 or g = 2.0011 for Fe-TPyP/PMAA with Fe-TPyP = 100 and 50 ȝmol respectively, are attributed to high-spin Fe(III)-porphyrin. From this spectra, is shown that there shift toward a higher g-value confirming the interaction between Fe-TPyP and the polymer matrix. With increasing amount of Fe-TPyP loading from 50–100 ȝmol, intensity of the signal at g ~ 2.00 was also found to be correspondingly higher. 73 K-M / a. u Fe-TPyP=100 ȝmol Fe-TPyP=50 ȝmol Fe-TPyP=25 ȝmol Fe-TPyP=10 ȝmol Fe-TPyP=5 ȝmol 400 500 600 700 800 Wavelength / nm Figure 4.9 UV-Vis DR spectra of Fe-TPyP/PMAA with various of amount of Fe-TPyP loading 74 Intensity / a. u Fe-TPyP Fe-TPyP = 100ȝmol Fe-TPyP = 50ȝmol 100 200 300 400 500 B / mT Figure 4.10 ESR spectra of Fe-TPyP and Fe-TPyP/PMAA (containing 50 and 100 µmol Fe-TPyP) Furthermore, in order to determine the binding ability of the Fe-TpyP complex in the polymer support, the intensity of the spectra was measured. Tong et al. [45] have also used this technique to evaluate the binding abilities of the polymer to histamine. Measurement of the luminescence intensities of the polymer support (PSSS-Po) in the absence and in the presence of quencher (azulene) has been shown to confirm the occurrence of quenching of the support by the quencher [65]. 75 The luminescence emission spectra of the PMAA, Fe-TPyP and FeTPyP/PMAA are presented in Figure 4.11. As can be seen in this figure, the PMAA and Fe-TPyP exhibit the emission bands at 574 nm and 556 nm, respectively. More significantly, the quenching effect of the Fe-TPyP luminescence caused a decrease of the intensity of the band at around 572 nm. Figure 4.12 show the luminescence emission spectrum of Fe-TPyP/PMAA with different amount of Fe-TPyP loading. All the spectra exhibit bands at around 572 nm and two additional bands at range 650 – 750 nm. It can be seen that the luminescence intensity of Fe-TPyP/PMAA at 572 nm and 650-750 nm decreases with increasing amount of Fe-TPyP loading. The efficiency of the energy transferred from PMAA to Fe-TPyP (Ȥ) was calculated using Equation 4.1 and the experimental data. For example, under the experimental condition (amount of Fe-TPyP loading = 100 ȝmol), the value of Ȥ was found to be 0.373 (I0 = 552 and I = 206, at Ȝ = 572 nm). This means that 37.3% of the energy absorbed by PMAA is transferred to Fe-TPyP and this indicates that PMAA can act as an efficient energy donor [65]. Intensity / a. u 574 572 (a) (b) 556 (c) 400 500 600 700 800 Wavelength / nm Figure 4.11 The luminescence emission spectra of (a) as-synthesized PMAA, (b) Fe-TPyP/PMAA and (c) Fe-TPyP (Ȝex = 333 nm) 76 Fe-TPyP=0µmol Intensity / a. u Fe-TPyP=5µmol Fe-TPyP=10µmol Fe-TPyP=25µmol Fe-TPyP=50µmol Fe-TPyP=100µmol 400 500 600 700 800 Wavelength / nm Figure 4.12 The luminescence emission spectra of as-synthesized PMAA and Fe-TPyP/PMAA with different of amount of Fe-TPyP loading (Ȝex = 333 nm) 77 The quenching effect can be described by the Stern-Volmer kinetics equation expressed as a dependence of the ratio of the luminescence intensities (I0/I) of PMAA on the amount of the Fe-TPyP loading (Figure 4.13). It is shown that the luminescence intensity of the luminescent Fe-TPyP/PMAA decreases when PMAA bound with Fe-TPyP. The considerable static interaction between PMAA and Fe-TPyP exists in all I0/I values. The results confirm that the Fe-TPyP on PMAA is engaged in high-affinity binding. 3 = 0.0162x++1.1895 1.1895 y =y 0.0162x 2 R2R= =0.9431 0.9431 2.5 I0/I I 0 /I 2 1.5 1 0.5 0 0 20 40 60 80 100 120 Amount of Fe-TPyP Loading / ȝmol Amount of Fe-TPyP Loading / µmol Figure 4.13 The Stern-Volmer kinetics: the dependence of the ratio of the luminescence intensities (I0/I) on the Fe-TPyP concentration The 13 C CP/MAS NMR spectra of Fe-TPyP complexes, as-synthesized PMAA and Fe-TPyP/PMAA are shown in Figure 4.14. The effects of encapsulation are appreciable for the lineshape of the carboxyl carbon (-COOH) of PMAA. The shift of the lineshape of the carboxyl carbon of PMAA to higher magnetic field may be attributed to the formation of hydrogen bonding between unpaired electron of –N= in Fe-TPyP and –COOH group of PMAA. To examine fully the lineshape of the carboxyl carbon of PMAA in the Fe-TPyP/PMAA, we have shown the expansion of the carboxyl region in Figure 4.14 and the assigment of the 13C the chemical shifts in Table 4.2. 78 * Impurities Relative Intensity / a. u * * * * * * * * * * * * * * * * (a) * (b) * (c) * (d) C CH2 COOH * * 200 CH3 * (e) * 150 100 50 0 Chemical shift / ppm Figure 4.14 13 C CP/MAS spectra of Fe-TPyP/PMAA with various of amount of Fe-TPyP loading (a) 100 ȝmol, (b) 50 ȝmol, (c) 25 ȝmol, (d) 5 ȝmol and (e) as-synthesized PMAA 79 Table 4.2 : Assignment of chemical shifts of 13 C CP/MAS NMR spectra of as-synthesized PMAA and Fe-TPyP/PMAA with various of amount of Fe-TPyP loading Chemical shift (ppm) Sample CH3 C CH2 COOH Polymethacrylicacid (PMAA) 16.8 43.4 60.8 173.6 Fe-TPyP/PMAA (Fe-TPyP= 5ȝmol) 17.1 44.2 61.3 172.9 Fe-TPyP/PMAA (Fe-TPyP= 25ȝmol) 16.0 43.1 60.7 175.3 Fe-TPyP/PMAA (Fe-TPyP= 50ȝmol) 15.3 42.9 60.0 175.9 Fe-TPyP/PMAA (Fe-TPyP=100ȝmol) 13.6 40.5 58.2 176.0 The elemental analyses of the Fe-TPyP/PMAA samples were performed by means of AAS. Table 4.3 summarized the values obtained from AAS measurements of the iron content in the polymer matrix. It is surprising that the values found are below the expected values of the Fe loaded in the samples. The difference is probably due to leaching of Fe-TPyP into solution during the encapsulation procedure. However, the table clearly demonstrates the increase of the iron content with increasing Fe-TPyP loading in the samples. Table 4.3: Iron content (%Fe) of Fe-TPyP/PMAA with different amount of Fe-TPyP loading determined by AAS Sample Fe (%) Fe-TPyP/PMAA (Fe-TPyP= 100 ȝmol) 0.0185 Fe-TPyP/PMAA (Fe-TPyP= 50ȝmol) 0.0110 Fe-TPyP/PMAA (Fe-TPyP= 25 ȝmol) 0.0073 Fe-TPyP/PMAA (Fe-TPyP= 10 ȝmol) 0.0059 Fe-TPyP/PMAA (Fe-TPyP= 5 ȝmol) 0.0043 80 The thermal degradation behavior of as-synthesized PMAA, Fe-TPyP/PMAA and Fe-TPyP complexes were studied in the range 50 – 900 °C. Figure 4.15 shows two main stages of PMAA degradation with maximum decomposition rates at 240 and 380 °C. The first stage of decomposition is due to the weight loss of adsorbed water and anhydride formation reaction. In the second degradation stage, the polymer decomposes with the elimination of CO and CO2 by way of abundant backbone scission and formation of a small concentration of unsaturation. TGA results of Fe-TPyP show three weight loss regions below 150 °C, 450-500 °C and 530-600 °C. In first stage, endothermic weight losses were observed below 150 °C, which can be reasonably assigned to the desorption of water. Further, the two significant weight losses within 450-500 °C and 530-600 °C are due to the decomposition or burning of iron-porphyrin. 100 90 80 70 Weight / % 60 50 (a) 40 30 (b) 20 (c) 10 0 300 600 900 Temperature / oC Figure 4.15 TGA thermograms of (a) Fe-TPyP complexes, (b) Fe-TPyP/PMAA and (c) as-synthesized polymethacrylic acid (PMAA) 81 The TGA profile of Fe-TPyP/PMAA show three stages of decomposition within intervals 50–150 °C, 150–280 °C and 280– 450 °C. It can be seen from Figure 4.16 that the first peak on the curve of Fe-TPyP/PMAA represents evolution of water molecules bound to Fe-TPyP and anhydride formation of uncomplexed carboxylate groups of PMAA. The second peak on the curve is broad and likely represents overlapping of two processes: the release of CO2 and CO of carboxylate groups of PMAA and initial decomposition of porphyrin. The shift of the second peak of Fe-TPyP/PMAA decomposition to a higher temperature range in comparison with PMAA may be caused by dissociation of hydrogen bonds between functional groups of PMAA with functional groups of Fe-TPyP complex. The shift of the second stage of Fe-TPyP decomposition is not higher than the temperature range of Fe-TPyP decomposition, obviosly because amount of Fe-TPyP loading in the polymer matrix is very small. The third degradation stage is similar to as-synthesized PMAA, where unsaturated products and some CO2 and CO are released. Table 4.3 shows the surface properties of PMAA-supported Fe-TPyP catalysts determined from surface area analysis. A decrease in the surface area was observed as the amount of iron loading onto the polymer increased up to 100 µmol, which might be due to the blocking of pore of the support as well as due to the steric hindrance [79]. Table 4.3 : Surface properties of PMAA-supported Fe-TPyP with different amount of Fe-TPyP loading Samples Surface area (m2g-1) PMAA 127 Fe-TPyP/PMAA (Fe-TPyP= 5 ȝmol) 112 Fe-TPyP/PMAA (Fe-TPyP= 10 ȝmol) 98 Fe-TPyP/PMAA (Fe-TPyP= 25 ȝmol) 64 Fe-TPyP/PMAA (Fe-TPyP= 50 ȝmol) 48 Fe-TPyP/PMAA (Fe-TPyP= 100 ȝmol) 23 82 CHAPTER 5 SINGLE-STEP SYNTHESIS OF PHENOL FROM BENZENE OVER Fe-TPyP/Al-MCM-41 AND Fe-TPyP/PMAA CATALYSTS 5.1 Reaction Mechanism of Benzene Oxidation to Phenol Oxidation of benzene to phenol represents a critical test for the reactivity of transition metalloporphyrin in catalytic oxidations using hydrogen peroxide as oxidant. It has been demonstrated that a least five products of the benzene oxidation may be resulted (Scheme 5.1). The reaction mechanism for the oxidation of aromatic compounds employing transition metalloporphyrin has been previously studied [80]. The reaction path proposed for the present study involves a first stage, where the interaction of ironporphyrin supported catalyst with hydrogen peroxide yields the P-Fe-OOH species, via redox mechanism. Iron porphyrin peroxide further converted to iron-oxo species (P-Fe(V)=O) as shown in Scheme 5.2, which are similar to the hypervalent species of iron(V)-oxo observed in cytochrome P-450. This iron-oxo species is active enough to insert its “oxygen” atom into the C-H bond leading to hydroxylation product, therefore displays oxo chemistry feature. 83 OH OH O HO • + OH H2O O OH OH OH OH Scheme 5.1 The probable products of benzene oxidation (phenol, hydroquinone, catechol, resorcinol and benzoquinone) For the oxidation of benzene, the iron-oxo species attack the aromatic ring to form the benzyl radical, and the so-called oxygen rebound mechanism to form the phenol. For the formation of quinines, the benzyl radical is converted into the phenyl radical by a second attack of iron-oxo species. From phenyl radical, hydroquinone could be formed together with iron(III)-porphyrin. 5.2 The Single-Step Synthesis of Phenol from Benzene The catalytic experiments were performed in a batch reactor with continuous stirring in the liquid-phase at 70 °C. Benzene was used without further purification. A typical oxidation procedure is as follows: 2 mL of benzene (22.5 mmol), 50 mg of catalysts, and hydrogen peroxide (1 mL) were mixed together and stirred. The liquidphase reactor was a 50 mL two-necked round bottle flask equipped with a condenser. 84 For optimization of the catalysts, the catalytic activity of the various catalysts was compared after of 1, 3, 5 and 20 hours of reaction. The activities were characterized by the yield of phenol in the oxidation of benzene. To investigate the effect of temperature, the reaction was repeated and carried out at ambient temperature (30 oC), 70 oC and 100 oC. For the effect of solvent on catalytic activity, the reaction was carried out in different solvent, namely, methanol and acetic acid and solvent-free. The solid catalysts were separated from the mixture by centrifugation. H - OH (Z) O FeIII.P + (E) OH OH P.FeV 4b O 5 OH OH P.FeIII OH OH H O O H 4a 1 H (Z) FeIII.P O + (Z) H2O H - P.FeIII O O OH 2 3 P.FeV O OH Schema 5.2 Proposed reaction path for the oxidation of benzene H 85 5.3 Analysis of Reaction Products The resulting products were analyzed periodically using gas chromatography (GC). Gas chromatograph-mass spectroscopy (GC-MS) and high performance liquid chromatograph (HPLC) was also used to verify the resulting products. All the products were analyzed using GC to determine the amount of product while the components of the products were identified by using GC-MSD. 5.3.1 Gas Chromatography (GC) A sample of the mixture to be separated is introduced into this gas stream just before it encounters the stationary phase; the components are separated by elution and detected as they emerge in the gas at the other end of the column. They are distinguished by the different times which the take to pass through the column- the reaction times [81]. The basic concept of such an instrument has remained unchanged since the first one was built, although there has been much refinement and an amazing improvement in performance. The main components are shown in Figure 5.1. Experimental procedure: GC (Hewlett Packard 5890 GC Series II) was used to identify the reaction product equipped with a flame ionization detector (FID) and a polar column (carbowax). The oven temperature was programmed according to the conditions tabulated in Table 5.1. 86 Figure 5.1 Block diagram of a gas chromatograph Table 5.1 : GC-FID oven-programmed set up for identifying phenol 5.3.2 GC Parameter Temperature / Time Oven Temperature 50 oC Initial Temperature 50 oC Initial Time 5 min Rate 10 oC/min Final Temperature 200 oC Hold Time 5 min Gas Chromatography – Mass Spectrometry Analysis (GC-MS) Mass spectrometers, in their simplest forms, are designed to perform three basic functions. These are (1) to vaporize compounds of widely varying volatility,(2) to produce ions form the neutral molecules in the gas phase, and (3) to separate ions according to their mass-to-charge ratios (m/z), detect, and record them [82]. Any device featuring electrical detection and having the ability to separate gaseous positive to as a mass spectrometer, whereas a mass spectrograph is an instrument in which the focused ion beams are recorded on photographic plate. 87 The purpose of the mass spectrometer part of a GC-MS system is to provide some definite information about the compounds as they elute from the gas chromatograph, in general terms, the GC-MS has to perform one of two tasks: either identification of unknown compounds or the detection of a known compound that may or may not be present. The mass spectrometer provides information necessary to fulfill these functions. The techniques of gas chromatography and mass spectrometry, both ideally suited for the analysis of complex mixtures, share many common factors. The principal requirement which dictates the method of coupling the gas chromatograph to the mass spectrometer is the pressure at which the ion source must operate [83]. Experimental procedure: GC-MS (Agilent 6890N-5973 Network Mass Selective Detector) is equipped with HP-5MS column (30m x 0.251 mm x 0.25 µm), diffusion pump and turbo molecular pump. Sample was analyzed on splitless method with helium (He) as the carrier gas. 0.2 µL of those samples were injected to GC using 10 µL syringes at initial temperature 60 oC without hold time, with rate 15 oC/min until 250 oC, and hold 2 minutes. 5.3.3 High Performance Liquid Chromatography (HPLC) High performance liquid chromatography (HPLC) is a technique that has arisen from the application to liquid chromatography (LC) of theories and instrumentation that were originally developed for gas chromatography (GC). It was known from gas chromatographic theory that efficiency could be improved if the particle size of the stationary phase materials used in LC could be reduced [84]. High performance liquid chromatography arose gradually in the late as these high efficiency materials were produced, and as improvements in instrumentation allowed 88 the full potential of these materials to be realized. As HPLC has developed, the particle size of the stationary phase materials used in LC has become progressively smaller. The stationary phases used today are called micro particulate column packing and are commonly uniform, porous silica particles, with spherical or irregular shape, and nominal diameters of 10, 5 or 3 µm. In analytical HPLC the mobile phase is normally pumped through the column at a flow rate of 1 – 5 cm3 min-1. The mobile phase in HPLC may be water, organic solvents or buffers either on their own or mixed with one another. Experimental procedure: The products were analyzed by HPLC (Perkin Elmer Series 200) using a Water system equipped with two Series 200 LC Pump and a Series 200 UV.Vis Detector. A methanol / water gradient, starting at 70 / 30 (v/v) and reaching 100% methanol within 15 minutes was used for elution. The samples were prepared by solubilization in methanol /water 70 / 30 (v/v). A 0.1 mL sample was collected over the needle and injected into the HPLC equipment. Their UV-Vis spectra were recorded and compared with authentic samples The results of the experiments are reported in terms of total conversion, X and the product selectivity, S, where % conversion and % selectivity are defined as follows: Conversion, X (%) = Amount of benzene reacted Amount of benzene input Amount of phenol resulting Selectivity, S, (%) = Amount of total product x 100% x 100% 89 5.4 Results and Discussion 5.4.1 Catalytic Activity The single-step synthesis of phenol from benzene over Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA has been investigated. This single-step benzene oxidation with hydrogen peroxide can be considered as a potential commercial alternative to the broadly practiced three-step synthesis route involving cumene as an intermediate [85]. However, phenol is oxidized more easily than benzene and thus, many byproducts like hydroquinone and benzoquinone may be obtained In the present study, the main product of the liquid-phase oxidation of benzene over the various catalysts is phenol and the by-product is hydroquinone. The turnover number (TON), defined as the molar ratio of phenol to the loaded Fe for the reaction with Fe-TPyP/PMAA, was higher than that of Fe-TPyP/Al-MCM-41 (Table 5.2). If the surface area of the catalysts is taken into consideration, the TON per surface area for the reaction with Fe-TPyP/PMAA becomes much higher compared to that of Fe-TPyP/Al-MCM-41. It strongly confirms that the direct oxidation of benzene to phenol is efficiently catalyzed by Fe-TPyP/PMAA. Table 5.2 : Catalytic activity of single-step synthesis of phenol from benzenea a Catalyst Fe/ ȝmol Surface area of catalyst / m2 g-1 Phenol yield / ȝmol TON per Fe Fe-TPyP Al-MCM-41 Fe-TPyP/Al-MCM-41 (Fe-TPyP =100ȝmol) Fe-TPyP/PMAA (Fe-TPyP = 100ȝmol) Fe-TPyP/Al-MCM-41 (Fe-TPyP = 100ȝmol)b Fe-TPyP/PMAA (Fe-TPyP = 100ȝmol)b 0.38 813 383 0.18 10.45 17.87 47 0.020 48 40.23 2011 - - 15.97 - - - 34.58 - All reaction were carried out at 70 oC for 20 hours with benzene (2 mL), 30% H2O2 (1 mL), and catalyst (50mg) with vigorous stirring. bThe reaction was performed after washing and drying of the catalyst until third times. 90 Figure 5.2 shows the activities of the iron-porphyrins supported on molecular sieve Al-MCM-41 and PMAA as catalysts for the single-step synthesis of phenol from benzene. Unexpectedly, the reaction system containing unsupported Fe-TPyP catalyst (homogeneous system) is much less reactive than Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA systems. 50 Phenol Yield / ȝmol 45 40 35 30 25 20 15 10 5 0 Al-MCM-41 FeTPyP FeTPyP/AlMCM-41 FeTPyP/PMAA Catalysts Figure 5.2 Effect of the different catalyst on the phenol yield for 20 hours reaction The higher activity of the later systems possibly arises from Fe-TPyP coordination to the molecular sieve or polymer, which renders it more resistant to oxidative self-destruction. From the results, it is evident that Fe-TPyP/PMAA catalyst gives higher phenol yield than Fe-TPyP/Al-MCM-41. This may be due to the different coordination modes of Fe-TPyP complexes when composite with molecular sieve and polymethacrylic acid (PMAA). 91 5.4.2 The Selectivity of Products Figure 5.3 shows the product selectivity of Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA catalysts toward the oxidation of benzene. GC and GC-MS analyses indicate that phenol was the only product of the oxidation of benzene over Fe-TPyP/Al-MCM-41 and other probable by-products, such as hydroquinone, catechol and benzoquinone were not detected. It is known that the Al-MCM-41 can act as alkylation catalyst due to the presence of the Brönsted and Lewis acidity. Since cumene is an alkylation product of benzene, was not produced in the reaction containing Fe-TPyP/Al-MCM-41 composite, it implies that the acid properties of Al-MCM-41 were suppressed by the presence of Fe-TPyP. The lack of the acid properties may be attributed to the interaction between the Brönsted acid site in Al-MCM-41 and the lone-pair electrons of Fe-TPyP. On the other hand, the product of Fe-TPyP/PMAA toward the production of phenol was ca. 75%, since ca. 25% hydroquinone was also produced. The structure of the supports seems to have a strong influence on the selectivity of the catalysts toward phenol. The results suggest that the rigid ordered structure of Fe-TPyP/ Al-MCM-41 may have contributed to the very high selectivity for phenol. 120 Fe-TPyP-Al-MCM-41 Selectivity / % 100 Fe-TPyP-PMAA 80 60 40 20 0 Phenol Hydroquinone Reaction Products Figure 5.3 The product selectivity of single-step synthesis of phenol in aqueous hydrogen peroxide using Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA catalysts 92 The results (Figure 5.3) also suggest that the selective synthesis of phenol from benzene requires Fe-TPyP in the limited pore channels of Al-MCM-41 to suppress the formation of bulkier hydroquinone derivatives. Figure 5.4 shows that the percentage (%) conversion of benzene to phenol at 70 °C over Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA. The results indicate that the percentage conversion of benzene over Fe-TPyP/PMAA is higher than that of Fe-TPyP/Al-MCM-41. In this case, the hydrophobic nature of Fe-TPyP/PMAA provides for the higher activity and hence 100 % conversion of benzene to phenol Conversion / % 120 100 80 60 40 20 0 Fe-TPyP/AlMCM-41-100 Fe-TPyP/AlMCM-41-50 Fe-TPyP/ PMAA-100 Fe-TPyP/ PMAA-50 Catalyst Figure 5.4 The percentage conversion of benzene to phenol in aqueous hydrogen peroxide using Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA catalysts 5.4.3 Regenerability of The Catalysts The regenerability of the catalysts was studied at 70 °C for 3 cycles. The Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA could be easily recovered from the reaction products and regenerated by washing and drying in air. They were reused in fresh reactant mixtures. The selectivity of encapsulated Fe-TPyP in Al-MCM-41 did not change after three times of reusing and showed 100% activities (Table 5.3), but the catalytic activities of Fe-TPyP/PMAA decreased to 75% after three cycles. 93 A decrease of the phenol yields between the initial reactions and the first reusing is observed for benzene oxidation catalyzed by Fe-TPyP/Al-MCM41 and Fe-TPyP/PMAA, giving an indication that the extraction procedure-using methanol deactivates the catalysts through leaching of the Fe-TPyP complex into solution. This was also observed for Fe-TPyP/PMAA, which presented a decrease in its activity from the fresh reaction compared with the recycle. A decrease in the activity of the catalyst from the first to the third recycle could be attributed to the leaching or decomposition of Fe-TPyP complex under the present reaction condition. The hydrogen peroxide as oxidant may have contributed to the leaching of the active sites of the catalysts. Table 5.3 : The catalytic activity of Fe-TPyP supported in Al-MCM-41 and polymethacrylic acid (PMAA) during the recycling in single-step synthesis of phenol from benzene Benzene Oxidation Number of Catalyst Selectivity (%) ȝmol) Recycle Fresh Phenol Yield 17.87 100 1 15.97 100 2 16.32 100 3 15.66 100 40.23 75 1 34.58 70 2 32.00 75 3 31.70 75 Fresh Fe-TPyP/Al-MCM-41-100 Fe-TPyP/PMAA-100 Although Fe-TPyP/PMAA showed higher activity compared to Fe-TPyP/ Al-MCM-41, the selectivity and the regenerability of Fe-TPyP/PMAA is not as good as that of Fe-TPyP/Al-MCM-41. Based on the discussion, one considers that the hydrophobic nature of Fe-TPyP/PMAA may account for the high catalytic activity, while the ordered structure of Fe-TPyP/Al-MCM-41 provides for a high selectivity in oxidation of benzene with aqueous hydrogen peroxide. 94 5.4.4 Optimization of Catalyst Optimization of the catalyst activity of the supported Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA catalyst was further explored by investigating the effect of reaction time, solvent, the loading amount of Fe-TPyP and reaction temperature. (a) Effect of Reaction Time The effect of reaction time on single-step synthesis of phenol from benzene with hydrogen peroxide in the presence of various catalysts is shown in Figure 5.5. It is seen that the step increase in the phenol yield occurs beginning at 1 hour reaction time over both catalysts. After which, it can be observed that the phenol yield increased almost linearly with the increase in the reaction time up to around 20 hours. (b) Effect of Solvent Figure 5.6 shows the effect of different solvent on the phenol yield in the presence Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA as catalysts at 70 °C for 20 hours of reaction time. The highest phenol yield was obtained when the reaction is performed without solvent. With solvent, the activity of the catalysts may have been hindered, due to leaching of the iron-porphyrin complexes into solution during the reaction process. For reaction that was carried out in a solvent, evidently, the more polar acetic acid is better solvent than methanol for the synthesis of phenol. The yield of phenol was found to increase with increasing polarity of the solvent. 95 45 Phenol Yield / ȝmol 40 35 30 25 20 15 Fe-TPyP-Al-MCM-41 (Fe-TPyP = 100 ȝmol) Fe-TPyP-Al-MCM-41 (Fe-TPyP = 50 ȝmol) Fe-TPyP-PMAA (Fe-TPyP = 100 ȝmol) Fe-TPyP-PMAA (Fe-TPyP = 50 ȝmol) 10 5 0 0 5 10 15 20 25 Reaction Time / h Figure 5.5 Effect of reaction time on the phenol yield in the single-step synthesis of phenol from benzene over Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA catalysts 45 Fe-TPyP/Al-MCM-41 Phenol Yield / µmol Phenol Yield / ȝmol 40 Fe-TPyP/PMAA 35 30 25 20 15 10 5 0 Solvent-Free Acetic Acid Methanol Solvent Solvent Figure 5.6 Effect of different solvent on the phenol yield using Fe-TPyP/ Al-MCM-41 and Fe-TPyP/PMAA as catalysts at 70 °C 96 (c) Effect of Loading Amount of Fe-TPyP It can be seen in Figure 5.7 that samples (Fe-TPyP/Al-MCM-41 or Fe-TPyP/PMAA) with the higher amount of Fe-TPyP loading give rise to higher yields of phenol at 70 °C after 20 hours of reaction. The results show that the product yield is greatly influenced and determined by the presence of Fe-TPyP as active sites. Furthermore, it obvious that the amount of Fe-TPyP sites plays an important role in the direct oxidation of benzene to phenol. Phenol Yield / ȝmol 45 40 Fe-TPyP-Al-MCM-41 35 Fe-TPyP-PMAA 30 25 20 15 10 5 0 50 100 Amount of Fe-TPyP loading / ȝmol Figure 5.7 Effect of amount of Fe-TPyP loading on the phenol yield in solvent- free at 70 °C for 20 hours reaction time over Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA catalysts. 97 (d) Effect of Reaction Temperature The effect of temperature on phenol yields was studied at room temperature and 70 °C over Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA as catalysts. The results are presented in Figure 5.8. Comparison of the results at room temperature and 70 °C illustrated that higher phenol yield was obtained for the latter than the former due to higher activity of hydrogen peroxide at room temperature. At a higher temperature of 100 °C over both catalysts, the phenol yield was found to be much less, which is probably because of coking. The higher temperature may have resulted in side reactions such as alkylation and oligomerization. The side products could have poisoned the active sites, blocked the pores and decreased the activity and selectivity of the catalysts. Phenol Yield / ȝmol 45 40 Fe-TPyP/Al-MCM-41 35 Fe-TPyP/PMAA 30 25 20 15 10 5 0 30 R.T 70 100 o Temperature / C Figure 5.8 Effect of reaction temperature on phenol yield in solvent-free for 20 hours reaction time over Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA catalysts. 98 CHAPTER 6 CONCLUSION AND RECOMMENDATION The encapsulation of bulky iron(III)-5,10,15,20-tetra-(4-pyridyl) porphyrin (Fe-TPyP) complexes within the ordered structure of mesoporous molecular sieve Al-MCM-41 (Fe-TPyP/Al-MCM-41) and polymethacrylic acid (Fe-TPyP/PMAA) as inorganic and organic supports, respectively, were successfully achieved by sequential synthesis of Fe-TPyP via treatment of FeCl3 with 5,10,15,20-tetra-(4pyridyl) porphyrin (TPyP) into the pores of Al-MCM-41 and polymerizing a monomer methacrylic acid (MAA) with a cross-linker around the Fe-TPyP complexes. The immobilized Fe-TPyP systems have demonstrated excellent activity and selectivity for the single-step synthesis of phenol from benzene. The physical properties of Fe-TPyP/Al-MCM-41 and Fe-TPyP/PMAA were investigated by means of several spectroscopy characterization techniques. The materials obtained were characterized by X-ray Diffraction (XRD), Fourier Transform Infrared (FTIR), Ultraviolet Visible Diffuse Reflectance (UV-Vis DR), Electron Spin Resonance (ESR), luminescence and 13 C CP/MAS NMR spectroscopies, Thermogravimetric Analysis (TGA) and wet chemical analysis. The powder XRD data confirmed that the ordered structure of mesoporous Al-MCM-41 remained intact after encapsulation process. Characterization of Fe-TPyP composites with Al-MCM-41 and PMAA using FTIR, UV-Vis DR and ESR analysis confirmed that the structure of Fe-TPyP in inorganic and polymer supports is similar with bare Fe-TPyP. The three peaks in the range of 500–700 and at 462 nm in the UV-Vis DR spectrum are observed in UV-Vis DR of Fe-TPyP/Al-MCM-41 which are typical of 99 high-spin Fe(III)-porphyrin and the axial electrostatic interaction between cationic Fe-TPyP and anionic Al-MCM-41. The band at 589 nm in the spectrum of Fe-TPyP/PMAA corresponds to the coordination of the Fe-TPyP OH-containing PMAA. The specific interaction of Fe-TPyP in Al-MCM-41 and/or PMAA was studied by ESR, 13 C CP/MAS NMR and luminescence spectroscopies. The ESR spectra of Fe-TPyP/PMAA and Fe-TPyP/Al-MCM-41 composites showed that there is a shift towards a higher g-value confirming the interaction between Fe-TPyP and the supports is occurred. By quenching of the luminescence spectra of Fe-TPyP/PMAA with difference in the concentration of Fe-TPyP, it is proven that there is interaction between Fe-TPyP and PMAA, presumably by the formation of hydrogen bonding between Fe-TPyP and the polymer. Further support of the interaction was obtained by 13 C CP/MAS NMR which shows that the peak of carboxyl of PMAA is shifted to high magnetic field. The change in the position of the carboxyl carbon may be attributed to the formation of hydrogen bonding between unpaired electron of –N= bond in Fe-TPyP and –COOH group of PMAA. Single-point BET surface area analysis was used to determine specific surface area of the composites. It is revealed that surface area of Fe-TPyP/ Al-MCM-41 composites is decreased with an increase in the loading amount of Fe-TPyP, suggesting the encapsulation of complex in the pores of Al-MCM-41 has been achieved. With mesoporous molecular sieve (Al-MCM-41) and the polymer (PMAA) as supports, the immobilized iron-porphyrin system has demonstrated excellent activity for the single-step synthesis of phenol from benzene under mild reaction conditions. The effect of reaction time, solvent, amount of Fe-TPyP loading, temperature and the performance of the recovered catalysts have been studied. The immobilized iron-porphyrin in PMAA (Fe-TPyP/PMAA) gives a higher activity compared with Fe-TPyP supported on Al-MCM-41 (Fe-TPyP/Al-MCM-41). However, the product selectivity of Fe-TPyP/PMAA is not as good as that of Fe-TPyP/Al-MCM-41. One considers that the hydrophobic nature of Fe-TPyP/PMAA may account for the high activity, and the ordered structure of 100 Fe-TPyP/Al-MCM-41 provides for the high selectivity in the single-step synthesis of phenol from benzene in the present study. In conclusion, the study presented in this thesis is sufficient to establish the remarkable selectivity of Fe-TPyP/Al-MCM-41 as catalyst in single-step synthesis of phenol from benzene. As a global guide for future actions, this work opens new perspectives for the use of a more hydrophobic ordered structure materials containing Fe-TPyP as a catalyst for synthesis of phenol from benzene. 101 REFERENCES 1. Sheldon, R.A. Metalloporphyrins in Catalytic Oxidations. New York: Marcel Dekker Inc. 1994, p. 13-14, 340-341. 2. Mayer, J. M. Hydrogen Atom Abstraction by Metal-oxo Complexes: Understanding the Analogy with Organic Radical Reactions. Accounts of Chemical Research, 1998. 8 (31): 441-450. 3. Iamamoto, Y., Ciuffi, K.J., Sacco, H.C., Iwamoto, L.S., Nascimento, O.R. and Prado, C.M.C. Characterization and Catalytic Activity of 2.6dichlorophenyl Substituted Iron(III) Porphyrin Supported on Silica Gel and Imidazole Propyl Gel. J. Molecular Catalysis A: Chemical, 1997. 116: 405420. 4. Groves, J.T. Key Elements of the Chemistry of Cytochrome P-450. The Oxygen Rebond Mechanism. Journal of Chemical Education, 1985. 62: 928931. 5. Prado-Manso, C..M.C., Vidoto, E.A., Vinhado, F.S., Sacco, H.C., Ciuffi, K.J., Martins, P.R., Ferreira, A.G., Smith, J.R.L., Nascimento, O.R. and Iamamoto, Y. Characterization and Catalytic Activity of Iron(III) mono(4methyl pyridyl)tris-(halophenyl) Porphyrins in Homogeneous and Heterogeneous Systems. J. Molecular Catalysis A: Chemical, 1999. 150: 251-266. 6. Rebelo, S.L.H., Simoes, M.M.Q., Neves, M.G.P.M.S. and Cavaleiro, J.A.S. Oxidation of Alkylaromatics with Hydrogen Peroxide Catalysed by Manganese(III) Porphyrins in the Presence of Ammonium Acetate. J. Molecular Catalysis A: Chemical, 2003. 201: 9-22. 7. Paul-Roth, C., Montigne, F.D., Rethore, G., Simonneaux, G., Gulea, M. and Masson, S. Cyclopropanation of Alkenes with Diisopropyl Diazomethylphosphonate Catalyzed by Ruthenium Porphyrin Complexes. J. Molecular Catalysis A: Chemical, 2003. 201: 79-91. 102 8. Terazono, Y. and Dolphin, D. Synthesis of the Hemin of β-tetrakis (trifluoromethyl)-meso-tetraphenylporphyrin and its Evaluation as a P-450 Mimic. Inorganica Chimica Acta, 2003. 346: 261-264. 9. Liu, J.Y., Li, X.F., Guo, Z.X. and Li, Y.Z. Comparative Study on HemeContaining Enzyme-like Catalytic Activities of Water-Soluble Metalloporphyrins. J. Molecular Catalysis A: Chemical, 2002. 179: 27-33. 10. Schiavon, M.A., Iamamoto, Y., Nascimento, O.R. and Assis, M.D. Catalytic activity of nitro- and carboxy-substituted iron porphyrins in hydrocarbon oxidation homogeneous solution and supported systems. J. Molecular Catalysis A: Chemical, 2001. 174: 213-222. 11. Chatterjee, D., and Mitra, A. Olefin Epoxidation Catalyzed by Schiff-base Complexes of Mn and Ni Heterogenised-Homogeneous Systems. J. Molecular Catalysis A: Chemical, 1999. 144: 363-367. 12. Drechsel, S. M., Kamirski, R. C. K., Nakagaki, S. and Wypych, F. Encapsulation of Fe(III) and Cu(II) Complexes in NaY Zeolite. J. Colloid and Interface Science, 2004. 277: 138-145. 13. Bonoldi, L., Busetto, C., Congiu, A., Marra, g., Ranghino, G., Salvalaggio, M., Spano, G. and Giamello, E. An ESR Study of Titanium-Silicalite in Presence of H2O2. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2002. 58: 1143-1154. 14. Skrobot, F.C., Valente, A.A., Neves, G., Rosa, I., Rocha, J. and Cavaleiro, J.A.S. Monoterpenes Oxidation in the Presence of Y-Zeolite-Entrapped Manganese(III) tetra (4-N-benzylpyridyl) porphyrin. J. Molecular Catalysis A: Chemical, 2003. 201: 211-222. 15. Li, Z., Xia, C-G. and Zhang, X-M. Preparation and Catalysis of DMY and MCM-41 Encapsulated Cationic Mn(III)-porphyrin Complex.. J. Molecular Catalysis A: Chemical, 2002. 185: 47-56. 16. Thomas, J.M. Molecular Sieves- the Chemistry of Crystalline Sponges. Nature, 1998. 368: 289-290. 17. Beck, J.S., Vartuli, J.C., Roth, W.J., Leonowicz, M.E., Kresge, C.T., Schmitt, K.D., Chu, C.T-W., Olson, D.H., Sheppard, Z.W., Mc Cullen, S.B., Higgins, J.B. and Schlenker, J.L. A New Family of Mesoporous Molecular Sieves Prepared with liquid Crystal Template. J. American Chemical Society, 1992. 114: 10834-10843. 103 18. Maschmeyer, T., Rey, F., Sankar, G and Thomas, J.M. Heterogeneous Catalyst obtained by Grafting Metallocene Complexes onto Mesoporous Silica. Nature, 1995. 378: 159-162. 19. Navijanti, V. and Hamdan, H. Sintesis Zeozim Cu (III)/Fe(III)-salen-AlMCM-41 dan Penggunaannya sebagai Mangkin bagi Tindak Balas Pempolimeran Bisfenol-A. Master Thesis. Universiti Teknologi Malaysia; 2002. 20. Liu, C.J., Yu, W., Li, S. G. and Che, C. M. Ruthenium Porphyrin Encapsulated in Modified Mesoporous Molecular Sieves MCM-41 for Alkene Oxidation. Chemical Modification, 1997. 387: 65-66. 21. Yoo, S-K., Han, J.H., Lee, S.J., Ryu, J.Y., Kim, C., Jin, S.W., Kim, Y.M. and Nam, W.W. Conversion of Olefins into trans-diols or trans-diol mono-ethers by using Iron(III)porphyrin Complex and H2O2. Inorganic Chemistry Communications, 2003. 6:1148-1151. 22. Gerhartz, W. Ullmann¶s Encyclopedia of Industrial Chemistry. Weinheim, Germany: VCH, 1985. 23. Juttu, G. Modified Microporous Aluminosilicates as Novel Solid Acid Catalysts. Ph.D. Thesis. University of Delaware; 2001. 24. Miyahara, T., Kanzaki, H., Hamada, R., Kuroiwa, S., Nishiyama, S. and Tsuruya, S. Liquid-phase Oxidation of Benzene to Phenol by CuO-Al2O3 Catalysts Prepared by co-precipitation Method. J. Molecular Catalysis A: Chemical, 2001. 176: 141-150. 25. Ivanov, A.A., Chernyacsky, V.S., Gross, M.J., Kharitonov, A.S. Uriarte, A.K. and Panov, G.I. Kinetics of Benzene to Phenol oxidation over Fe-ZSM-5 Catalyst. Applied Catalysis A: General, 2003. 249: 327-343. 26. Passoni, L.C., Luna, F.J., Wallau, M., Buffon, R. and Schuchardt, U. Heterogenization of H6PMo9V3O40 and Palladium Acetate in VPI-5 and MCM-41 and their use in the Catalytic Oxidation of Benzene to Phenol. J. Molecular Catalysis A: Chemical, 1998. 134: 229-235. 27. Passoni, L. C., Cruz, A. T., Buffon, R. and Schuchardt, U. Direct Selective Oxidation of Benzene to Phenol with Molecular Oxygen in the Presence of Palladium and Heteropolyacids. J. Molecular Catalysis A: Chemical, 1997. 120: 117-123. 104 28. Othani, T., Nishiyama, S., Tsuruya, S. and Masai, M. Liquid-Phase Benzene Oxidation to Phenol with Molecular Oxygen Catalyzed by Cu-Zeolites. J. Catalysis, 1995. 155: 158. 29. Okamura, J., Nishiyama, S., Tsuruya, S., and Masai, M. Formation of Cusupported Mesoporous Silicates and Aluminosilicates and Liquid-Phase Oxidation of Benzene Catalyzed by the Cu-mesoporous Silicates and Aluminosilicates. J. Molecular Catalysis A: Chemical, 1998. 135: 133. 30. Zhan, B. Z. Nanoscale Confined Biomimetic Catalysts: Synthesi,s Characterization and Catalysis. Ph.D. Thesis. Hong Kong University of Science and Technology; 1998. 31. Haber, J., Pamin, K. and Poltowicz, J. Cationic Metalloporphyrin and other Macrocyclic Compounds in Zeolite Matrix as Catalysts for Oxidation with Dioxigen. J. Molecular Catalysis A: Chemical, 2004. 224: 153-159. 32. Hartley, F. R. Supported Metal Complexes, In Catalysis by Metal Complexes. Dodrecht: D. Reidel Pub. 1985. p. 3, 34-36. 33. Rosa, I. L. V., Manso, C. M. C. ‘P., Serra, O. A. and Iamamoto, Y. Biomimetical Catalytic of Iron(III) Porphyrins Encapsulated in the Zeolite X. J. Molecular Catalysis A: Chemical, 2000. 160: 199-208. 34. Khan, T.A., and Hriljac, J.A. Hydrothermal Synthesis of Microporous Materials with the Direct Incorporation of Porphyrin Molecules. Inorganica Chimica Acta, 1999. 294: 179-182. 35. Parton, R., De Vos, D. and Jacobs, P.A. Enzymes Mimicking with Zeolites. In Derouane, E.G. (editor), Zeolite Microporous Solid: Synthesis, Structure, and Reactivity. Elsevier. 1998, p. 555-577. 36. Ganesan, R. and Viswanathan, B. Synthesis, Characterization and Catalytic Activity of ȝ-oxo Bridge Dinuclear iron 1,10 phenanthroline Complex Encapsulated in MCM-41. J. Molecular Catalysis A: Chemical, 2002. 181: 99-107. 37. Ernst, S. and Selle, M. Immobilization and Catalytic Properties of perfluorinated Ruthenium phthlocyanine Complexes in MCM-41-type Molecular Sieves. Microporous and Mesoporous Materials, 1999. 27: 355363. 38. Szostak, R. Molecular Sieves: Principle of Synthesis and Identification. Reinhold. 1989. p. 2-5. 105 39. Corma, A. From Microporous to Mesoporous Molecular Sieves Materials and their Use in Catalysis. Chemical Review, 1997. 97. 40. Clercg, B. D., Lefebvre, F. and Verpoort, F. Immobilization of Multifunctional Schiff Base Containing Ruthenium Complexes on MCM-41. Applied Catalysis, 2003. 247: 345-364. 41. Schufth, F. Ordered Mesoporous Materials-State of the Art and Prospects. International Zeolite Conference. Montpellier, France. 2001. 42. Sung-Suh, H. M. Photoionization of Porphyrins in Vesicles and Porous Inorganic Materials. Ph.D. Thesis. University of Houston; 1997. 43. Sawant, K. R. Imprinting the Surface of Mesoporous Aluminosilicates using Organic Structure-Directing Agents. Ph.D. Thesis. University of Delaware; 2004. 44. Kobayashi, S. and Nagayama, S. A. Microencapsulated Lewis Acid. A New Type of Polymer-Supported Lewis Acid Catalyst of Wide Utility in Organic Synthesis. J. American Chemical Society, 1998. 120: 2985-2986. 45. Tong A., Dong, H. and Li, L. Molecular Imprinting-based Fluorescent Chemosensor for Histamine Using Zinc(II)-Protoporphyrin as a Functional Monomer. Analytica Chimica Acta, 2002. 466: 31-37. 46. Haber, J., Klosowski, M. and Poltowicz, J. Co-oxidation of Styrene and isobutyraldehyde in The Presence of Polyaniline-supported Metalloporphyrin. J. Molecular Catalysis A: Chemical, 2003. 201: 167-168. 47. Leeder, S.M. Solid-Phase Media for Use in Heterogeneous Catalysis: A New Strategy for Differentiating Reactants Based on Sorption Differences. Ph.D. Thesis. University of North Carolina; 2004. 48. Hiemer, U., Klemm, E., Scheffler, F., Selvam, T., Schwieger, W. and Emig, G. Microreaction Engineering Studies of The Hydroxylation of Benzene with Nitrous Oxide. J. Chem. Engg., 2004. 101: 17-22. 49. Masumoto, Y-k, Hamada, R., Yokotu, K., Nishiyama, S. and Tsuruya, S. Liquid-Phase Oxidation of Benzene to Phenol by Vanadium Catalysts in Aqueous Solvent with High Acetic Acid Concentration. J. Molecular Catalysis A: Chemical, 2002. 184: 215-222. 50. Kubánek, P., Wichterlová, B. and Sobalik, Z. Nature of Active in the Oxidation of Benzene to Phenol with N2O over H-ZSM-5 with Low Fe Concentration. J. Catalysis, 2002. 211: 109-118. 106 51. Lemke, K., Ehrich, H., Lohse, U., Berndt, H. And Jáhnisch, K. Selective Hydroxylation of Benzene to Phenol over Supported Vanadium Oxide Catalysts. Applied Catalysis A: General, 2003. 243, 41-51. 52. Burch, R. and Howitt, C. Investigation of Zeolite Catalyst for the Direct Oxidation of Benzene to Phenol. Applied Catalysis A: General, 1993. 103: 135-162. 53. Zhang, W., Wang, J., Tanev, P. T. and Pinnaváia, T. J. Catalytic Hydroxylation of Benzene over Transition Metal Substituted Hexagonal Mesoporous Silicas. Chemical Communication, 1996. 8: 979-980. 54. Chen, Y-W. and Lu, Y-H. Characteristics of V-MCM-41 and its Catalytic Properties in Oxidation of Benzene. Ind. Eng. Chem. Res., 1999. 38: 18931903. 55. Passoni, L., Tadini, A., Cruz, Buffon, R. and Schuchardt, U. Direct Selective Oxidation of Benzene to Phenol using Molecular Oxygen in Presence of Palladium and Heteropolyacids. J. Molecular Catalyst A: Chemical, 1997. 120: 117-123. 56. George, W. O. and Mclntyre, P.S. Infrared Spectroscopy. Analytical Chemistry by Open Learning (ACOL), Chichester: John Wiley & Sons. 1987. p. 83-84. 57. Frei, R. W. and MacNeil, J.D. Diffuse Reflectance Spectroscopy in Environmental Problem-Solving. Cleveland, Ohio: CRC Press. 1973. 58. Sinlapadech, S. Photoionization Organic Molecules in MCM-41, Al-MCM-41 and Metal Contining Al-MCM-41 Mesoporous Materials. Ph. D. Thesis. University of Houston; 2003. 59. Fierro, J.L.G. Spectroscopy Analysis of Heterogeneous Catalysts 57 part A. Amsterdam: Elsevier Sc. 1990, p. A18, A28. 60. Ranby, B. and Rabek, J. F. ESR Spectroscopy, A Working Manual with Exercises. London: Harwood Academic Publisher, p. 1-3. 61. Skoog, D. A. Principles of Instrumental Analysis. 3 rd ed. Philadelphia, PA: Saunders, College Pub. 1985. p. 160-224. 62. Sequera, C. A. and Hudson, M. J. Multifunctional Mesoporous Inorganic Solids. Dordrecht: Kluwer Academic Publisher, p. 4-7. 107 63. Endud, S. Secondary Aluminated Mesoporous Molecular Sieve Al-MCM-41: Synthesis, Characterization and Catalytic Activity. Ph.D. Thesis. University Teknologi Malaysia; 2000. 64. Marfunin, A. S. Spectroscopy, Luminescence and Radiation Centers in Minerals. Berlin: Springer-Verlag. 1979. 65. Maria, N., Karewicz, A., Nikolai, L. and Guillet, J. E. Studies of the Antenna in Polymer Molecules. 29. Synthesis and Properties of Poly(Sodium Styrene aulfonate-co-(4-acryolyloxy phenyl)-10, 15, 20-tritolyporphyrin) in Aqueous Solution. Polymer, 2002. 43: 2003-2009. 66. Breitmaier, E. and Bauer, G. 13C NMR Spectroscopy, A Working Manual with Exercises. London: Harwood Academic Publishers. 1984. p.1-3. 67. Kim, J.M., Kwak, J.H., Jun, S. and Ryoo, R. Ion Exchange and Thermal Stability of MCM-41. Physical Chemistry, 1995. 99: 16742-16747. 68. Ren, T., Yan, L., Zhang, X. and Suo, J. Selective Oxidation of Benzene to Phenol with N2O by Unsupported and Supported FePO4 Catalysts. Applied Catalysis A: General, 2003. 244: 11-14. 69. Birnbaum, E. R., Lacheur, R. M. Le., Horton, A. C. and Tumas, W. Metalloporphyrin-Catalyzed Homogeneous Oxidation Supercritical Carbon Oxide. J. Molecular Catalyst A: Chemical, 1999. 139: 11-24. 70. Nakagaki, S., Mangrich, A. S., Xavier, C-R. and Machado, A. M. Synthesis of Metallotetraphenyl-Porphyrin in the Presence of Zeolite. Meeting of Brasilian Chemistry Society XVIII; 1995. 71. Chan, H. B. S., Budd, P. M. and Naylor, T. deV. Control of Mesostructured Silica Particle Morphology. J. Mater. Chem., 2001. 11: 951-957. 72. Ciuffi, K. J., Sacco, H. C., Valim, J. B., Manso, C. M. C. P., erra, O. A., Nascimento, O. R., Vidoto, E. A. and Iamamoto, Y. Polymeric OrganicInorganic Hybrid Material Containing Iron(III) porphyrin using Sol-gel Process. J. Non-Crystalline Solids, 1999. 247: 146-152. 73. Chang, Z., Liub, G. and Zhanga, Z. Effects of Electric Fields in Polymerization on Enthalpy of PMAA Anhydridization. Thermochimica Acta, 2004. 411: 95-100. 74. Tamayo, F.G., Casilas, J.L., and Esteban, A.M. Highly Selectivity FenorenImprinted Polymer with a Homogeneous Binding Site Distribution Prepared 108 by Precipitation Polymerization and its Application to be Clean up of Fenoren in Plant Sample. Analytica Chimica Acta, 2003. 482: 165-173. 75. Hentze, H. –P. and Antonieeti, M. Template Synthesis of Porous Organic Polymers. Current Opinion in Solid State and Materials Science, 2001. 5: 343-353. 76. Cárdenas, G., MuĖoz, C. and Carbacho, H. Thermal Properties and TGAFTIR Studies of Polyacrylic and Polymethacrylic Acid Doped with Metal Clusters. European Polymer Journal, 2000. 36: 1091-1099. 77. Azhgozhinova, G. S., Güven, O., Pekel, N., Dubolazov, A. V., Mun, G. A. and Nurkeeva, Z. S. Complex Formation of Linear Poly(methacrylic acid) with Uranyl Ions in Aqueous Solutions. Journal of Colloid and Interface Science, 2004. 278: 155-159. 78. Miyoshi, T., Takegoshi, K. and Hikichi, K. High-Resolution Solid-State 13C Nuclear Magnetic Resonance Study of a Polymer Complex: Poly(methacrylic acid) / Poly(ethylene oxide). Polymer, 1996. 37: 11-18. 79. Ito, H., Imae, T., Nakamura, T., Sugiura, M. and Oshibe, Y. Self-Association of Water-Soluble Fluorinated Diblock Copolymers in Solutions. Journal of Cooloid and Interface Science, 2004. 276: 290-298. 80. Goor, G., Edwards, J. and Curci, R., in: Strukul, G. (Ed.). Catalytic Oxidations with Hydrogen Peroxide as Oxidant. Dordrecht: Kluwer Academic Publisher.1992. 81. Willett, J. E. Gas Chromatography. Analytical Chemistry by Open Learning (ACOL). Chichester: John Wiley & Sons. 1987. p. 12-3, 10-12. 82. Howe, I., Williams, D. H. and Bowen, R. D. Mass Spectrometry, Principles and Applications. Advanced Book Program. 2nd. ed. New York: Mc GrawHill. 1981. p. 1-5. 83. Gudzinowicz, J. B., Gudzinowicz, M. J. and Martin, H. F. Fundamentals of Integrated GC-MS, Part II: Mass Spectrometry. Chromatographic Science Series, Volume 7. New York: Marcel Dekker. Inc. 1976. p. 1-2. 84. Meyer, V. R. Practical High-Performance Liquid Chromatography. 2nd ed. Chichester: John Wiley& Sons. 1988. p. 1-3. 85. Roland, E. and Kleinschmit, P. Ullmann’s Encyclopedia of Industrial Chemistry, Verlagsgesell: VCH. 1996, Vol. A19, p. 301. 109 APPENDIX A ULTRAVIOLET VISIBLE DIFFUSE REFLECTANCE (UV-Vis DR) 1. UV-Vis diffuse reflectance spectroscopic data of Fe-TPyP/Al-MCM-41 samples with different amount of Fe-TPyP loading (see Chapter 3). Samples Characteristic bands (nm) Fe-TPyP 424, 510, 585, 644 Fe-TPyP/Al-MCM-41 (Fe-TPyP = 5ȝmol) 424, 454, 521, 590, 644 Fe-TPyP/Al-MCM-41(Fe-TPyP = 10ȝmol) 421, 462, 519, 590, 644 Fe-TPyP/Al-MCM-41(Fe-TPyP = 25ȝmol) 416, 454, 520, 590, 644 Fe-TPyP/Al-MCM-41(Fe-TPyP = 50ȝmol) 421, 460, 518, 589,644 Fe-TPyP/Al-MCM-41(Fe-TPyP = 100ȝmol) 414, 462, 517, 589, 644 2. UV-Vis diffuse reflectance spectroscopic data of Fe-TPyP/PMAA samples with different amount of Fe-TPyP loading (see Chapter 4). Samples Characteristic bands (nm) Fe-TPyP 424, 510, 585, 644 Fe-TPyP/PMAA(Fe-TPyP = 5ȝmol) 417, 515, 589, 644 Fe-TPyP/PMAA (Fe-TPyP = 10ȝmol) 418, 513, 588, 644 Fe-TPyP/PMAA (Fe-TPyP = 25ȝmol) 418, 512, 587, 644 Fe-Fe-TPyP/PMAA (Fe-TPyP = 50ȝmol) 418, 515, 590, 644 Fe-TPyP/PMAA (Fe-TPyP = 100ȝmol) 418, 517, 590, 644 110 APPENDIX B LUMINESCENCE SPECTROSCOPY (LS) 1. Luminescence intensity change of Fe-TPyP/PMAA with Fe-TPyP binding. Fe-TPyP (ȝmol) I I0/I ȋ = 1 - I/I0 * 0 552 1 0 5 452 1.221 0.627 10 382 1.445 0.548 25 334 1.653 0.489 50 249 2.217 0.395 100 206 2.680 0.181 * I and I0 represent the luminescence intensity in the presence or absence of Fe-TPyP. (I0 = 552) 2. The Stern-Volmer kinetics: the dependences of the ratio of the luminescence intensities (I0/I) on the Fe-TPyP concentration. 3 y = 0.0162x + 1.1895 y = 0.0162x + 1.1895 R2 = 0.9431 2 R = 0.9431 2.5 I0 /I 2 1.5 1 0.5 0 0 20 40 60 80 Amount of Fe-TPyP Loading / ȝmol 100 120 111 APPENDIX C SCANNING ELECTRON MICROSCOPY (SEM) 1. Scanning electron micrographs of magnifications scales show: (a) 5µm and (b) 2µm) (a) (b) Fe-TPyP/Al-MCM-41 (2 112 3. Scanning electron micrographs of (a) Fe-TPyPAl-MCM-41 (Fe-TPyP = 100 ȝmol) and (b) Fe-TPyP/Al-MCM-41 (Fe-TPyP = 50 ȝmol) (a) (b) 113 APPENDIX D GAS CHROMATOGRAPHY (GC) 1. Gas Chromatography Data of Authentic sample Calibration for phenol No. Concentration (mmol) Area 1. 0.003 12277 2. 0.004 13310 3. 0.005 14641 4. 0.006 17625 5. 0.008 20817 6. 0.01 34017 7. 0.02 76414 8. 0.03 93879 9. 0.04 145490 10. 0.05 161522 11. 0.06 244752 12. 0.08 389370 13. 0.1 454572 14. 0.5 3063656 15. 1 5166445 114 Calibration Curve of Phenol Standard 1.2 Phenol Concentration / mmol 2. 1 y = 2E-07x + 0.0088 Ry2==2E-07x 0.9919 + 0.0088 2 R = 0.9919 0.8 0.6 0.4 0.2 0 0.E+00 0 2.E+06 2 4.E+06 4 AreaArea (x 10-6) 6.E+06 6 115 5.766 Chromatograms of Substrates Benzene tR (min) Methanol Phenol 16.071 17.327 Hydrogen peroxide tR (min) 24.387 4.688 3. 116 4. Verification of phenol by GC technique a. Chromatograms of (a) phenol standard sample and (b) an example reaction product from oxidation benzene with hydrogen peroxide at 70 oC for 3 hours using Fe-TPyP/Al-MCM-41. Phenol Methanol (a) Benzene Methanol Phenol (b) 117 b. GC Chromatograms of (a) phenol standard sample and (b) an example reaction product from oxidation benzene with hydrogen peroxide at 70 oC for 3 hours using Fe-TPyP/PMAA. Phenol Methanol (a) Benzene Methanol Phenol (b) 118 APPENDIX E GAS CHROMATOGRAPHY-MASS SPECTROMETRY (GC-MS) 1. Total Ion Chromatograms of Benzene standard Abundance TIC: HD-14.D 1.8e+07 1.7e+07 1.6e+07 1.5e+07 1.4e+07 1.3e+07 1.2e+07 1.1e+07 1e+07 9000000 8000000 7000000 6000000 5000000 4000000 3000000 2000000 1000000 0 2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 Time--> Abundance Scan 85 (2.565 min): HD1.D 78 12000 11000 10000 9000 8000 7000 6000 5000 4000 3000 2000 52 1000 55 74 63 83 98 0 45 m/z--> 50 55 60 65 70 75 80 85 90 95 100 105 119 2. Total Ion Chromatograms of Phenol standard Abundance TIC: HD-8.D 7000000 6500000 6000000 5500000 5000000 4500000 4000000 3500000 3000000 2500000 2000000 1500000 1000000 500000 0 2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 Time--> Abundance Scan 587 (3.436 min): HD-12.D 94 350000 300000 OH 250000 200000 150000 66 100000 50000 50 55 50 55 62 74 79 45 m/z--> 60 65 70 75 80 104 108 90 0 85 90 95 100 105 110 115 120 3. Total Ion Chromatograms of Hydroquinone standard Abundance TIC: HD-5.D 3200000 3000000 2800000 2600000 2400000 2200000 2000000 1800000 1600000 1400000 1200000 1000000 800000 600000 400000 200000 0 2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 Time--> Abundance Scan 1097 (6.350 min): HD-5.D 110 750000 700000 650000 OH 600000 550000 500000 450000 400000 350000 300000 OH 250000 200000 81 150000 53 100000 50000 63 69 58 0 45 m/z--> 50 55 60 65 70 74 75 80 85 90 95 90 95 100105110115120125130135140 105 122 135 121 4. Total Ion Chromatograms of Products using Fe-TPyP/Al-MCM-41 as catalysts 70 oC for 3 hours. Abundance TIC: HD-12.D 1.7e+07 1.6e+07 1.5e+07 1.4e+07 1.3e+07 1.2e+07 1.1e+07 1e+07 9000000 8000000 7000000 6000000 5000000 4000000 3000000 2000000 1000000 0 2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 Time--> Abundance Scan 785 (4.567 min): HD-14.D 94 OH 3500 3000 2500 78 2000 1500 66 1000 51 500 55 63 69 104 74 0 45 m/z--> 50 55 60 65 70 75 80 85 90 95 100 105 110 122 5. Total Ion Chromatograms of Products using Fe-TPyP/PMAA as catalysts 70 oC for 3 hours. Abundance TIC: HD-13.D 1.8e+07 1.7e+07 1.6e+07 1.5e+07 1.4e+07 1.3e+07 1.2e+07 1.1e+07 1e+07 9000000 8000000 7000000 6000000 5000000 4000000 3000000 2000000 1000000 0 2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 Time--> Abundance Scan 591 (3.459 min): HD-13.D 94 220000 200000 OH 180000 160000 140000 120000 100000 80000 66 60000 40000 20000 50 55 50 55 62 74 78 82 0 45 m/z--> 60 65 70 75 80 105 110 85 90 95 100 105 110 115 123 6. Mass spectra of (a) hydroquinone standard sample and (b) hydroquinone from oxidation of benzene at 70 oC for 3 hours over Fe-TPyP/ PMAA. Abundance Scan 1097 (6.350 min): HD-5.D 110 750000 700000 650000 OH 600000 550000 500000 450000 400000 350000 300000 OH 250000 200000 81 150000 53 100000 50000 63 69 74 58 0 45 50 55 60 65 70 75 80 85 90 95 90 95 100105110115120125130135140 105 122 135 m/z--> (a) Abundance Scan 1308 (7.556 min): HD-9.D 110 18000 17000 16000 15000 14000 13000 12000 11000 10000 9000 8000 7000 6000 81 5000 4000 3000 54 2000 1000 63 253 95 0 50 60 70 80 90 100110120130140150160170180190200210220230240250 m/z--> (b) 124 APPENDIX F HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) 1. HPLC chromatograms of (a) phenol standard sample and (b) phenol 10.59 Phenol 10.44 Response (mV) 2.99 from oxidation benzene at 70 oC for 3 hours using Fe-TPyP/Al-MCM-41. Phenol Methanol Time (min) 2.88 Response (mV) (a) Methanol Time (min) (b) 125 2. HPLC chromatograms of (a) the mixture between phenol and hydroquinone standard samples and (b) phenol and hydroquinone from 10.44 Phenol 10.36 Response (mV) 2.85 oxidation benzene at 70 oC for 3 hours using Fe-TPyP/PMAA. Phenol Methanol 4.77 Hydroquinone Time (min) Methanol 4.61 3.05 Response (mV) (a) Hydroquinone Time (min) (b) 126 APPENDIX G REACTION PATH FOR THE OXIDATION OF BENZENE TO PHENOL 1. Reaction path for the oxidation of benzene to phenol over Fe-TPyP/Al-MCM-41 Step 1 P-Fe3+-Al-MCM-41 + H2O2 P-Fe4+(OH)-Al-MCM-41 P-Fe4+(OH)-Al-MCM-41 + H2O2 P-Fe4+(OOH)-Al-MCM-41 + H2O P-Fe4+(OOH)-Al-MCM-41 P-Fe5+=O-Al-MCM-41 + + -OH - OH Step 2 (Z) OH (Z) 5+ 4+ P-Fe =O-Al-MCM-41 + P-Fe -Al-MCM-41 OH P-Fe3+-Al-MCM-41 + and OH 2P-Fe4+(OH)-Al-MCM-41 + + OH 2P-Fe3+-Al-MCM-4 1 127 2. Reaction path for the oxidation of benzene to phenol over Fe-TPyP/ PMAA Step 1 O O O FeIII H + H FeIII O P H + H+ P H O + O H O -H2O FeIII FeV + R H P P Step 2 OH FeV 2 H FeIV + P P FeIII . O H + HO P and O FeIII H + HO P IV Fe P FeIII P OH P + HO OH . FeV OH 128 APPENDIX H LIST OF PUBLICATIONS 1. Nur, H., Hamid, H., Endud, S. and Ramli, Z., “Iron-porphyrin Encapsulated in poly(methacrylic acid) and Mesoporous Al-MCM-41 as Catalysts in the Oxidation of Benzene to Phenol”, Materials Chemistry and Physics, In Press, 2005. 2. Hamid, H., Endud, S., Nur, H. and Ramli, Z., “Synthesis and Characterization of Poly(methacrylic acid) (PMAA)-Iron(III) Porphyrin Hybrid Catalyst for Oxidation of Benzene to Phenol”, Paper presented at Symposium on Science and Mathematics 2004 (SSM 2004), Universiti Teknologi Malaysia, 2004. 3. Hamid, H., Endud, S., Nur, H. and Ramli, Z., “Comparative Study of ironporphyrin supported on Mesoporous Al-MCM-41 and Poly(methacrylic acid) (PMAA) : Characterization and their Catalytic Activities”, Poster presented at Annual Fundamental Science Seminar 2004 (AFSS 2004), Ibnu Sina Institute,Universiti Teknologi Malaysia, 2004. 4. Hamid, H., Endud, S., Nur, H. and Ramli, Z., “Encapsulation of IronPorphyrin within Ordered Mesoporous Al-MCM-41: Synthesis, Characterization and Catalytic Activity”, Report for Post-Graduate First Assessment, Pusat Pengajian Siswazah, Universiti Teknologi Malaysia, 2004.