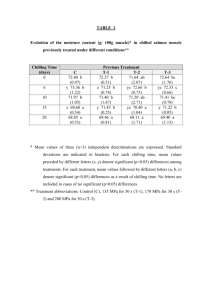
Environmental and Experimental Botany 70 (2011) 283–288
Contents lists available at ScienceDirect
Environmental and Experimental Botany
journal homepage: www.elsevier.com/locate/envexpbot
Chilling relieves corm dormancy in Calopogon tuberosus (Orchidaceae) from
geographically distant populations
Philip J. Kauth a,∗ , Michael E. Kane a , Wagner A. Vendrame b
a
b
Plant Restoration, Conservation, and Propagation Biotechnology Program, Environmental Horticulture Department, University of Florida, PO Box 110675, Gainesville, FL 32611, USA
Tropical Research and Education Center, University of Florida, 18905 SW 280th St, Homestead, FL 33031, USA
a r t i c l e
i n f o
Article history:
Received 17 November 2009
Received in revised form 4 October 2010
Accepted 6 October 2010
Keywords:
Corm
Dormancy
Ecotype
Orchid
a b s t r a c t
Many plant species require a chilling period to commence regrowth from overwintering structures such
as buds, corms, tubers, and rhizomes. While the effects of chilling have been thoroughly studied in a horticultural context, little information exists regarding the relationship between ecotypic differentiation and
chilling requirements. Effects of chilling storage organs on shoot emergence of widespread orchid species
has not been examined, and ecotypic differentiation in the Orchidaceae has also received little attention.
The effects of chilling on corm dormancy in Calopogon tuberosus, a widespread orchid of eastern North
America, were studied. Seeds were collected from south Florida, north central Florida, South Carolina, and
Michigan, and germinated in vitro to produce plants. After 20 weeks in vitro culture, corms were removed
from seedlings and chilled for 0, 2, 4, 6, and 8 weeks. Corms were subsequently planted in a soilless potting
mix and placed under ex vitro conditions in an environmental growth chamber. Shoot emergence was
monitored bi-weekly for 16 weeks, and shoot length, leaf number, leaf width, root number, root length,
and corm diameter were measured after 16 weeks. Longer chilling periods broke corm dormancy more
effectively than shorter chilling treatments regardless of population. Shoots of all populations sprouted
rapidly on corms after 6 and 8 weeks chilling. In addition, a higher percentage of shoots sprouted on corms
after 8 weeks chilling. After 16 weeks, north central Florida and South Carolina plantlets were larger than
Michigan and south Florida plantlets. Differing chilling requirements among C. tuberosus populations
may reflect ecotypic differentiation resulting from varying environmental conditions at each site.
© 2010 Elsevier B.V. All rights reserved.
1. Introduction
Calopogon tuberosus var. tuberosus is a terrestrial orchid native
to eastern North America with a large distribution from Florida
to maritime Canada. Throughout its range, C. tuberosus occupies a variety of habitats including bogs, fens, marl prairies, and
mesic roadsides. Both genetic and morphological variation have
been reported recently (Goldman et al., 2004a,b; Trapnell et al.,
2004), but whether ecotypes exist remains unclear. Recent studies exploring the in vitro ecology of seed germination and seedling
development determined that photoperiod, germination media,
and growing season length influenced the development of C.
tuberosus ecotypes (Kauth et al., 2008; Kauth and Kane, 2009).
Additionally, potential differences in the extent of corm dormancy
among widespread C. tuberosus populations may be influenced by
local adaptation.
Many temperate plant species form overwintering structures,
such as buds, tubers, rhizomes, and corms, before unfavorable
∗ Corresponding author. Tel.: +1 352 273 4864; fax: +1 352 392 1413.
E-mail address: pkauth@ufl.edu (P.J. Kauth).
0098-8472/$ – see front matter © 2010 Elsevier B.V. All rights reserved.
doi:10.1016/j.envexpbot.2010.10.003
growth conditions are encountered (Garbisch et al., 1995; Rohde
and Bhalerao, 2007), and remain dormant until favorable growth
conditions are encountered the following growing season (Garbisch
et al., 1995). In order to break dormancy, a chilling period is often
required (Rohde and Bhalerao, 2007). Longer chilling periods are
often required to break dormancy in tubers and corms of temperate
species, but extended periods often inhibit growth and development (Clark, 1995; Yañez et al., 2005; Fukai et al., 2006). However,
chilling period requirement may be different according to plant
provenance in that southern species may require shorter chill periods (Perry and Wang, 1960; Garbisch et al., 1995).
Chilling requirements as a function of ecotypic differentiation
have been explored in tree species (Perry and Wang, 1960; Kriebel
and Wang, 1962), aquatic species (Garbisch et al., 1996), and forage grasses (Silsbury, 1961; Cooper, 1964; Eagles, 1967a,b; MacColl
and Cooper, 1967). Acer rubrum ecotypes from Florida required
no chilling to break dormancy, but longer chill periods were
required to break dormancy in more northern ecotypes (Perry and
Wang, 1960). Acer saccharum ecotypes from Georgia and Tennessee
required shorter chill periods to break dormancy than ecotypes
in Michigan and Ohio (Kriebel and Wang, 1962). In several forage
grass species, relative growth rate of Mediterranean populations
284
P.J. Kauth et al. / Environmental and Experimental Botany 70 (2011) 283–288
was higher at cooler temperatures compared to north European
populations that had a higher growth rate at warmer temperatures (Cooper, 1964). Prolonged chilling decreased both survival
and shoot growth of aquatic plant ecotypes from Florida (Garbisch
et al., 1996).
C. tuberosus is a model orchid species to exam the relationship
between chilling period and corm dormancy in widespread populations. Studying corm dormancy, chilling period necessary to break
dormancy, and shoot emergence may provide insight into C. tuberosus ecotypic differentiation. The objectives were to: (1) assess the
effect of chilling on the mechanism of corm dormancy and subsequent plantlet growth; (2) exam the response of four C. tuberosus
populations to low temperature storage; and (3) examine ecotypic
differentiation in C. tuberosus. Our hypotheses were: (1) all populations will require a period of chilling to break the mechanism
of corm dormancy; and (2) corms from more northern populations will require a longer chilling period compared to southern
populations.
2. Materials and methods
Seeds were collected from the following locations: Upper Peninsula Michigan (Menominee County, Michigan, USA), upstate South
Carolina (Greenville County, South Carolina, USA), north central
Florida (Levy County, Florida, USA), and south Florida (Collier
County, Florida, USA). Seed capsules from all populations were
collected before complete dehiscence and were stored at 23 ◦ C
over silica gel for 2 weeks. Seeds were then removed from capsules, pooled by geographic population, and stored dry in the
dark at −11 ◦ C until used. Further information about the environmental conditions for each site is supplied in supplemental
Table 1.
Seeds were surface disinfected in sterile scintillation vials for
3 min in a solution of 5 mL absolute ethanol, 5 mL 6% NaOCl,
and 90 mL sterile distilled, deionized water. Seeds were rinsed
with sterile dd water after surface sterilization. Solutions were
removed with sterile Pasteur pipettes. Seeds were transferred
with a 10 L sterile inoculating loop onto BM-1 Terrestrial Orchid
Medium (PhytoTechnology Laboratories, Shawnee Mission, KS)
contained in 100 mm × 15 mm Petri plates. The medium was supplemented with 1% activated charcoal. Medium pH was adjusted
to 5.7 with 0.1 N KOH prior to autoclaving for 40 min at 117.7 kPa
and 121 ◦ C. Ten replicate Petri plates with 30 mL medium each
were used for each seed source with approximately 100 seeds
per plate. Cultures were placed in an environmental growth
incubator (#I-35LL; Percival Scientific, Perry, IA, USA) under coolwhite fluorescent lights in a 12 h photoperiod at 24.2 ± 0.2 ◦ C and
40 mol m−2 s−1 .
After 8 weeks culture, seedlings were transferred to larger
culture vessels for further growth and development. Nine
seedlings were transferred to individual PhytoTech Culture Boxes
(PhytoTechnology Laboratories, Shawnee Mission, KS) containing
100 mL of BM-1 Terrestrial Orchid Medium. Five replicate vessels
were prepared for each treatment and seed source combination for
a total of 45 seedlings per treatment. A total of 25 vessels with a
total of 225 seedlings were prepared for each seed source. A total of
900 seedlings were transferred. Seedlings grew in vitro for another
12 weeks, for a total of 20 weeks culture. Environmental conditions
were the same as described previously.
After the 20 weeks, shoots and roots on seedlings were removed
so that only corms remained. The nine corms in each PhytoTech box
were transferred to Sigma Phytatrays I (#P1552, Sigma–Aldrich, St.
Louis, MO) containing 100 mL of moist, sterilized vermiculite. Five
Phytatrays I (114 mm × 86 mm × 63.5 mm) were prepared for each
treatment. Cultures containing the corms were subsequently stored
at 10 ± 0.3 ◦ C for 2, 4, 6, and 8 weeks in complete darkness; a control
(no cold storage) was also used. Five culture vessels per seed source
were allocated to each chilling period.
The 20 culture boxes (five replications per each of the four populations) allocated to the five chilling treatments were subsequently
removed after the chilling period. Corms were subsequently
planted in a 9-cell pack containing Fafard 2 (Conrad Fafard, Inc.,
Agawam, MA, USA). Corms were planted in a randomized complete block design with block designated as the chill treatment so
that block 1 was the control, etc. Each seed source was allocated
to each block, and blocks were replicated five times. Corms were
buried approximately 1 cm below the soil line. Trays were placed
in a walk-in growth chamber under a 16/8 h L/D photoperiod at
27 ± 2.2 ◦ C and an average relative humidity of 85%. Four 400-W
metal halide bulbs (Sylvania, Danvers, MA, USA) provided a light
level of 167 mol m−2 s−1 . Corms were watered as needed and as
frequently as daily.
Shoot emergence date was recorded by the presence of the
new shoot emerging from the soil. Every 2 weeks, starting upon
emergence and continuing until week 16, shoot length was measured from the soil surface to the shoot apex. At the final data
collection, leaf number, leaf width, shoot height, root number, root
length, corm diameter, and axillary shoot formation were recorded.
Axillary shoots (Fig. 1) grow from storage organs to form new storage organs (Dixon and Pate, 1978; Hollick et al., 2001). Percent
shoot emergence and percent survival, noted by the presence of a
corm beneath the soil surface, were recorded. Logistic regression
was used to assess the affect of chilling treatment and population on percent shoot emergence, percent survival, and percent
axillary shoot formation using the generalized linear mixed model
procedure (proc glimmix macro) in SAS v9.1. Least-square means
(lsmeans) were used to assess mean separation. Endpoint measurement data were analyzed using the general linear procedure (proc
glm), ANOVA (see supplemental Table 2), and least-square means
in SAS v9.1.
3. Results
3.1. Effects of chilling on shoot emergence
The main effects (population and chilling period), and their
interaction all significantly influenced the number of days to shoot
emergence. The average number of days to shoot emergence postchilling was less under the longer chill periods of 6 and 8 weeks,
regardless of population (Fig. 2A). Corms subjected to the control or
the 2-week chilling period exhibited the slowest shoot emergence.
South Carolina and south Florida corms chilled longer than 6 weeks
exhibited the quickest shoot emergence. Michigan corms required
4 weeks or longer for quickest shoot emergence, while shoots
emerged faster when north central Florida corms were chilled for
8 weeks (Fig. 2A).
Percent shoot emergence was highly influenced by the main
effect of chilling period, but the main effect of population and the
interaction between chilling treatment and population were not
significant. Lower percent shoot emergence was observed when
corms were chilled for shorter periods (Fig. 2B). Less than 20% shoot
emergence was observed in unchilled corms and following the 2week chilling period among all populations. In fact, only one shoot
from South Carolina emerged in the control and only one shoot from
north central Florida emerged in the 2-week chilling treatment.
Chilling periods longer than 6 weeks provided the highest percent
shoot emergence in Michigan corms (Fig. 2B), while 8 weeks chilling
provided the highest shoot emergence for all other populations.
Approximately 90% shoot emergence occurred on South Carolina,
north central Florida, and south Florida corms, compared to 78% for
Michigan corms (Fig. 2B).
P.J. Kauth et al. / Environmental and Experimental Botany 70 (2011) 283–288
285
Fig. 1. Ex vitro growth comparison of representative C. tuberosus plantlets. Plantlets represent average size after 16 weeks growth in a walk-in growth chamber. Plantlets
were generated after chilled corms were planted under ex vitro conditions. (A) Michigan plantlet with axillary shoot formed between the original and new corm. (B) South
Carolina plantlet with axillary shoot formed between the original and new corm. (C) North central Florida plantlet. (D) South Florida plantlet. Scale bars = 1 cm.
Fig. 2. Effects of chilling corms at 10 ◦ C on shoot emergence after 16 weeks of ex vitro growth of C. tuberosus plantlets. (A) Mean number of days to shoot emergence postchilling. (B) Percent shoot emergence recorded by the presence of a shoot emerged from the soil. (C) Percent survival measured by the presence of a corm beneath the soil.
(D) Percent axillary shoot formation. Each histobar represents the mean response of five replications with nine plantlets for a total of 45 plantlets per treatment × population.
Means with the same letter are not significantly different at ˛ = 0.05.
286
P.J. Kauth et al. / Environmental and Experimental Botany 70 (2011) 283–288
Fig. 3. Effects of chilling corms at 10 ◦ C on growth and development of C. tuberosus plantlets. Data were collected after 16 weeks ex vitro growth. Data from south Florida
ecotype does not appear in the control due to shoot senescence prior to data collection endpoint. (A) Shoot length measured from the soil surface to the tip of the longest leaf. (B)
Leaf number. (C) Leaf width measured at the widest point of the widest leaf. (D) Root number. (E) Root length of the longest root. (F) Corm diameter of the new corm measured
horizontally at the widest point. Each histobar represents the mean response of five replications with nine plantlets for a total of 45 plantlets per treatment × population.
Means with the same letter are not significantly different at ˛ = 0.05.
3.2. Effects of chilling on corm survival
After 16 weeks, corm survival was high regardless of population
or chilling period. Survival was measured by the presence of a viable
corm below the soil surface, and not the presence the emerged
shoot. The original corm often remained viable, but did not form a
shoot by the end of the experiment. Major differences in survival
were not clearly evident according to population, chilling period, or
their interaction. Corm survival was only different within Michigan
and south Florida populations. Survival of south Florida propagules
was highest with 4–8 weeks chilling, while Michigan corm survival
was highest after 2 weeks chilling (Fig. 2C). Near 100% survival
was observed in South Carolina and north central Florida corms
regardless of treatment (Fig. 2C).
3.3. Effects of chilling on axillary shoot formation
Only the main effect of population significantly influenced axillary shoot formation, while chilling period and the interaction
between population and chilling period were not significant. Axillary shoots were considered formed by the presence of a shoot
connecting the original corm to the new shoot (Fig. 1A, B). Axil-
lary shoot formation was prevalent in Michigan and South Carolina
populations after 16 weeks (Fig. 2D). Axillary shoot formation was
higher on corms that were chilled for 6 or 8 weeks compared to
shorter chilling periods in Michigan and South Carolina plantlets
(Fig. 2D). No axillary shoots formed on north central Florida and
south Florida corms in the control, 2 weeks, and 4 weeks chilling
periods (Fig. 2D). Axillary shoot formation in both Florida populations was highest when corms were chilled for 6 weeks, while 8
weeks chilling suppressed formation.
3.4. Effects of chilling on shoot growth
The main effects (population and chilling period) and their interaction all significantly influenced shoot growth. No differences in
shoot length occurred among chilling periods in the Michigan and
south Florida populations (Fig. 3A). Few differences were observed
in South Carolina and north central Florida populations. Michigan
and south Florida shoots were generally the shortest of all populations and north central Florida the highest (Fig. 3A). Shoots on
south Florida ecotypes did emerge in the control treatment (Fig. 2),
but shoots dehisced by week 16 and no data was collected due to
shoot-die back.
P.J. Kauth et al. / Environmental and Experimental Botany 70 (2011) 283–288
The interaction between the population and chilling period and
main effect of chilling period did not significantly influence leaf
number. However, population significantly influenced leaf development. Michigan had the highest leaf number after 16 weeks
followed by South Carolina and both Florida populations (Fig. 3B).
Less than two leaves were generally present on all plantlets regardless of population.
Leaf width was significantly affected by the interaction between
the main effects of population and chilling period as well as population alone. However, chilling period did not significantly influence
leaf width. Average leaf width was lowest on south Florida plantlets
(Fig. 3C) while the widest leaves grew on north central Florida and
South Carolina plantlets (Fig. 3C). No differences among treatments
were observed on South Carolina and south Florida. Wider leaves
were observed in chilling periods longer than 4 weeks on Michigan plantlets. Widest leaves were observed on north central Florida
plantlets from the control, 2 weeks, and 8 weeks chill period.
3.5. Effects of chilling on root growth
The main effects (population and chilling period) and their interaction all significantly influenced root development. No differences
were observed in root number for both South Carolina and Michigan populations, but more roots were observed in shorter chilling
periods for both Florida populations (Fig. 3D). The highest root
number was observed in the control treatment in north central
Florida plantlets, but South Carolina plantlets had the greatest root
number on average.
The main effects and their interaction all significantly influenced
root length. Longer chilling periods promoted the longest roots on
Michigan plantlets (Fig. 3E). No difference was observed on South
Carolina plantlets. Chilling periods less than 8 weeks promoted
the longest roots on north central Florida plantlets. South Florida
plantlets from the no chill treatment did not form roots, while few
differences were observed among chilling treatments (Fig. 3E).
3.6. Effects of chilling on corm development
Population significantly influenced new corm development
while chilling period and the main effects interaction were not significant. Few differences were observed among chilling treatments
within each population with the exception of South Carolina and
north central Florida where the largest corms occurred in the control and control/2 weeks chilling treatment, respectively (Fig. 3F).
Regardless of treatment, smallest corms south Florida plantlets
had the smallest corms, and new corms did not form on south
Florida plantlets in the control treatment. The largest corms grew
on South Carolina and north central Florida plantlets in the control
and 2 weeks chilling treatment, while Michigan and South Carolina plantlets had the largest corms in the 4, 6, 8 week chilling
treatments.
4. Discussion
This is the first study comparing the role of chilling in relieving corm dormancy in an orchid species. The results supported
our first hypothesis that chilling will effectively break corm dormancy. However, our second hypothesis was not supported. The
response of Florida corms to longer chilling periods was surprising. The results aid in our understanding of the ecological growth
strategy of C. tuberosus as well as ecotypic differentiation.
Previous research examining chilling of storage organs focused
on the effects of temperature and chilling period in relation to shoot
growth and flowering of horticultural crops (Clark, 1995; Kim et al.,
1996; González et al., 1998; Yañez et al., 2005; Fukai et al., 2006),
but little information exists focusing on local adaptation to chilling
287
(Cooper, 1964; Eagles, 1967a,b; MacColl and Cooper, 1967). While
few differences in chilling were found among populations, local
adaptation to different habitats and environmental conditions may,
in part, explain the chilling requirement.
Comparative influence of chilling storage organs such as tubers
and corms resulting from ecotypic differentiation has received little
attention. Chilling dormant buds of tree ecotypes has been investigated previously (Perry and Wang, 1960; Kriebel and Wang, 1962;
Myking and Heide, 1995; Li et al., 2003, 2005), but correlating chilling response of buds with underground storage organs may be
difficult due to location of plant parts. Regardless, chilling requirements should be considered in a restoration context if plants are
moved from their home-site since southern ecotypes may not be
cold-hardy (Garbisch et al., 1996).
Chilling of C. tuberosus corms followed a similar pattern to
bud chilling in birch species (Betula pendula and Betula pubescens).
Longer chilling treatments reduced days to bud break regardless
of population latitude, and the number of days to bud break was
more pronounced for southern ecotypes (Myking and Heide, 1995).
Longer chilling periods increased shoot emergence and reduced the
number of days to shoot emergence regardless of chilling period
in ecotypes of C. tuberosus. Longer chilling treatments had a more
pronounced influence on emergence days of southern C. tuberosus ecotypes, yet corms from northern populations generally broke
dormancy earlier than southern ecotypes. This may have been
caused by the southern ecotypes inability to initiate growth at
10 ◦ C and a higher base temperature required for dormancy break
(Myking and Heide, 1995).
The requirement for a chill period longer than 6 weeks for the C.
tuberosus Michigan population was expected. The long winters and
relatively constant temperatures below freezing require plants to
maintain dormancy until environmental conditions are appropriate (Garbisch et al., 1996). No difference in shoot emergence was
observed between the 6- and 8-week chilling period for Michigan
plants, and shoot emergence was lower than all other populations
in the 8-week chilling treatment. Being subjected to longer winters,
Michigan plants may require a chill period longer than 8 weeks for
maximum shoot emergence.
The longer chilling period required for shoot emergence
on southern C. tuberosus ecotypes was surprising, but may be
explained by temperature sensitivity. Winter temperatures in the
south often exceed 17 ◦ C, but temperatures may drop suddenly in
subsequent days. South Florida temperatures do fall below 0 ◦ C,
albeit for shorter periods than northern climates, reflecting the
south Florida ecotype’s inability to grow at lower temperatures
(Myking and Heide, 1995). Longer chilling periods may ensure
Florida plants do not initiate shoot emergence until the threat of
freezing temperatures is surpassed (Garbisch et al., 1996). Early
shoot dehiscence on south Florida shoots in the control treatment
may have been caused by an inadequate chilling period. IwayaInoue et al. (1996) reported starch digestion and respiration rate
was greater in tulip bulbs that received a chilling treatment. Similarly, south Florida corms in the non-chilled treatment may not
have had sufficient chilling that promoted starch digestion and
water movement to the developing shoot meristem (Iwaya-Inoue
et al., 1996).
An interesting morphological feature observed in Michigan and
South Carolina populations was the formation of an axillary shoot
between the new and old corms. These axillary shoots, often
referred as droppers, grow downward to form replacement storage organs for the next season’s growth (Dixon and Pate, 1978;
Hollick et al., 2001). However, the axillary shoots on C. tuberosus corms were different because they grew upward toward the
surface of the soil. This may be an ex situ phenomenon because
axillary shoots have not been seen on wild plants from any location (personal observation). Michigan and South Carolina plantlets
288
P.J. Kauth et al. / Environmental and Experimental Botany 70 (2011) 283–288
were more prone to form axillary shoots, and more axillary shoots
formed with longer chill periods regardless of plantlets population.
The exact ecological role of droppers and storage organ axillary
shoots are uncertain. Michigan and South Carolina ecotypes may
be more prone to forming axillary shoots to take advantage of a
shorter growing season by forcing new growth toward the soil surface. Because winter temperatures are more variable in Florida,
longer chilling requirements may influence axillary shoot growth
to avoid frost damage. Further investigation into the dynamics of
axillary shoot formation is warranted.
Clear differences in growth and development of plantlets after
corm chilling were not evident. However, longer chilling periods
effectively broke dormancy of all populations. Several factors may
have influenced the inconsistent plantlets growth and development results. Plantlet numbers were low in the control and shorter
chill treatments, thus error was much larger creating fewer significant results. The few plantlets that did emerge and develop in the
shorter chilling treatments had a longer time to develop compared
to those in the longer chilling treatments contributing to the larger
sized plantlets.
Chilling corms of C. tuberosus ecotypes effectively promoted
shoot emergence upon removal from the chilling treatments. The
differential response to chilling length by C. tuberosus ecotypes may
be in part due to temperature sensitivity, and the ability to successfully utilize carbohydrate reserves after chilling. Additionally,
shoot emergence was influenced more by longer chilling periods
regardless of population. This study combined with our previous
results indicates that a variety of environmental conditions including growing season length, photoperiod, and soil nutrient analysis
are influencing ecotypic differentiation among C. tuberosus populations.
Acknowledgements
We thank the following for collecting seed: Larry Richardson
(Wildlife Biologist; Florida Panther National Wildlife Refuge), Jim
Fowler, and Kip Knudson. We also thank Mary Bunch (South Carolina Heritage Preserve Program) for issuing collection permits. We
thank Dr. Charles Guy (University of Florida) for use of the walkin growth chamber. Brand names are provided as references; the
authors do not solely recommend or endorse these products. Partial funding was provided through U.S. Fish and Wildlife and the
Florida Panther National Wildlife Refuge.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in
the online version, at doi:10.1016/j.envexpbot.2010.10.003.
References
Clark, G.E., 1995. Effects of storage temperature and duration on the dormancy of
Sandersonia aurantiaca tubers. N. Z. J. Crop Hort. 23, 455–460.
Cooper, J.P., 1964. Climatic variation in forage grasses. I. Leaf development in climatic
races of Lolium and Dactylis. J. Appl. Ecol. 1, 45–61.
Dixon, K.W., Pate, J.S., 1978. Phenology, morphology, and reproductive biology of
tuberous sundew, Drosera erthryorhiza Lindl. Aust. J. Bot. 26, 441–454.
Eagles, C.F., 1967a. The effect of temperature on vegetative growth in climatic races
of Dactylis glomerata in controlled environments. Ann. Bot. 31, 31–39.
Eagles, C.F., 1967b. Variation in the soluble carbohydrate content of climatic races
of Dactylis glomerata (Cocksfoot) at different temperatures. Ann. Bot. 31, 645–
651.
Fukai, S., Kanechika, R., Hasegawa, A., 2006. Effect of low temperature on breaking dormancy and flowering of Arisaema sikokianum (Araceae). Sci. Hortic. 111,
97–100.
Garbisch, E.W., McIninch, S.M., Swartz, H.J., Salvaggio, G.J., 1995. Chilling responses
for some herbaceous wetland plants. Wetland J. 7, 16–20.
Garbisch, E.W., McIninch, S.M., Swartz, H.J., Salvaggio, G.J., 1996. The effects of controlled chilling on five wetland herbaceous plant species. Wetland J. 8, 20–
29.
Goldman, D.H., Jansen, R.K., van den Berg, C., Leitch, I.J., Fay, M.F., Chase, M.W.,
2004a. Molecular and cytological examination of Calopogon (Orchidaceae, Epidendroideae): circumscription, phylogeny, polyploidy, and possible hybrid
speciation. Am. J. Bot. 91, 707–723.
Goldman, D.H., van den Berg, C., Griffith, M.P., 2004b. Morphometric circumscription
of species and infraspecific taxa in Calopogon R. Br. (Orchidaceae). Plant Syst.
Evol. 247, 37–60.
González, A., Bañón, S., Fernández, J.A., Franco, J.A., Casas, J.L., Ochoa, J., 1998. Flowering responses of Gladiolus tristis (L.) after exposing corms to cold treatment.
Sci. Hortic. 74, 279–284.
Hollick, P., Senaratna, T., McComb, J., Bunn, E., Dixon, K.W., 2001. Response to
paclobutrazol of symbiotic mycorrhizal fungi and dropper (tuber stalk) formation of host orchid seedlings. Plant Growth Regul. 00, 1–9.
Iwaya-Inoue, M., Motooka, K., Ishida, N., Koizumi, M., Kano, H., 1996. Chilling
effects for normal growth of tulip bulbs estimated by MRI. Acta Hort. 440, 407–
412.
Kauth, P.J., Kane, M.E., Vendrame, W.A., Reinhardt-Adams, C., 2008. Asymbiotic germination response to photoperiod and nutritional media in six populations of
Calopogon tuberosus var. tuberosus (Orchidaceae): evidence for ecotypic differentiation. Ann. Bot. 102, 783–793.
Kauth, P.J., Kane, M.E., 2009. In vitro ecology of Calopogon tuberosus var. tuberosus (Orchidaceae) seedlings from distant populations: implications assessing
for ecotypic differentiation. J. Torrey Bot. Soc. 136, 433–444.
Kim, H.H., Ohkawa, K., Sakaguchi, K., 1996. Effects of storage temperature and duration on flower bud development, emergence and flowering of Zephyra elegans
D. Don. Sci. Hortic. 67, 55–63.
Kriebel, H.B., Wang, C.W., 1962. The interaction between provenance and degree of
chilling in bud-break of sugar maple. Silvae Genet. 11, 125–130.
Li, C., Junttila, O., Heino, P., Palva, E.T., 2003. Different responses of northern and
southern ecotypes of Betula pendula to exogenous ABA application. Tree Physiol.
23, 481–487.
Li, C., Wu, N., Liu, S., 2005. Development of freezing tolerance in different altitudinal
ecotypes of Salix paraplesia. Biol. Plant. 49, 65–71.
MacColl, D., Cooper, J.P., 1967. Climatic variation in forage grasses. III. Seasonal
changes in growth and assimilation in climatic races of Lolium, Dactylis and
Festuca. J. Appl. Ecol. 4, 113–127.
Myking, T., Heide, O.M., 1995. Dormancy release and chilling requirement of buds
of latitudinal ecotypes of Betula pendula and B. pubescens. Tree Physiol. 15,
697–704.
Perry, T.O., Wang, C.W., 1960. Genetic variation in the winter chilling requirement
for date of dormacy break for Acer rubrum. Ecology 41, 790–794.
Rohde, A., Bhalerao, R.P., 2007. Plant dormancy in the perennial context. Trends Plant
Sci. 12, 217–223.
Silsbury, J.H., 1961. A study of dormancy, survival and other characteristics in Lolium
perenne L. at Adelaide, S.A. Aust. J. Agric. Res. 12, 1–9.
Trapnell, D.W., Hamrick, J.L., Giannasi, D.E., 2004. Genetic variation and species
boundaries in Calopogon (Orchidaceae). Syst. Bot. 29, 308–315.
Yañez, P., Ohno, H., Ohkawa, K., 2005. Temperature effects on corm dormancy and
growth of Zephyra elegans D.Don. Sci. Hortic. 105, 127–138.