Document 14471323
advertisement

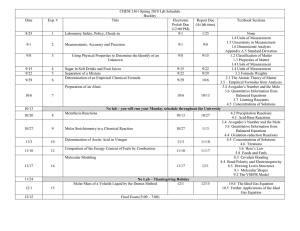
Learning Goals for Unit 3: Quantities in Chemical Reactions By the end of this unit, I will be able to: • explain the difference between qualitative and quantitative analysis and provide examples of both in society • explain the concept of a mole • calculate molar mass of a substance • calculate mass, amount or entities based on the relationships between Avogadro’snumber, the mole concept, and the molar mass of any given substance • calculate the % composition experimentally (from mass data) or theoretically (from molar mass data and the chemical formula) • explain the relationship between the empirical formula and the molecular formula of a chemical compound • determine the empirical formulae and molecular formulae of various chemical compounds, given molar masses and percentage composition or mass data • calculate the corresponding mass or amount in moles for any given reactant or product in a balanced chemical equation as well as for any other reactant or product in the chemical reaction • solve problems related to quantities in chemical reactions by performing calculations involving percentage yield and limiting reagents • use factor label method to solve problems • use correct mathematical format (factor label and/or formulae), units and significant digits when solving problems • Vocabulary – p. 306, 344 D3. Understanding Basic Concepts