White light is a mixture of all wavelengths. When white... diffraction grating it is broken up into a continuous distribution... SPECTRA
advertisement

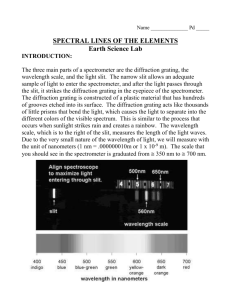
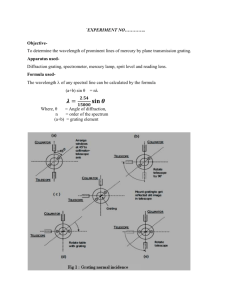
SPECTRA White light is a mixture of all wavelengths. When white light is sent through a prism or a diffraction grating it is broken up into a continuous distribution of colors called a spectrum. The relation between wavelength and color is given in the following table. color wavelength (nm) violet 400 - 450 blue 450 - 480 blue-green 480 - 510 green 510 - 550 yellow-green 550 - 570 yellow 570 - 590 orange 590 - 630 red above 630 A rainbow is a spectrum produced when sunlight is refracted through raindrops. Any thin slice of color in a continuous spectrum is called a spectrally pure color and is characterized by its wavelength . Spectrally pure colors are rare in most of our natural environment but they are easily produced in the laboratory. When an electric current is sent through a diffuse transparent gas the gas is heated and glows. If the light from such a gas is passed through a prism or diffraction grating, a discrete set of bright colors will typically be seen. When a diffraction grating is used the colors appear to be narrow lines parallel to the slits of the grating and are called spectral lines. A collection of these lines (usually in the form of a table or graph) for a gas composed of a given chemical element is called the line spectrum of the element. A very different sort of spectrum is produced by a hot opaque object like a lightbulb filament. Instead of discrete spectral lines this incandescent light source produces a broad distribution of colors. Every color in the visible range is represented in such a spectrum and the relative intensities of various colors depends on the temperature of the hot object. 3.1 Experiment 1: Grating Spectroscope Apparatus: CENCO grating spectroscope, variac-controlled light bulb, sodium and mercury vapor lamps. Procedure: Hold the device so that its right angle is on your left. View through the grating fixed to the aperture at the short end. Illuminate the vertical slit on the left with the light to be studied. The nominal wavelength is read on the scale to the right of the slit. A. The spectroscope is not accurately calibrated; the nominal wavelength appearing on the scale is not the true wavelength. Use the fluorescent lights in the room to make a table of true vs. nominal wavelength The actual wavelengths, in nm, are: violet blue green orange red 436 489 545 585 612 Compare to the observed lines. Make a table of corrections to be used for subsequent measurements. B. Use the spectroscope to find the wavelengths of light from sodium and mercury vapor lamps. C. Examine the light from the filament of an unfrosted light bulb. Begin by turning the current up to a significant value but not so high that its light is painful. Use the spectroscope to compare the intensity of the violet light to that of the red light. As the current is turned down, all intensities decrease. Can you see a relative difference in the intensity of the violet light compared to the red light? Which color’s intensity decreases fastest as the current is turned down? Repeat a few times to make sure of your result. D. Take the instrument home for a day or two. Look at and report your findings for light from: a gas flame, a candle flame, automobile headlights, a room lamp, commercial neon lights and the sun at midday and at sunrise or sunset (of course you should not use light directly from the disk of the sun). 3.2 Experiment 2 : Prism Spectrometer Apparatus: Prism spectrometer, mercury and sodium vapor lamps, hydrogen discharge tube, helium-neon laser The prism spectrometer consists of an equilateral flint prism fixed to a stand containing an adjustable slit, an objective lens that focuses the light from the slit onto the prism, and a telescope consisting of an objective lens and an adjustable ocular with built-in cross-hair. The telescope sits on a platform that is free to rotate about a vertical axis through the prism. Gross rotation angle of the telescope is changed by moving the telescope support with the hand while fine adjustments are made with a screw on the right side of the support. Angle of the telescope is measured on a principal scale and on a vernier scale equipped with a viewing lens to read minutes of arc. Procedure: A. Calibration of Angles Light enters the device through the slit. A typical experiment consists of measuring the angular positions of spectral lines produced when light has gone through the prism. This light emerges as colored images of the slit. The slit should first be opened relatively wide and placed so as to view the light from a mercury vapor lamp. The line spectra can be located by gross rotation of the telescope housing. Then close down the slit until only narrow spectral lines remain. These will often be slightly curved, forming a bow rather than a straight line. Use the fine adjust to line up the vertical cross-hair with the center of the bow of each spectral line. There will be a dim line far in the violet (404.7nm), a bright violet line (435.8nm), green (546.1nm), and a yellow doublet (577.0nm and 579.1nm). Make a table of the angular position of the telescope for each of these lines. The yellow doublet lines are so close together that, unless you feel confident that you have resolved them into two separate lines, it is better to regard them as a single line at the average wavelength. The work should be done by at least two students and the results averaged. Then do the same with a hydrogen discharge tube, recording the position of the bright red line (656.3nm) and the blue line (486.1nm). B. Mathematical Description of the Calibation You now have six spectral lines that span most of the visible spectrum. Use the pointplot function in MAPLE to graph this data and re-measure any points that look like they do not lie on a smooth curve. To use the prism spectrometer to measure the wavelength of unknown lines it is necessary to fit the data to a formula. Use the least squares fit command in MAPLE to fit the wavelengths to a polynomial in the angles. It is first necessary to convert to decimal angles. It is suggested that instead of demanding a fit directly to the angle, which is often a large number of degrees that varies only slightly over the visible range, you first subtract from all angles the angular position of the green line. Then your angular positions will be small positive and negative numbers. The fit will benefit greatly in accuracy from this procedure. When using your formula you will have to remember to 3.3 always make this change to any measured angles. Your formula should reproduce all of the wavelengths to an accuracy of 4nm or better. Modify the fitting procedure or return to the angle calibration if this has not been achieved. C. Making Measurements Now use the sodium vapor lamp and determine the wavelength of the bright yellow lines. Then prepare the red beam from helium-neon laser for viewing by sending the beam through a pair of crossed polarizers. These do not block all of the light since they are not 100% efficient but the little light that goes through them both will usually be dimmed sufficiently that it cannot cause damage to the eye. When using an especially strong laser it may be necessary to use three polarizers. The way to check that no damage can be done to the eye is to be sure that the beam emerging from the polarizers does not cast a visible spot on the hand or on a screen. If it is too dim to be seen in diffuse reflection it is too dim to damage the eye. Adjust the laser and/or spectrometer until the dim red beam falls on the slit. Compare your results with the known values: sodium 589.5nm, He-Ne laser 632.8nm. 3.4 Experiment 3 : PC Spectrometer This device, manufactured by Ocean Optics, sends light through a fiber optic cable to illuminate 600 lines of a diffraction grating. The resulting dispersed light passes through a semiconductor photodetector which produces an electrical signal that is, within limits, proportional to the light intensity. The grating and detector are mounted on a card connected directly to the pc bus inside the computer housing. A. The software is activated by clicking the OOI icon. The end of the fiber cable used as a light detector is kept screwed into the black end of a rigid container when not in use. A blue colored cylindrical screw driver is provided and it is a good idea to use this as a housing to protect the end of the cable in all applications. The container has a number of functions, including protecting the sensitive cable and providing a dust cover. Please remember to store the cable in this container when it is not in use. Fiber optic cables are glass structures that are easily damaged and cannot withstand much mechanical loading. Never clamp such a cable tightly. Unscrew the cable from the black end of the container and, keeping it inside the blue screwdriver, sample the light from the overhead fixtures. You may lightly clamp the screwdriver to a lab stand for support. Take some time to familiarize yourself with the features of this spectrometer. In particular, you should note the freeze-frame feature (camera icon), pixel width feature for averaging (summation icon), integration time (integral icon), graph scale adjust (icon with vertical and horizontal arrows) and peak locator (icon looks like a graph of a bellshaped curve with a colored vertical line running through the graph). The arrow icons move the peak indicator display over the spectrum. B. Take a spectrum of the mercury and sodium vapor lamps and the hydrogen discharge tube. Compare your results to the published values of these wavelengths as a check on the calibration of the instrument and note any discrepancies. C. Next find the wavelength of a He-Ne laser using a polarization-damped beam. Do not put an unfiltered laser beam directly into the cable. D. Take at least five spectra of the variac-controlled light bulb at different intensities. For each spectrum, note the variac setting and find the intensity at which the spectrum is most luminous and also the intensities at 450nm, 550nm, and 650nm. Find the ratios of blue/green, blue/red, and red/green. Plot the wavelength of maximum brightness vs variac setting (use the pointsplot feature in MAPLE) and comment on regularities. Do the same for the intensity ratios. 3.5 Spectral Lines Niels Bohr explained spectral lines as due to the relaxation of excited atoms. There are only a discrete number of stable orbits in which bound atomic electrons can move about the nucleus. When an atom absorbs energy from its environment the internal state of motion of the atom changes from its most relaxed state (called the “ground state”) to one of its excited states. These states are different for different chemical elements but the same for every atom of a given element1. It is common to diagram the states of an atom using a vertical energy scale with a horizontal line segment drawn for each state. The figure illustrates the energy level diagram for hydrogen, the simplest atom. An atom in an excited state will eventually relax into a state of lesser energy and release some of its excess energy, often in the form of electromagnetic radiation. The energy radiated depends on the difference between the energies of the initial and final atomic states. That is (3.1) energy radiated = energy of initial state - energy of final state Ei - Ef The radiated energy appears as a pulse of electromagnetic radiation called a photon. The energy of the photon is directly proportional to its frequency f (3.2) energy radiated = photon energy = h f where h is a constant of nature called Planck’s constant, with the approximate value h = 6.626 × 10-34 Js. Therefore, higher energy photons have larger frequencies (shorter wavelengths). The energies of atomic states are very well defined, usually to within a part in 105 or less, so that the frequencies (and therefore the wavelengths) of atomic radiations are correspondingly well defined. The resulting sharp spectral lines are as close as unaided nature comes to providing spectrally pure light. Problem: The first excited state of neutral sodium occurs at an energy of 2.10eV. When electrons in this state emit radiation and decay into the ground state, what wavelength radiation is formed? 1 Actually, there are slight differences in the spectra of atoms of the same element that have different numbers of neutrons in the nucleus, i.e. different isotopes of the same element. 3.6 What color is the resulting illumination? 3.7