Annals of Botany 102: 783 –793, 2008
doi:10.1093/aob/mcn163, available online at www.aob.oxfordjournals.org
Asymbiotic Germination Response to Photoperiod and Nutritional Media in Six
Populations of Calopogon tuberosus var. tuberosus (Orchidaceae): Evidence for
Ecotypic Differentiation
P HI L IP J . K A UT H 1, *, MICH AE L E . K AN E 1 , WAG NER A . VEN DR AME 3 and
CAR R I E R E I NH AR D T - AD AM S 2
1
Plant Restoration, Conservation and Propagation Biotechnology Program, 2Restoration and Plant Ecology
Program, Environmental Horticulture Department, University of Florida, PO Box 110675, Gainesville, FL 32611,
USA and 3Tropical Research and Education Center, University of Florida, 18905 SW 280th Street, Homestead,
FL 33031-3314, USA
Received: 25 April 2008 Returned for revision: 8 July 2008 Accepted: 30 July 2008 Published electronically: 30 August 2008
Key words: Asymbiotic germination, corm development, Calopogon tuberosus, ecotypic differentiation, native orchid,
orchid seed germination, seedling development.
IN TROD UCT IO N
Ecotypic differentiation has recently been recognized as an
important issue in several plant sciences including conservation, restoration and population genetics (Hufford and
Mazer, 2003). Ecotypic differentiation enables species to
survive diverse habitats and environmental conditions
across its geographical range, but the specific functions
they serve in ecosystems remain unclear (Seliskar et al.,
2002). For this reason, using local plant material for
restoration purposes or reintroductions may be necessary
to maintain ecosystem health (Linhart, 1995). Introducing
poorly adapted ecotypes into unsuitable habitats may lead
to reduced plant population fitness (Hufford and Mazer,
2003; McKay et al., 2005).
Common garden studies are often utilized to detect local
adaptation (Sanders and McGraw, 2005), but obtaining
permits to collect and transplant protected, rare, threatened
* For correspondence. E-mail pkauth@ufl.edu
or endangered species is often difficult. Alternatively, studying
the ecology and physiology of seed germination and seedling
development from widespread populations may provide
insight into ecotypic differentiation (Singh, 1973). Currently,
little information exists on seed germination among
geographically distinct orchid populations as well as orchid
ecotypic differentiation. Studies of orchid seed germination
ecology are needed to support reintroduction programmes
that typically use seed germination as a propagation tool.
Calopogon tuberosus var. tuberosus is a terrestrial orchid
of eastern North America from Florida to Canada and west
to Texas, and occupies habitats including alkaline prairies,
pine flatwoods, mesic roadsides, fens and sphagnum
bogs (Luer, 1972). Goldman et al. (2004) defined three
C. tuberosus geographic clines. Northern plants in glaciated
areas are differentiated from southern plants by labellum
apex shape, reduced flower size and reduced leaf and
inflorescence height. South-west populations west of the
Mississippi Embayment differ from those in the south-east
# The Author 2008. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved.
For Permissions, please email: journals.permissions@oxfordjournals.org
Downloaded from aob.oxfordjournals.org at University of Florida on May 26, 2011
† Background and Aims Ecotypic differentiation has been explored in numerous plant species, but has been largely
ignored in the Orchidaceae. Applying a specific germination protocol for widespread seed sources may be unreliable
due to inherent physiological or genetic differences in localized populations. It is crucial to determine whether ecotypic differentiation exists for restoration and conservation programmes. Calopogon tuberosus var. tuberosus, a
widespread terrestrial orchid of eastern North America, is a model species to explore ecotypic differences in germination requirements, as this species occupies diverse habitats spanning a wide geographical range.
† Methods Mature seeds were collected from south Florida, north central Florida, three locations in South Carolina,
and the upper Michigan peninsula. Effects of three photoperiods (8/16, 12/12, 16/8 h L/D) were examined on asymbiotic in vitro seed germination and seedling development of C. tuberosus. Germination and early development was
monitored for 8 weeks, while advanced development was monitored for an additional 8 weeks. In an additional
experiment, asymbiotic seed germination and development was monitored for 8 weeks on six culture media
(BM-1 terrestrial orchid medium, Knudson C, Malmgrem, half-strength MS, P723, and Vacin and Went). A tetrazolium test for embryo viability was performed.
† Key Results Short days promoted the highest germination among Florida populations, but few differences among
photoperiods in other seed sources existed. Different media had little effect on the germination of Michigan and
Florida populations, but germination of South Carolina seeds was higher on media with higher calcium and magnesium. Tetrazolium testing confirmed that South Carolina seeds exhibited low viability while viability was
higher in Florida seeds. Seed germination and corm formation was rapid in Michigan seeds across all treatments.
Michigan seedlings allocated more biomass to corms compared with other seed sources.
† Conclusions Rapid germination and corm formation may be a survival mechanism in response to a compressed
growing season in northern populations. Ecotypic differentiation may be occurring based on seed germination
and corm formation data.
784
Kauth et al. — Seed Germination and Seedling Development of Calopogon
M AT E R I A L S A N D M E T H O D S
Seed source
Intact seed capsules (slightly yellow in colour) of Calopogon
tuberosus (L.) Britton, Sterns & Poggenb. var. tuberosus were
collected before dehiscence approx. 2 months after peak flowering throughout summer 2006. Capsules were collected from
the Florida Panther National Wildlife Refuge (Collier County,
Florida, USA), Goethe State Forest (Levy County, Florida,
USA), Ashmore Heritage Preserve (Greenville County,
South Carolina, USA), Eva Chandler Heritage Preserve
(Greenville County, South Carolina, USA), site ‘C’ near
Eva Chandler Heritage Preserve (Greenville County, South
Carolina, USA) and Carney Fen (Menominee County,
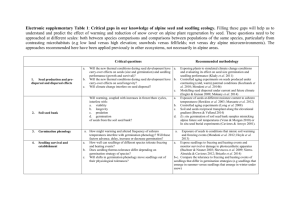
Michigan, USA; Fig. 1). The populations from site ‘C’ and
Eva Chandler occupy cataract bogs, which form when
streams flow over granite out-cropping resulting in
sphagnum-filled depressions (Porcher and Rayner, 2001);
for further site-specific information see Table 1.
Non-dehisced capsules were collected to reduce the potential
for surface contamination of individual seeds. Upon collecting and receiving capsules, they were stored at 23 8C over
silica desiccant for 2 weeks. After 2 weeks, seeds were
removed from the capsules and stored over silica desiccant
at –11 8C until use.
Seed viability test
A seed viability test (Lakon, 1949) was performed on all
populations by staining embryos with 2,3,5-triphenyl tetrazolium chloride (TTC). Seeds were scarified in an aqueous
5 % CaOCl2 solution for 0 min, 30 min, 1 h, 2 h or 3 h. Two
replications of approx. 100 seeds each were used per treatment. After scarification, seeds were rinsed twice in
distilled-deionized (dd) water and suspended in sterile
water for 24 h in darkness at 23 + 2 8C. Water was replaced
with TTC and seeds were soaked for 24 h at 30 8C in darkness. After the TTC soak, embryos were scored as viable if
any degree of red staining was observed.
Media and seed preparation
Media were prepared in 1000-ml batches, and the pH was
adjusted to 5.7 with 0.1 N KOH prior to autoclaving for
40 min at 117.7 kPa and 121 8C. Aliquots (40 mL) of
sterile medium were dispensed into square 100 15 mm
Petri plates with a 36-cell bottom (IntegridTM Petri Dish;
Becton Dickinson and Co., Franklin Lakes, NJ, USA).
Mature seeds were surface sterilized in sterile scintillation
vials for 3 min in a solution of 5 mL absolute ethanol,
5 mL 6 % NaOCl and 90 mL sterile dd water. Seeds were
rinsed twice with sterile dd water after surface sterilization.
Solutions were removed from the vials with sterile Pasteur
pipettes. Seeds were then placed on the surface of the germination media with a sterile inoculating loop. The interior
16 cells of the Petri plates were used for subreplications to
avoid uneven media drying at the edges. Petri plates were
sealed with one layer of Nescofilm (Karlan Research
Products, Santa Rosa, CA, USA). Seed germination and
seedling development (Table 2) were monitored weekly
for 8 weeks according to the six developmental stages
described by Kauth et al. (2006).
Photoperiod effects on asymbiotic germination and early
seedling development
A 6 3 factorial design was used with six seed sources
and three photoperiods including a short day (SD ¼ 8/16 h
L/D), neutral day (ND ¼ 12/12 h L/D), long day (LD ¼ 16/
8 h L/D). PhytoTechnology Orchid Seed Sowing Medium
(#P723; PhytoTechnology Laboratories, Shawnee Mission,
KS, USA) was used based on previous success with
C. tuberosus seed germination and development (Kauth
et al., 2006). Ten replicate Petri plates with five randomly
selected subreplications (48.5 + 17.9 seeds) were used per
seed source and photoperiod treatment. Culture vessels
were placed under cool-white fluorescent lights (F96712,
General Electric) at an average of 33.3 + 7 (12/12 photoperiod), 31.6 + 5 (8/16 photoperiod) and 31.6 + 6 (16/8
photoperiod) mmol m22 s21, and incubated at 25 + 0.4 8C.
Photoperiod effects on advanced in vitro seedling development
After 8 weeks, seedlings in the photoperiod experiment
were transferred from Petri plates to PhytoTech culture
boxes (95 95 100 mm) containing 100 mL P723
medium; seedlings were maintained in corresponding
photoperiods. Ten seedlings were transferred to each
culture box. After an additional 8 weeks and 16 weeks
total, five culture vessels per treatment (50 total seedlings)
were randomly selected. Seedling percentage biomass
Downloaded from aob.oxfordjournals.org at University of Florida on May 26, 2011
by larger flowers and inflorescence heights. Morphological
variation may be caused by environmental factors or
cross-pollination avoidance with other Calopogon species
(Goldman et al., 2004).
Given that C. tuberosus is a commonly recognized orchid
in North America, information exists regarding ecology,
pollination and seed germination for this species. However,
seed germination information is often conflicting. Different
environmental conditions for seed germination of
C. tuberosus have been recommended ranging from complete
darkness to light incubation (Stoutamire, 1974; Whitlow,
1996; Kauth et al., 2006). Likewise, different germination
media have also been recommended (Henrich et al., 1981;
Arditti et al., 1985; Anderson, 1990; Kauth et al., 2006).
Differences in germination and seedling development
might be the result of local adaptation to specific environmental conditions. Attributing ecotypic differentiation to
germination differences is difficult since seed source is
rarely reported in C. tuberosus seed germination studies,
and basing recommendations for seed germination of
C. tuberosus on one population is tenuous. Evaluation of
in vitro seed germination from diverse populations may
clarify whether ecotypic differentiation occurs among
C. tuberosus populations. In this paper, the effects of photoperiod and culture media on asymbiotic seed germination
and seedling development are compared among widespread
populations of C. tuberosus.
Kauth et al. — Seed Germination and Seedling Development of Calopogon
785
Downloaded from aob.oxfordjournals.org at University of Florida on May 26, 2011
F I G . 1. Habitat and location of Calopogon tuberosus populations used in the present study: (A) Calopogon tuberosus flower; (B) fen habitat on the
upper Michigan peninsula; (C) cataract bog in South Carolina; (D) roadside habitat in north central Florida; (E) prairie habitat in south Florida;
(F) population locations.
allocation was determined by dividing corm, root and shoot
weights by the total seedling weight. Culture conditions
were the same as previously described.
Asymbiotic germination media evaluation
A 6 6 factorial design with six germination media
(Table 3) and six seed sources was used. Five media
commercially prepared by PhytoTechnology Laboratories
were used: BM-1 Terrestrial Orchid Medium (BM-1;
#B141; van Waes and Debergh, 1986a), Knudson C Orchid
Medium (KC; #K400; Knudson, 1946), Malmgren
Modified Terrestrial Orchid Medium (MM; #M482;
Malmgren, 1996), Orchid Seed Sowing Medium (#P723),
and Vacin and Went Modified Orchid Medium (VW;
#V895; Vacin and Went, 1949). Murashige and Skoog
Medium in half-strength (MS; #M5524; Murashige and
Skoog, 1962) was commercially prepared by Sigma-Aldrich
786
Kauth et al. — Seed Germination and Seedling Development of Calopogon
TA B L E 1. Location, habitat, and basic environmental conditions of Calopogon tuberosus seed sources used in the present
study
Population location and
(designation)
Co-ordinates
Habitat
Panther Refuge (south Florida)
268100 0600 N, 818210 5100 W
Goethe State Forest (north
central Florida)
Ashmore Preserve (South
Carolina 1)
Site ‘C’ (South Carolina 2)
298090 1800 N, 828370 1200 W
Chandler Preserve (South
Carolina 3)
Carney fen (Michigan)
TA B L E
Cataract bog
0
00
00
0
0
00
00
35805 03 N, 82836 27 W
Cataract bog
458340 4700 N, 878390 3800 W
Fen
Description
Imbibed seed, swollen and greening still covered or partially
covered by testa
Enlarged seed without testa
Protocorm with pointed shoot apex and rhizoids
Protocorm with emerging leaf and developing rhizoids
Seedling with one elongated leaf and one developing root
Seedling with evident roots and two or more leaves
(St Louis, MO, USA). BM-1 and VW were further supplemented with 0.1 % charcoal, KC was further
supplemented with 0.1 % charcoal and 0.8 % TCw agar
(PhytoTechnology Laboratories), MM was further supplemented with 0.8 % TCw agar, and 0.5MS was further supplemented with 0.1 % charcoal, 0.8 % TC agar, and organics
found in P723. All media contained 2 % sucrose. Five replicate Petri plates with three randomly selected subreplications
(62.6 + 15.2 seeds) were used per treatment. Germination
and development were monitored biweekly for 8 weeks.
Culture vessels were placed under ND conditions, cool-white
fluorescent lights at 33.3 + 7 mmol m22 s21, and 25 + 0.4 8C.
Statistical nalysis
Germination percentages were calculated by dividing the
number of germinated seeds by the total number of seeds
with an embryo in each subreplication. The percentage of
protocorms and seedlings in a developmental stage was calculated by dividing the number of seeds in a stage by the
total number of seeds with an embryo. Germination
counts were arcsine transformed to normalize variation.
Germination and seedling development data were analysed
using general linear model procedures and least square
means at a ¼ 0.05 in SAS v. 8.02.
R E S U LT S
Seed viability
For all populations except south Florida (no difference in
pretreatment time), the highest percentage of viable
Long day
Short day
Ochopee fine sandy loam (fine sandy
loam)
Smyrna fine sand (fine sand)
13 h 47 min
10 h 30 min
14 h 02 min
10 h 16 min
Congaree (sphagnum/fine sandy loam)
14 h 30 min
9 h 49 min
Ashe-Cleveland association (sphagnum/
sandy loam)
Ashe-Cleveland association (sphagnum/
sandy loam)
Lupton-Cathro association (sphagnum/
muck)
14 h 30 min
9 h 49 min
14 h 30 min
9 h 49 min
15 h 42 min
8 h 46 min
embryos was observed after 3 h of calcium hypochlorite
pretreatment. Maximum embryo viability for each population was as follows: 85.4 % south Florida; 66.7 % north
central Florida; 25.0 % South Carolina 1; 38.1 % South
Carolina 2; 42.1 % South Carolina 3; and 50.3 % Michigan.
Photoperiod effects on germination and early development
Total seed germination percentage (Fig. 2) was highest
under SD conditions for north central Florida (60.2 %)
and south Florida (48.5 %) populations. There was no
difference in germination among the three photoperiods
for Michigan seeds (11.6 %, 12.5 % and 9.9 %).
Germination percentages in all South Carolina populations
did not exceed 4 %.
Seeds from Michigan germinated and developed more
quickly compared with other populations, with imbibition
occurring 1 week after inoculation. By week 8 .95 % of
the germinated protocorms in all photoperiods developed
to stage 6. Protocorm development was similar among
photoperiods (Fig. 2A).
South Carolina protocorm development was unpredictable. South Carolina 1 (Fig. 2B) and South Carolina 2
(Fig. 2C) protocorms developed slowly with ,1 % developing to leaf-bearing stages. Only South Carolina 1 protocorms under ND developed to stage 6 while South
Carolina 2 protocorms under both ND and LD conditions
developed to stages 5 and 6. Development of South
Carolina 3 (Fig. 2D) protocorms was more advanced than
other South Carolina populations.
Seeds from north central Florida germinated quickly
and corms formed after week 8. Greater than 16 % of the
protocorms in each photoperiod developed to an advanced
leaf-bearing stage (stage 6) by week 8 (Fig. 2E).
Although germination of south Florida seeds was highest
under SD conditions, the majority of seeds did not
develop past the imbibition stage by week 8 (Fig. 2F).
Fewer than 5 % of the south Florida seeds under SD conditions developed past imbibition after 8 weeks culture.
Approximately 10 % of the seeds under both ND and LD
conditions developed past imbibition. A low percentage
of south Florida seedlings in all photoperiods developed
to advanced leaf-bearing stages (stages 5 and 6).
Downloaded from aob.oxfordjournals.org at University of Florida on May 26, 2011
2
3
4
5
6
358050 0200 N, 828350 5100 W
0
2. Six stages of orchid seed development (from
Kauth et al., 2006)
Stage
1
35805 13 N, 82834 46 W
Alkaline
prairie
Mesic
roadside
Lake bog
Soil designation and (composition)
Kauth et al. — Seed Germination and Seedling Development of Calopogon
787
TA B L E 3. Comparative mineral salt content of asymbiotic orchid seed germination media: BM-1 Terrestrial Orchid Medium
(BM-1), Knudson C (KC), Malmgren Modified Terrestrial Orchid Medium (MM), Murashige and Skoog (MS),
PhytoTechnology Orchid Seed Sowing Medium (P723), Vacin and Went (VW)
MM
0.73
0.81
0.0021
0.83
0.55
1.03
0.92
0.20
2.20
2.20
1.10
0.20
100
10
0.05
400
0.5
2.0
100
4.35
n/a
161.7
0.105
0.10
100.2
147.9
1.03
34.8
0.05
500
0.5
100
2.0
100
5.0
0.5
0.5
6.98
n/a
Photoperiod effects on advanced seedling development
After 16 weeks culture, Michigan seedlings began to
senesce while South Carolina and Florida seedlings continued to grow. Corm formation was limited in south Florida
seedlings, while Michigan, South Carolina and north
central Florida seedlings all formed corms (Fig. 3).
Biomass allocation was similar among photoperiods
within each seed source (Fig. 4). Maximum dry weight allocation to corms was observed in Michigan seedlings
(Fig. 4A). Although north central Florida seedlings
formed large corms, the percentage dry biomass allocation
was more evenly distributed among shoots, corms and roots
than other populations (Fig. 4C). The greatest seedling
shoot biomass allocation was observed in South Carolina
3 and south Florida populations (Fig. 4B, D).
Media effects on germination and early development
Michigan seeds germinated and protocorms developed
quickly on all media, but the highest germination percentages occurred on P723 (34.1 %). With the exception of
KC and VW, over 90 % of the protocorms on all other
media developed to stage 6 (Fig. 5A).
Seed germination for South Carolina 1 was highest on
VW, but germination was only 4.9 % (Fig. 5B). No clear
P723
5.15
0.75
1.50
0.62
9.85
5.62
0.31
0.71
0.10
26.7
0.026
0.025
50
1.25
25
0.26
9.22
100
1.0
2000
1.0
10
24.72
15.00
VW
7.57
1.93
1.01
5.19
7.03
3.77
8.71
0.20
KC
0.5MS
13.82
2.12
3.35
1.01
10.49
5.19
1.84
8.69
10.31
1.50
3.1
0.75
19.70
10.89
0.63
0.86
0.10
100
90
30
30
35.54
12.76
46.72
24.31
50
0.053
0.5
50
2.50
50
0.52
14.95
100
1.0
2000
1.0
10
48.01
30.01
differences in germination were observed in South
Carolina 2 (Fig. 5C), but germination on P723 was
significantly lower than all other media. Germination on
KC (39.7 %) and MS (30.4 %) was highest for South
Carolina 3 seeds, while lowest germination occurred on
P723 (Fig. 5D).
In both Florida populations, few differences in total germination existed among media; however, subsequent development differed greatly. For north central Florida, higher
numbers of stage 4, 5 and 6 seedlings were observed on
BM-1, MS, P723 and VW (Fig. 5E). The highest germination percentage of north central Florida seeds was observed
on MM, but the majority of seeds remained in stage 1 after
8 weeks. The highest percentage of stage 4, 5 and 6 protocorms was observed on BM-1, P723 and VW for south
Florida (Fig. 5F). Germination percentages were high on
KC and MM for south Florida seeds, but no stage 6 seedlings developed within 8 weeks and considerably fewer
stage 5 seedlings developed compared with all other media.
Media effects on corm development
Corm development on BM-1, MS and P723 was superior
in all populations (Fig. 6). Seedling development of
Michigan, South Carolina 3, and north central Florida
seedlings was superior to other populations (Fig. 6).
Downloaded from aob.oxfordjournals.org at University of Florida on May 26, 2011
Macronutrients (mM)
Ammonium
Calcium
Chlorine
Magnesium
Nitrate
Potassium
Phosphate
Sulfate
Sodium
Micronutrients (mM)
Boron
Cobalt
Copper
Iron
Iodine
Manganese
Molybdenum
Zinc
Organics (mg L21)
Biotin
Casein hydrolysate
Folic acid
L-Glutamine
Glycine
myo-Inositol
Nicotinic acid
Peptone
Pyridoxine HCl
Thiamine HCl
Total mineral salt concentration (mM)
Total inorganic N (mM)
BM-1
14
Michigan
a
A
12
a
a
10
8
6
4
2
0
C
1·2
South Carolina 2
a
a
1·0
0·8
0·6
0·4
a
0·0
North Central Florida
70 E
60
a
b
50
b
40
30
20
10
0
8/16
12/12
16/8
0·5
South Carolina 1
B
a
Stage 1
Stage 2
Stage 3
Stage 4
Stage 5
Stage 6
0·4
0·3
0·2
0·1
b
0·0
4
South Carolina 3
D
a
3
2
b
1
b
0
70
South Florida
F
60
50
a
40
b
b
12/12
16/8
30
20
10
0
8/16
Photoperiod
Photoperiod
F I G . 2. Photoperiod effects on seed germination and subsequent development of Calopogon tuberosus from different populations after culture on P723
medium for 8 weeks: (A) upper Michigan peninsula population; (B) South Carolina population from Ashmore; (C) South Carolina population from site
‘C’; (D) South Carolina population from Eva Chandler; (E) north central Florida population; (F) South Florida population. Histobars within each seed
source with the same letter are not significantly different (a ¼ 0.05). See Table 2 for stages of germination and development.
However, development of Michigan, South Carolina 3, and
north central Florida seedlings differed markedly. Corm
formation was more pronounced in seedlings from northern
latitudes. Thus by week 8, no corm formation was observed
in Florida seedlings while early and advanced corm
formation was observed in South Carolina and Michigan
seedlings, respectively.
DISCUSSION
Based upon differences in seed germination, seedling
development and, particularly, corm development among
C. tuberosus populations, further evidence for ecotypic
differentiation beyond morphological variation is provided.
Goldman et al. (2004) reported that morphological
variation in C. tuberosus correlating to geographic location
was likely to be caused by different selection pressures and
abiotic factors, but these selective pressures were not
specifically explored with respect to ecotypic
differentiation.
Seed viability and quality
Differences in seed germination responses are often
attributed to seed viability and quality. Comparisons of
orchid seed germination among populations of the same
species have been reported, but C. tuberosus has not been
examined. Symbiotic germination and mycorrhizal specificity among populations rather than ecotypic differentiation
were examined in these studies (Zettler and McInnis, 1992;
Zettler and Hofer, 1998; Sharma et al., 2003). However,
differences in seed germination and viability among populations were described which might be accounted for by
ecotypic differentiation.
Downloaded from aob.oxfordjournals.org at University of Florida on May 26, 2011
0·2
Germination and development (%) Germination and development (%) Germination and development (%)
Kauth et al. — Seed Germination and Seedling Development of Calopogon
Germination and development (%) Germination and development (%) Germination and development (%)
788
Kauth et al. — Seed Germination and Seedling Development of Calopogon
South Florida
North Central Florida
South Carolina
789
Michigan
8/16
12/12
16/8
Percent total dry weight
80
A
Michigan
B
South Carolina 3
North Central Florida
D
South Florida
Shoot
Corm
Root
70
60
50
40
30
20
10
Percent total dry weight
0
80
C
70
60
50
40
30
20
10
0
8/16
12/12
Photoperiod
16/8
8/16
12/12
16/8
Photoperiod
F I G . 4. Percentage dry weight biomass allocation of Calopogon tuberosus seedlings after 16 weeks in vitro culture: (A) upper Michigan peninsula
population; (B) South Carolina population from Eva Chandler; (C) north central Florida population; (D) south Florida population. Histobars represent
the mean response of 50 seedlings + s.e.
Downloaded from aob.oxfordjournals.org at University of Florida on May 26, 2011
F I G . 3. Effects of photoperiod on in vitro seedling development of Calopogon tuberosus from different populations after 16 weeks total culture (8 weeks
in Petri dishes/8 weeks in PhytoTech culture boxes): (A–D) seedlings cultured under an 8/16 h L/D photoperiod; (E –H) seedlings cultured under a 12/
12 h L/D photoperiod; (I–L) seedlings cultured under a 16/8 h L/D photoperiod; (A, E, I) South Florida seedlings; (B, F, J) North Central Florida seedlings; (C, G, K) South Carolina seedlings from Eva Chandler; (D, H, L) upper Michigan peninsula seedlings. Scale bars ¼ 1 cm.
Kauth et al. — Seed Germination and Seedling Development of Calopogon
35
A
B
Michigan
a
30
25
ab
20
b
b
b
b
15
10
5
Germination and development (%)
Germination and development (%)
790
South Carolina 1
Stage 1
Stage 2
Stage 3
Stage 4
Stage 5
Stage 6
8
6
a
4
b
2
b
b
0
a
6
4
a
a
ab
2
a
b
0
70
Germination and development (%)
South Carolina 2
C
North Central Florida
E
a
a
60
ab
50
a
ab
b
40
30
20
10
Germination and development (%)
Germination and development (%)
8
40
D
a
South Carolina 3
ab
30
b
20
c
c
10
0
70
d
F
South Florida
a
60
ab
ab
abc
bc
50
c
40
30
20
10
0
0
BM-1
KC
MM
MS
P723
VW
Germination media
BM-1
KC
MM
MS
P723
VW
Germination media
F I G . 5. Effects of culture media on seed germination and early development of Calopogon tuberosus from different populations after 8 weeks culture
under a 12/12 h L/D photoperiod: (A) upper Michigan peninsula population; (B) South Carolina population from Ashmore; (C) South Carolina population
from site ‘C’; (D) South Carolina population from Eva Chandler; (E) north central Florida population; (F) south Florida population. Histobars within each
seed source with the same letter are not significantly different (a ¼ 0.05). For media abbreviations and formulae see Table 3. See Table 2 for stages of
germination and development.
Population size and inbreeding depression may influence
low seed germination of several C. tuberosus populations
as well as differences in seed viability. Lower germination percentages in small populations of Platanthera
integrilabia, compared with larger populations, were
attributed to lower seed viability (Zettler and McInnis,
1992). Similarly, Platanthera clavellata seed germination
differences were attributed to inbreeding depression
(Zettler and Hofer, 1998). Reduction in pollinator
numbers at different sites may lead to seed viability
differences in C. tuberosus as reported for Platanthera
leucophaea and P. praeclara (Bowles et al., 2002;
Sharma et al., 2003).
Another plausible explanation regarding differences in
seed viability may be self-pollination. Calopogon tuberosus
is a non-rewarding/out-crosser pollinated by Bombus,
Xylocopa and Megachile bees through deception (van der
Pijl and Dodson, 1966; Thien and Marcks, 1972;
Dressler, 1981). Self-pollination in C. tuberosus may be
common as Firmage and Cole (1988) reported in Maine
populations. Self-pollination in Calypso bulbosa, and probably C. tuberosus, was mediated by bumble bees since a
Downloaded from aob.oxfordjournals.org at University of Florida on May 26, 2011
Germination and development (%)
0
Kauth et al. — Seed Germination and Seedling Development of Calopogon
BM-1
P723
791
0·5MS
North
Central
Florida
South
Carolina
F I G . 6. Culture media effects on early seedling development of Calopogon tuberosus from different populations after culture for 8 weeks: (A–C) north
central Florida seedlings; (D– F) South Carolina seedlings from Eva Chandler; (G –I) Upper Michigan peninsula seedlings; (A, D, G) seedlings cultured
on BM-1 Terrestrial Orchid Medium; (B, E, H) seedlings cultured on P723 Orchid Seed Sowing Medium; (C, F, I) seedlings cultured on half-strength MS.
Scale bars ¼ 1 cm.
mechanism for autogamy does not exist (Alexandersson
and Agren, 2000). While fruit set is generally not affected
by self-pollination, reduced seed viability or embryo production can be reduced (Tremblay et al., 2005). Low seed
viability and germinability in certain C. tuberosus populations may be caused by higher levels of self-pollination;
however, further investigation is warranted.
Differences in viability may be explained by varying
degrees of testa permeability or hardness that warrants
investigation. van Waes and Debergh (1986b) reported
various optimal pretreatment times from 45 min to 16 h in
calcium hypochlorite in 31 species of terrestrial orchids,
thus differences in C. tuberosus viability are not surprising.
Differences in the testa structures among C. tuberosus
populations are likely since dry seeds among populations
appear different ( pers. obs.). Seeds from South Carolina
have an opaque testa with rounded ends. Seeds from
Michigan are long, narrow, and have tapered ends. Both
Michigan and South Carolina seeds appear to have thick
testas. Seeds from north central Florida are small and
transparent, while seeds from south Florida contain large
embryos and are also transparent. Because northern
populations had lower viabilities, longer pretreatment in
calcium hypochlorite may be required to weaken the less
permeable testas.
The correlation between TTC determined seed viability
and the corresponding observed percentage germination
is often variable and species specific (St-Arnaud et al.,
1992; Shoushtari et al., 1994; van Waes and Debergh,
1986a). Tetrazolium testing can overestimate viability
because this test does not detect inactive enzymes that
may become active during germination (Lauzer et al.,
1994). For this reason, fluorescein diacetate (FDA) is used
with results often correlating with germination (Pritchard,
1985; Vendrame et al., 2007). Lower germination percentages compared with viability may reflect non-optimal
temperatures with seeds from northern climates requiring
cooler temperatures in vitro or stratification to germinate;
these concerns are currently being addressed in separate
experiments. In addition, seeds that do not germinate
in vitro may have an intrinsic dormancy mechanism.
Embryo damage during surface sterilization is also a
likely scenario that may have reduced germination.
Photoperiod
As far as is known, no other published articles exist that
compare photoperiodic effects on North American orchid
seed germination spanning several populations of the
same species. For non-orchid ecotypes, however, photoperiod is reported to be an important factor on germination
(Singh, 1973; Seneca, 1974; Probert et al., 1985).
Due to latitudinal differences in location, populations
experience different seasonal variations in photoperiod,
temperature regimes and growing season. Calopogon
tuberosus flowers in early June to mid-July in the north
Downloaded from aob.oxfordjournals.org at University of Florida on May 26, 2011
Michigan
792
Kauth et al. — Seed Germination and Seedling Development of Calopogon
Calopogon tuberosus from Eva Chandler Heritage
Preserve in South Carolina is found in proximity to the
rare Parnassia grandiflora, indicating high calcium and
magnesium content in granite outcroppings (Porcher and
Rayner, 2001). Although South Carolina 3 seed germination was low, higher germination occurred on VW and
KC, which contain higher concentrations of both calcium
and magnesium. Soil analysis from each C. tuberosus population may provide insight into differences in soil nutrient
availability, and ultimately seed germination. Soil analysis
from each C. tuberosus population may provide insight
into differences in soil nutrient availability, and ultimately
seed germination.
Conclusions
This study provides insight into physiological and
developmental aspects that are important aspects for
ecotypic differentiation. Based on in vitro seed germination
studies, ecotypic differentiation may be occurring within
C. tuberosus, evident by rapid germination and subsequent
seedling development, as well as immediate corm formation in northern populations. Rapid corm development
in northern plants may be a consequence of the relatively
shorter growing season experienced by these populations.
Conversely, southern plants display greater shoot biomass
allocation and a slower tendency to form corms. Ecotypic
differentiation does not extend only to distant populations
(i.e. Florida and Michigan), but also within close proximity
(north central and south Florida populations approx.
400 km apart).
In vitro seed germination is only one technique that can
be utilized to differentiate ecotypes. Combining in vitro
results with in situ data may provide more understanding
into ecotypic differentiation since conditions experienced
in the field differ from those in vitro. Other techniques
should be integrated along with in vitro techniques such
as in situ germination, cytological examination and
genetic analysis, to gain a more complete understanding.
These topics are being examined in subsequent studies.
Media screen
P723 proved to be an adequate medium for germinating
Florida and Michigan seeds, but discrepancies between
germination and viability may have been caused by using
a non-optimal medium for other seed sources. Abundant
literature exists on mineral nutrition of orchid seeds, and
how media composition influences germination and
development (Curtis, 1947; Spoerl and Curtis, 1948;
Raghavan, 1964; van Waes and Debergh, 1986a; Kauth
et al., 2006). However, site-specific differences in soil
nutrient availability could explain differences in germination and development as found in Dactylorhiza incarnata
by Dijk and Eck (1995). Seedlings from coastal areas
grew faster in vitro and were more tolerant of exogenous
ammonium and nitrate compared with seedlings from
inland populations. Coastal populations inhabit calcareous
areas where high nutrient levels are found due to the
introduction of fertilizers and poor drainage (Dijk and
Eck, 1995).
ACK N OW L E D G E M E N T S
We thank the following for collecting seed: Larry
Richardson (Wildlife Biologist; Florida Panther National
Wildlife Refuge); Jim Fowler (South Carolina populations);
Kip Knudson (Carney Fen population). We also thank Mary
Bunch (South Carolina Heritage Preserve Program) for
issuing collection permits. Brand names are provided as
references; the authors do not solely recommend or
endorse these products. We also thank the US Fish and
Wildlife-Florida Panther National Wildlife Refuge for
assisting with partial financial support.
L I T E R AT U R E CI T E D
Alexandersson R, Agren J. 2000. Genetic structure in the nonrewarding,
bumblebee-pollinated orchid Calypso bulbosa. Heredity 85: 401–409.
Anderson AB. 1990. Asymbiotic germination of seeds of some North
American orchids. In: Sawyers CE, ed. North American native
Downloaded from aob.oxfordjournals.org at University of Florida on May 26, 2011
and mid-May to early June in the south (Luer, 1972).
In Florida, seed capsules dehisce and seeds are disbursed
in July when photoperiods are approx. 13– 14 h.
Short-day conditions promoted the highest germination percentage for both Florida populations. At both Florida
locations, the shortest natural photoperiods do not approach
8 h, but approx. 10 h. Whether Florida seeds are somewhat
light sensitive during germination remains unclear without
also conducting in situ germination studies.
Development of protocorms in all three photoperiods for
north central Florida was very rapid compared with south
Florida protocorms. A large percentage of south Florida
seeds germinated only to the imbibition stage by week 8,
perhaps due to the longer growing season in south
Florida. In north central Florida, lower daily surface temperatures in winter can drop below freezing point, while low
daily winter temperatures in south Florida rarely drop below
5 8C. The warmer conditions in south Florida may aid in
slower protocorm development, assuming that temperatures
within the soil where protocorms reside are also different.
After 16 weeks culture, south Florida seedlings were
small and did not form corms, while north central Florida
seedlings were larger and readily formed corms.
Although total germination was low in Michigan seeds,
seedling development and corm formation was more rapid
than those from the southern populations. Imbibition
occurred after 1 week, and corm initiation began by week
6. Regardless of photoperiod, after 16 weeks culture the
large seedling corm : shoot : root ratios generated in seedlings from the Michigan population suggest that a high
percentage of carbohydrates are allocated to corms. Rapid
seed germination, seedling development and corm formation in northern populations may be indicative of a
photoperiod-insensitive seedling developmental sequence
that ensures rapid corm development during short northern
growing seasons and increases winter survival. Kane et al.
(2000) similarly reported more rapid corm development in
more northern ecotypes of the wetland non-orchid species
Sagittaria latifolia.
Kauth et al. — Seed Germination and Seedling Development of Calopogon
Pritchard HW. 1985. Determination of orchid seed viability using
fluorescein diacetate. Plant, Cell & Environment 8: 727–730.
Probert RJ, Smith RD, Birch P. 1985. Germination responses to light
and alternating temperatures in European populations of Dactylis
glomerata L. New Phytologist 99: 305– 316.
Raghavan V. 1964. Effects of certain organic nitrogen compounds on
growth in vitro of seedlings of Cattleya. Botanical Gazette 125:
260– 267.
Sanders S, McGraw JB. 2005. Population differentiation of a threatened
plant: variation in response to local environment and implications for
restoration. Journal of the Torrey Botanical Society 132: 561– 572.
Seliskar DM, Gallagher JL, Burdick DM, Mutz LA. 2002. The regulation of ecosystem functions by ecotypic variation in the dominant
plant: a Spartina alterniflora salt-marsh case study. Journal of
Ecology 90: 1 –11.
Seneca ED. 1974. Germination and seedling response of Atlantic and Gulf
coasts populations of Spartina alterniflora. American Journal of
Botany 61: 947 –956.
Sharma J, Zettler LW, Van Sambeek JW, Ellersieck MR, Starbuck
CJ. 2003. Symbiotic seed germination and mycorrhizae of federally
threatened Platanthera praeclara (Orchidaceae). American Midland
Naturalist 149: 104 –120.
Shoushtari BD, Heydari R, Johnson GL, Arditti J. 1994. Germination
and viability staining of orchid seeds following prolonged storage.
Lindleyana 9: 77–84.
Singh KP. 1973. Effect of temperature and light on seed germination of
two ecotypes of Portulaca oleracea L. New Phytologist 752:
289– 295.
Spoerl E, Curtis JT. 1948. Studies of the nitrogen nutrition of orchid
embryos. III. Amino acid nitrogen. American Orchid Society
Bulletin 17: 307–312.
St-Arnaud M, Lauzer D, Barabé D. 1992. In vitro germination and early
growth of seedlings of Cypripedium acaule (Orchidaceae). Lindleyana
7: 22–27.
Stoutamire WP. 1974. Terrestrial orchid seedlings. In: Withner CL, ed.
The orchids: scientific studies. New York, NY, John Wiley and
Sons, 101–128.
Thien LB, Marcks BG. 1972. The floral biology of Arethusa bulbosa,
Calopogon tuberosus, and Pogonia ophioglossiodes (Orchidaceae).
Canadian Journal of Botany 50: 2319– 2325.
Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. 2005.
Variation in sexual reproduction in orchids and its evolutionary
consequences: a spasmodic journey to diversification. Biological
Journal of the Linnean Society 84: 1– 54.
Vacin EF, Went FW. 1949. Some pH changes in nutrient solutions.
Botanical Gazette 110: 605– 613.
Vendrame WA, Carvalho VS, Dias JMM. 2007. In vitro germination and
seedling development of cryopreserved Dendrobium hybrid mature
seeds. Scientia Horticulturae 114: 188–193.
van Waes JM, Debergh PC. 1986a. In vitro germination of some Western
European orchids. Physiologia Plantarum 67: 253–261.
van Waes JM, Debergh PC. 1986b. Adaptation of the tetrazolium method
for testing the seed viability, and scanning electron microscopy study
of some Western European orchids. Physiologia Plantarum 66:
435– 442.
Whitlow CE. 1996. Mass production of Calopogon tuberosus. In: Allen C,
ed. North American native terrestrial orchids: propagation and
production. Germantown, MD: North American Native Terrestrial
Orchid Conference, 5 –10.
Zettler LW, Hofer CJ. 1998. Propagation of the little club-spur orchid
(Platanthera clavellata) by symbiotic seed germination, and its
ecological implications. Environmental and Experimental Botany
39: 189 –195.
Zettler LW, McInnis TM. 1992. Propagation of Platanthera integrilabia
(Correll) Luer, and endangered terrestrial orchid, through symbiotic
seed germination. Lindleyana 7: 154 –161.
Downloaded from aob.oxfordjournals.org at University of Florida on May 26, 2011
terrestrial orchids: propagation and production. Chadds Ford, PA:
Brandywine Conservancy, 75–80.
Arditti J, Oliva AP, Michaud JD. 1985. Practical germination of North
American and related orchids. 3. Calopogon tuberosus, Calypso
bulbosa, Cypripedium species and hybrids, Piperia elegans var.
elata, Piperia maritima, Platanthera hyberborea, and Platanthera
saccata. American Orchid Society Bulletin 54: 859 –872.
Bowles ML, Jacobs KA, Zettler LW, Delaney TW. 2002. Crossing
effects on seed viability and experimental germination of the
federal threatened Platanthera leucophaea (Orchidaceae). Rhodora
104: 14–300.
Curtis JT. 1947. Studies on the nitrogen nutrition of orchid
embryos. I. Complex nitrogen sources. American Orchid Society
Bulletin 16: 654–660.
Dijk E, Eck N. 1995. Ammonium toxicity and nitrate response of axenically grown Dactylorhiza incarnata seedlings. New Phytologist 131:
361– 367.
Dressler R L. 1981. The orchids: natural history and classification.
Cambridge, MA: Harvard University Press.
Firmage DH, Cole FR. 1988. Reproductive success and inflorescence size
of Calopogon tuberosus (Orchidaceae). American Journal of Botany
75: 1371–1377.
Goldman DH, van den Berg C, Griffith MP. 2004. Morphometric
circumscription of species and infraspecific taxa in Calopogon
R. Br. (Orchidaceae). Plant Systematics and Evolution 247: 37–60.
Henrich JE, Stimart DP, Ascher PD. 1981. Terrestrial orchid seed germination in vitro on a defined medium. Journal of the American Society
for Horticultural Science 106: 193– 196.
Hufford KM, Mazer SJ. 2003. Plant ecotypes: genetic differentiation in
the age of ecological restoration. Trends in Ecology & Evolution
18: 147 –155.
Kane ME, Gillis R, Philman N, Campbell S. 2000. Seasonal differences
in ex vitro growth and corm formation between two micropropagated
Sagittaria latifolia ecotypes. Acta Horticulturae 520: 229– 237.
Kauth PJ, Vendrame WA, Kane ME. 2006. In vitro seed culture and
seedlings development of Calopogon tuberosus. Plant Cell, Tissue
and Organ Culture 85: 91– 102.
Knudson L. 1946. A new nutrient solution for the germination of orchid
seed. American Orchid Society Bulletin 15: 214– 217.
Lakon G. 1949. The topographical tetrazolium method for determining the
germinating capacity of seeds. Plant Physiology 24: 389– 394.
Lauzer D, St-Arnaud M, Barabé D. 1994. Tetrazolium staining and
in vitro germination of mature seeds of Cypripedium acaule
(Orchidaceae). Lindleyana 9: 197– 204.
Linhart YB. 1995. Restoration, revegetation, and the importance of
genetic and evolutionary perspectives. In: Roundy BA, McArthur
ED, Haley JS, Mann DK, eds. Proceedings: wildland shrub and
arid land restoration symposium. Gen. Tech. Rep. INT-GTR-315.
Odgen, UT: US Department of Agriculture, Forest Service,
Intermountain Research Station, 271–287.
Luer CA. 1972. The native orchids of Florida. New York, NY: New York
Botanical Garden.
McKay JK, Christian CE, Harrison SH, Rice KJ. 2005. “How local is
local?”—a review of practical and conceptual issues in genetics of
restoration. Restoration Ecology 13: 432–440.
Malmgren S. 1996. Orchid propagation: theory and practice. In: Allen C,
ed. North American native terrestrial orchids: propagation and
production. Germantown, MD: North American Native Terrestrial
Orchid Conference, 63– 71.
Murashige T, Skoog F. 1962. A revised medium for rapid growth and
bioassays with tobacco tissue cultures. Physiologia Plantarum 15:
473– 497.
van der Pijl L, Dodson CH. 1966. Orchid flowers: their pollination and
evolution. Coral Gables, FL: University of Miami Press.
Porcher RD, Rayner DA. 2001. A guide to the wildflowers of South
Carolina. Colombia, SC: University of South Carolina Press.
793