Quantitative Chemistry Unit Planner: Mole Concept & Stoichiometry
advertisement
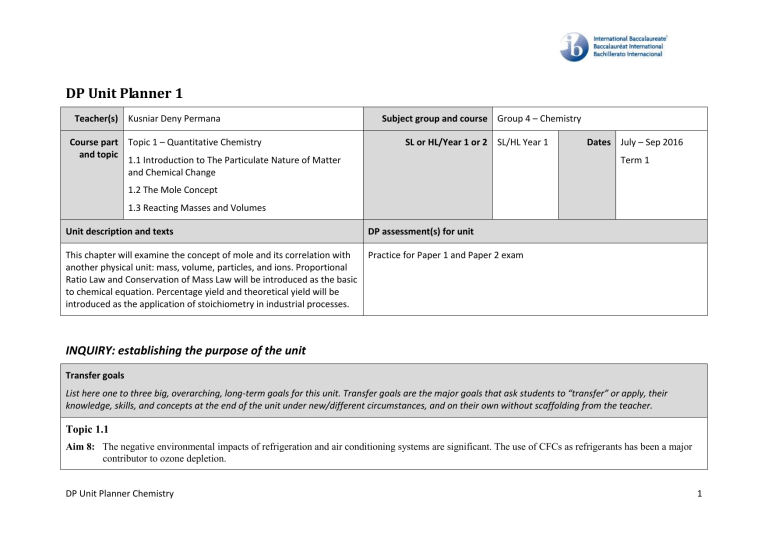
DP Unit Planner 1 Teacher(s) Kusniar Deny Permana Course part Topic 1 – Quantitative Chemistry and topic 1.1 Introduction to The Particulate Nature of Matter and Chemical Change Subject group and course Group 4 – Chemistry SL or HL/Year 1 or 2 SL/HL Year 1 Dates July – Sep 2016 Term 1 1.2 The Mole Concept 1.3 Reacting Masses and Volumes Unit description and texts DP assessment(s) for unit This chapter will examine the concept of mole and its correlation with another physical unit: mass, volume, particles, and ions. Proportional Ratio Law and Conservation of Mass Law will be introduced as the basic to chemical equation. Percentage yield and theoretical yield will be introduced as the application of stoichiometry in industrial processes. Practice for Paper 1 and Paper 2 exam INQUIRY: establishing the purpose of the unit Transfer goals List here one to three big, overarching, long-term goals for this unit. Transfer goals are the major goals that ask students to “transfer” or apply, their knowledge, skills, and concepts at the end of the unit under new/different circumstances, and on their own without scaffolding from the teacher. Topic 1.1 Aim 8: The negative environmental impacts of refrigeration and air conditioning systems are significant. The use of CFCs as refrigerants has been a major contributor to ozone depletion. DP Unit Planner Chemistry 1 Topic 1.2 Aim 6: Experiment could include percent mass of hydrates, burning of magnesium or calculating Avogadro number. Aim 7: Data loggers can be used to measure mass changes during reaction. Topic 1.3 Aim 6: Experimental design could include excess and limiting reactants. Experiments could include gravimetric determination by precipitation of an insoluble salt. Aim 7: Data loggers can be usd to measure temperature, pressure and volume changes in reactions or to determine the value of the gas constant, R. Aim 8: The unit part per million, ppm, is commonly used in measuring small levels of pollutants in fluids. This unit convenient for communicating very low concentrations, but is not a formal SI unit. ACTION: teaching and learning through inquiry Content/skills/concepts—essential understandings Learning process Check the boxes for any pedagogical approaches used during the unit. Aim for a variety of approaches to help facilitate learning. Students will know the following content: 1. Atom of different elements combine in fixed ratios to form compounds which have different properties from their component elements. 2. Mixture contain more than one element and/or compound that are not chemically bonded together and so retain their individual properties. 3. Mixture are either homogeneous or heterogeneous. 4. The mole is a fixed number of particles and refers to the amount, n, of substance. DP Unit Planner Chemistry Learning experiences and strategies/planning for self-supporting learning: Lecture Socratic seminar Small group/pair work PowerPoint lecture/notes Individual presentations 2 5. Masses of atoms are compared on a scale relative to 12C and are expressed as relative atomic mass (Ar) and relative formula/formula mass (Mr) 6. Molar mass (M) has the unit g mol-1. 7. The empirical formula and molecular formula of a compound give the simplest ratio and the actual number of atom present in a molecule. 8. Reactant can be either limiting or excess. 9. The experimental yield can be different from the theoretical yield. 10. Avogadro’s law enables the mole ration of reacting gases to be determined from volume of the gases. 11. The molar volume of an ideal gas is a constant at specified temperature and pressure. 12. The molar concentration of a solution is determined by the amount of solute and the volume of solution. 13. A standard solution is one of known concentration. Group presentations Student lecture/leading Interdisciplinary learning Details: Other/s: Formative assessment: Written checks for understanding, including balancing chemical equation, mole-related calculation, percentage yield, and limiting and excess reactant concept. Verbal checks for understanding, including mole concept, state symbols, basic concept of chemical changes contrast to physical changes. Students will develop the following skills: Summative assessment: 1. Students will be able to deduct of chemical equations when reactants and product are specified. Paper 1 and paper 2 practice exam (timed) 2. Student will be able to demonstrate the application of the state symbols (s), (l), (g), and (aq) in equations. 3. Student will be able to explain the observable changes in physical properties and temperature during changes of states. 4. Students will be able to calculate the molar masses of atoms, ions, molecules and formula unit. 5. Student will be able to solve a problem involving the relationships between the number of particles, the amount of substance in moles and the mass in grams. Value prior knowledge Student will be able to interconvert the percentage composition by mass and the empirical formula. Extend learning 6. DP Unit Planner Chemistry Differentiation: Affirm identity—build self-esteem Scaffold learning 3 7. Student will be able to determine the molecular formula of the compound from its empirical formula and molar mass. 8. Student will be able to obtain and use experimental data for deriving empirical formulas from reactions involving mass changes. 9. Students will be able to solve problems relating to reacting quantities, limiting and excess reactant, theoretical, experimental and percentage yield. 10. Student will be able to calculate reacting volumes of gases using Avogadro’s law. 11. Student will be able to solve problems and analyse graph involving the relationship between temperature, pressure and volume for a fixed mass of an ideal gas. 12. Student will be able to solve problems relating to the ideal gas equation. 13. Student will be able to explain the deviation of real gases from ideal behaviour at low temperature and high pressure. 14. Student will be able to obtain and use experimental values to calculate the molar mass of a gas from the ideal gas equation. 15. Student will be able to solve problems involving molar concentration, amount of solute and volume of solution. 16. Student will be able to use the experimental method of titration to calculate the concentration of a solution by reference to a standard solution. Details: Students will grasp the following concepts: 1. Chemical equations are the “language” of chemistry 2. Lavoisier’s discovery of oxygen, which overturned the phlogiston theory of combustion, is an example of a paradigm shift. 3. Mole is a concept of a number, unit less, like a dozen, a couple, etc. 4. Stoichiometric calculations are fundamental to chemical processes in research and industry, for example in the food, medical, pharmaceutical and manufacturing industries. 5. The molar volume for crystalline solids is determined by the technique of X-ray DP Unit Planner Chemistry 4 crystallography. 6. Gas volume changes during chemical reactions are responsible for the inflation of air bags in vehicles and are the basis of many other explosive reaction, such as the decomposition of TNT (trinitrotoluene). 7. The concept of percentage yield is vital in monitoring the efficiency of industrial processes. Approaches to learning (ATL) Check the boxes for any explicit approaches to learning connections made during the unit. For more information on ATL, please see the guide. Thinking Social Communication Self-management Research Details: Language and learning TOK connections CAS connections Check the boxes for any explicit language and learning connections made during the unit. For more information on the IB’s approach to language and learning, please see the guide. Check the boxes for any explicit TOK connections made during the unit Check the boxes for any explicit CAS connections. If you check any of the boxes, provide a brief note in the “details” section explaining how students engaged in CAS for this unit. Activating background knowledge Personal and shared knowledge Creativity Scaffolding for new learning Ways of knowing Activity Acquisition of new learning through practice Areas of knowledge Service DP Unit Planner Chemistry 5 Demonstrating proficiency The knowledge framework Details: Details: Details: AOK is natural sciences WOK is reason Resources List and attach (if applicable) any resources used in this unit IB Chemistry Syllabus Geoffrey Neuss, 2014, Chemistry for IB DIPLOMA Program, Oxford University Press Catrin Brown and Mike Ford, 2014, Pearson Baccalaureate Higher Level Chemistry 2nd Edition, Pearson Education Limited. Stage 3: Reflection—considering the planning, process and impact of the inquiry What worked well What didn’t work well Notes/changes/suggestions: List the portions of the unit (content, assessment, planning) that were successful List the portions of the unit (content, assessment, planning) that were not as successful as hoped List any notes, suggestions, or considerations for the future teaching of this unit DP Unit Planner Chemistry 6