Genecentric: Finding Graph Theoretic Structure in High- Throughput Epistasis Data Tufts University
advertisement

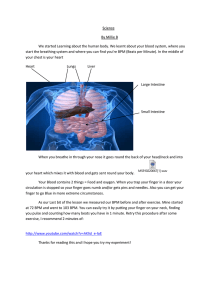
Genecentric: Finding Graph Theoretic Structure in HighThroughput Epistasis Data Andrew Gallant, Max Leiserson, M. Kachalov, Lenore Cowen, Ben Hescott Tufts University Protein-protein interaction High-throughput Interaction Data: aka ‘The Hairball’ What we want: What we have: Question: Can we infer anything about "real" pathways from the low-resolution graph model of pairwise interactions? The hairball: A simple graph model vertices ↔ genes/proteins edges ↔ physical interactions or genetic interactions simplifications: • undirected • loses temporal information • difficult to decompose into separate processes • conflates different PPI types into one class of "physical interactions" 1)Physical interactions 2) Genetic Interactions (epistasis) Interaction types • We distinguish here between two types of interaction: – physical interactions • genetic interactions Genetic interactions (epistasis) Only 18% of yeast genes are essential (the yeast dies when they’re removed). For the rest, we can compare the growth of the double knockout to its component single knockouts. Genetic interactions (epistasis) • For non-essential genes, we can compare the growth of the double knockout to its component single knockouts Picture: Ulitsky Nonessential Genes – Some genes are non-essential because they are only required under certain conditions (i.e. an enzyme to metabolize a particular nutrient). – Other genes are non-essential because the network has some built-in redundancy. • One gene (completely or partially) compensates for the loss of another. • One functional pathway (completely or partially) compensates for the loss of another. Redundant pathways and synthetic lethality Kelley and Ideker (2005): Between-Pathway Model (BPM) In reality, the data are very incomplete: Between-Pathway Model (BPM) Kelley and Ideker (2005) and Ulitsky and Shamir (2007) • Goal: detect putative BPMs in yeast interactome • Method: 1) find densely-connected subsets of the physical protein-protein interaction (PI) network (putative pathways) 2) check the genetic interaction (GI) network to see if patterns in density of genetic interactions correlate with these putative pathways 3) check resulting structures for overrepresentation of biological function (gene set enrichment) Kelley and Ideker (2005) and Ulitsky and Shamir (2007) (1) (2) enriched for function X enriched for function Y (3) Kelley and Ideker (2005) and Ulitsky and Shamir (2007) • Problems: – Sparse data limits the potential scope of discovery – independent validation is difficult Further work on this problem: Synthetic lethality: – Ulitsky and Shamir (2007) – Ma, Tarrone and Li (2008) – Brady, Maxwell, Daniels and Cowen (2009) – Hescott, Leiserson, Cowen and Slonim (2010) Epistasis (weighted) data: -- Kelley and Kingsford (2011) -- Leiserson, Tatar, Cowen and Hescott (2011) So: what is the right way to generalize BPMs to edge weights? Quantitative interaction data New methods generates high-throughput data for genetic interactions. -7.3556 -0.6347 E-MAP, Epistatic Miniarray Profile 3.69893 3.2723 Data is scalar (-22 to 15) -5.2571 -1.3668 -3.3368 -5.5312 Synthetic Lethal, < -2.5 Synthetic Sick, -2.5 < x < 0 0.5838 Synthetic Rescue, >+2.5 Allevating 0<x< 2.5 -6.3511 SGA, Synthetic Genetic Array (smaller weights, -1.1 to 0.8) Want most negative weight across 3.653986 6 3.23673 -7.32156 3.23723 -5.252571 -1.366879 -3.365368 -5.506312 3.68398 -3.36536 -0.66434 0.553838 -5.25271 -5.506312 -6.315511 2.73 0.53838 -1.36879 -6.31511 What is the Quality of a BPM? -7.321556 Once we obtain a candidate BPM we can score it using interaction data. Sum interactions within 3.685398 -3.365368 -0.664347 3.236723 -5.252571 2.13473 Sum interactions between Take the difference and normalize to create an interaction score 0.553838 0.13342 -1.366879 -6.315511 Genecentric takes the perspective of each gene in turn What is the ‘best’ candidate BPM that contains node g? -7.321556 3.685398 -3.365368 Consider a diverse set of GLOBAL partitions that try to MAXIMIZE our objective function over the whole graph. -0.664347 3.236723 -5.252571 2.13473 0.13342 0.553838 -1.366879 -6.315511 Which genes are consistently placed in the same (opposite) partition as g? So we can extract a gene’s best BPM from a diverse set of good global bipartitions Idea for constructing the global bipartitions: Maximal cut Create a random bipartition For every vertex (gene) assign to a partition at random Local search method Now for each gene, v, consider its interaction scores Unhappy vs happy vertices Flip Flip to the other side to make it happy! nowchange opposite(v) and or unhappy somesame(v) vertices is could to happy opposite(v) is same(v) Important properties Flip will always terminate - finite number of possible partitions - weight between partitions decreases with each flip - everyone is happy eventually - local optimum How we make a BPM from bipartitions -7.3215 3.6398 For every gene run weighted flip on the entire graph of interactions, M times (250 -3.3653 times) -0.66434 3.23672 -5.252571 Some genes will stay on same side for most runs. 2.1373 Some genes will stay0.55338 on the 0.13342 opposite side for most runs. -1.36679 Most will switch sides among-6.3151 the different runs BPM collection: Removing Redundancies -7.321556 Remove BPMs that are too large or small 3.685398 -3.365368 -0.664347 3.236723 -5.252571 Take the difference and divide by the size 2.13473 0.553838 0.13342 -1.366879 Sort by score, add to final output set if Jaccard index < .66 for all previously added BPMs -6.315511 Numbers chosen to match previous studies How do we measure results? • FuncAssociate to measure gene set enrichment Berriz, Beaver, Cenik, Tasan, Roth, “Next generation software for functional trend analysis,” Bioinformatics, 2009, 25(22): 3043-4. Location of physical interactions Our Results Comparison to previous methods: yeast ChromBio E-MAP #Modules / (%Enriched) #BPMs Enriched Same Function Bandyopadhyay et al. 37 (35) 96 41 (43%) 53 (55%) Ulitsky et al. 43 (43) 111 43 (39%) 71 (64%) Kelley et al. 40 (40) 98 35 (36%) 52 (53%) Genecentric 112 (103) 58 39 (67%) 43 (74%) Study Enriched Same or Similar Function How does Gencentric work with various data? SGA -7.3215 -0.66434 E-MAP (Cell Cycle) 3.6853 3.26723 -0.91511 -0.22314 0.54278 -0.687991 -5.252571 -1.366879 -3.365368 0.983123 0.253228 -5.506312 0.5538 0.404421 -6.315511 -6.31511 -7.22314 -3.12363 -1.687991 -6.63178 -5.7225 -0.22565 -0.55672 E-MAP -3.355371 (s. pombe) -2.404421 1.2833 4.51368 0.253228 1.23711 E-MAP (MAP-K) 5.22163 -7.137271 Genecentric on Various Data Sets Data Set #BPMs Enriched Same Function Collins et al. (Cell Cycle) 58 39 (67%) 43 (74%) Fiedler et al. (MAP-K) 5 0 (0%) 4 (80%) Tong et al. (SGA) 149 8 (5%) 17 (11%) 16 1 (6%) 1 (6%) Roguev et al, Enriched Same or Similar Function Consider physical interactions -7.3215 -0.66434 3.6853 3.236723 -5.252571 -1.366879 -3.365368 -5.506312 0.5538 -7.3556 -6.31511 3.5398 Physical Interactions -3.33368 -0.66347 genetic interactions 3.2723 -5.25371 2.13473 0.55838 -1.3689 -6.3111 Physical interactions in Local Cut BPMS PIs within Pathways Expected by chance within PIs between Pathways Expected by chance between Collins et al. 172 20 18 20 Fiedler et al. 13 1 1 1 147 41 17 39 Data Set Tong et al. Modifying the weights -7.321556 -0.664347 How does alleviating interaction data affect the results? 3.685398 3.236723 -5.252571 -3.365368 -5.506312 -1.366879 Does a continuum of possible weights change the results? 0.553838 -6.315511 Do extreme weights affect the quality of the results? Local Cut WeightEnriched Variants Enriched Same Weight scheme #BPMs Same Function or Similar Function Unchanged 58 39 (67%) 43 (74%) No alleviating 26 17 (65%) 19 (73%) Large values capped 68 4 (6%) 6 (9%) Alleviating +1 Aggravating -1 30 3 (10%) 7 (23%) Genecentric: try this at home • Project name: Genecentric • Project homepage: http://bcb.cs.tufts.edu/genecentric • Operating system: platform independent • Programming language: Python • Other requirements: Python 2.6 or higher • License: GNU Public License (GPL 2.0) Gencentric parameters • Set M (number of randomized bipartitions) default 250 • Set C (consistency of same side/opposite side for inclusion in g’s BPM) default 90% • Set J (Jaccard index, how much overlap before similar BPMs are pruned) default .66 • Do you want a min or max size module? (default 3-25) • FuncAssociate parameters: genespace, p-value Genecentric works out of the box • “New” E-MAP of plasma membrane genes from Aguilar et al. in 2010. • 374 genes including those known to be involved in endocytosis, signaling, lipid metabolism, eisome function. • Genecentric was run with default E-MAP parameters, except C was lowered from .9 to .8 to produce more BPMs (22 instead of 6) Genecentric on plasma membrane E-MAP : example BPM BPM1 BPM2 • COG6 COG5 COG8 PIB2 COG7 • ARL1 VPS35 GET3 ARL3 SYS1 GOT1 PEP8 SFT2 MNN1 VPS17 • Intra-Golgi vesicle-mediated transport, protein targeting to vacuole • Protein transport, Golgi apparatus, endsome transport, vesicle-mediated transport Genecentric on plasma membrane E-MAP : example BPM BPM1 BPM2 • SLT2 BCK1 CLC1 • PEX1 PEX6 EDE1 SKN7 ERG4 ADH1 PEX15 ARC18 EMC33 • Endoplasmic reticulum unfolded protein response • Protein import into peroxisome matrix, receptor recycling Biological Findings (cont.) • Some complexes come up again and again– could they be global mechanisms of fault tolerance? In Plasma Membrane; -- COG complex In Chrombio; – SWR-C complex (Chromatin remodeling) – Prefoldin complex (Chaperone) – MRE11 complex (DNA damage repair) Co-authors and collaborators • • • • Ben Hescott Max Leiserson Diana Tartar Maxim Kachalov thanks. A Graph Theory Problem • Our algorithm samples from the maximal bipartite subgraphs. With what distribution? Is it uniform? Proportional to the number of edges that cross the cut?? ??? • What are the properties of the stable bipartite subgraphs of the synthetic lethal network? Are they conserved across species? Approach • Run the partitioning algorithm 250 times on the yeast SL network (G). • For each gene g in G, – Construct a set A consisting of g and all nodes in G which wind up in the same set as g at least 70% of the time. – Construct another set B consisting of all nodes in G which wind up in the opposite set from g at least 70% of the time. • We call the subgraph of G defined by A and B the “stable bipartite subgraph of g”, and designate it as a candidate BPM. Delete a gene in pathway 1; see if changes in pathway 2 coherent BPM Deleted Gene Pathway restriction log10 ratio Sort Validation: Microarray Data • Rosetta compendium (Hughes et al, 2000): -- contains yeast expression profiles of 276 deletion mutants: i.e. for each gene in the yeast genome, measures how its expression levels change when particular gene g is deleted, as compared to wildtype yeast. At step i: N to 1 Calculate weighted percent of genes in pathway seen so far and precent of genes not in pathway: Score is max difference How to validate a pathway Using a permutation test we sample 99 random subsets of genes the same size as the pathway We calculate the cluster rank score for each of these 99 sets We sort the test plus the pathway score The p-value is the percentile A pathway is validated if its p-value is <=0.1 Delete a gene in pathway 1; see if changes in pathway 2 coherent We call a pathway “Validated” if its Cluster Rank Score has p-value < .1 Kelley-Ideker Histogram of the Lowest CRS per Pathway per BPM This histogram displays all the CRS scores from all of the results from Kelley and Ideker’s BPMs bucketed according to their lowest p value score. The p value scores <= 0.10 indicate a validated BPM. Ulitskyi Histogram of the Lowest CRS per Pathway per BPM This histogram displays all the CRS scores from all of the results from Ulitskyi’s BPMs bucketed according to their lowest p value score. The p value scores <= 0.10 indicate a validated BPM. Ma Histogram of the Lowest CRS per Pathway per BPM This histogram displays all the CRS scores from all of the results from Ma’s BPMs bucketed according to their lowest p value score. The p value scores <= 0.10 indicate a validated BPM. Brady Histogram of the Lowest CRS per BPM This histogram displays all the CRS scores from all of the results from Brady’s BPMs bucketed according to their lowest p value score. The p value scores <= 0.10 indicate a validated BPM. Clearly, Brady’s BPMs are disproportionately represented in the lower p value range. Results BPM dataset # paths hit knockouts Kelley-Ideker 160 (05) Ulitsky36 Shamir (07) Ma et al. 54 (08) Our results 959 # validated pathways 16 % validated pathways 10% 5 14% 6 11% 230 24% A Tantalizing Peek of What We can Do With More Data! • A heat map of the differential expression of yeast genes in pathway 2 in response to the deletion of two different genes (SHE4 and GAS1) from pathway 1 in a validated BPM of Ma et al. A random-gene validation test couples the two pathways together