What Have We Learned About Uterine
Contractions and Preterm Birth?
The HUAM Prediction Study
Jay D. Iams* for the National Institute of Child Health and Human
Development Maternal-Fetal Medicine Units Network
Measurement of uterine contraction frequency has been employed as a screening test to identify
women with increased risk of preterm birth, and as an aid in the early diagnosis of preterm labor. The
National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (NICHD
MFMU) Network performed a prospective, blinded observational study of uterine contraction
frequency to detect and predict preterm labor and birth, respectively. The goal of the study was to
assess the sensitivity, specificity, and positive and negative predictive value of various measures of
uterine contraction frequency. Data collected from 306 women revealed that contraction frequency
was significantly greater in women who would ultimately deliver before rather than after 35 weeks’
gestation. However, both sensitivity and positive predictive value of any measure of contraction
frequency to predict preterm birth were poor. Contraction frequency did not increase significantly
within 1 or 2 weeks of an episode of preterm labor. These results serve to explain the absence of an
association between contraction-based surveillance and preterm birth in randomized trials conducted
in women at risk of preterm birth.
© 2003 Elsevier Inc. All rights reserved.
etween 1994 and 1996, the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (NICHD
MFMU) Network conducted an observational
study of uterine contraction frequency in pregnant women, the Home Uterine Activity Monitoring (HUAM) Prediction Study.1 The study
was an effort to understand the relationship between uterine contraction frequency and the
onset of preterm labor, and to explain the failure of outpatient or ambulatory uterine contraction monitoring (also called HUAM) to reduce
the rate of preterm birth in published randomized trials.2,3
The study design and results are best understood when reviewed as part of a research chro-
B
From the *Division of Maternal-Fetal Medicine, Department of
Obstetrics and Gynecology, The Ohio State University College of
Medicine and Public Health, Columbus, OH.
This work was supported by grants from the National Institute of
Child Health and Human Development (U10-HD19897, U10-HD
21410, U10-HD 21414, U10-HD 21434, U10-HD 27860,
U10-HD 27861, U10-HD 27869, U10-HD 27883, U10-HD
27889, U10-HD 27905, U10-HD 27915, U10-HD 27917).
Reprints are not available.
© 2003 Elsevier Inc. All rights reserved.
0146-0005/03/2703-0004$30.00/0
doi:10.1016/S0146-0005(03)00018-1
204
nology that begins with the advent of attempts to
stop preterm labor. Remarkable as it may seem
today, prevention of preterm birth has not always been viewed as a desirable goal. Premature
labor was seen as the natural and probably desirable result of something abnormal about the
pregnancy that threatened the health of the
mother and/or fetus. There was considerable
reluctance to interfere with preterm labor for
both scientific and social reasons.
Two oft-cited comments by leading authorities reflect the prevailing sentiments of obstetricians from 1940 through the mid-1980s. The
first, by Eastman,4 remains as true today as it was
in 1947: “Only when the factors underlying prematurity are completely understood can any intelligent attempt at prevention be made.” The
second, by Taylor 5 was offered in response to a
presentation that described a prematurity prevention program: “I do not think that . . . prematurity can be reduced . . . by better obstetrics.
These problems are social, not obstetric.”
Against this history came arguments from
France6 and the United States7,8 that prematurity was a problem that should and could be
addressed without waiting for a complete explanation of the biochemical mechanisms involved.
Seminars in Perinatology, Vol 27, No 3 (June), 2003: pp 204-211
Uterine Contractions and Preterm Birth
History has shown that both camps made points
that remain valid today: There are interventions
that reduce the morbidity and mortality of prematurity, but progress has indeed been limited
by our incomplete understanding of the mechanisms that lead to preterm birth.
The use of tocolytics was advocated to prevent
preterm birth9 because the onset of preterm
labor was understood to begin with the coalescence of small uterine contractions into larger
contractions that then effected cervical change,
as observed in studies of primate models.10
When such use did not produce an obvious decline in the rate of preterm delivery, several
explanations were proposed:
1. The medications used were not optimal, either because of inadequate tocolytic effect or
unacceptable side effects.
2. The cause of the preterm labor was not understood and thus not appropriately treated
with medications directed at stopping uterine
muscle contractions.
3. Tocolytic medications were applied too late
to have any benefit.7,11
Those who favored Explanation 1 argued for
more and better tocolytics drugs; their descendants today advocate prophylactic and/or therapeutic tocolytics medication via either oral or
subcutaneous administration. Those who favored Explanation 2, the descendants of Eastman4 and Taylor,5 saw vindication in the failure
of tocolysis to reduce preterm birth rates, and
argue now for therapeutic nihilism until both
basic and clinical research reveals an unequivocally effective intervention. Many of this group
live, or lived for a time, in Dallas, TX; their views
continue to exert a substantial and important
influence on clinical practice. Those who favored Explanation 3 saw the failure of tocolysis
as an argument to improve the early identification of women with preterm labor so that tocolysis might be more efficacious. This group
spawned the March of Dimes Preterm Birth Prevention Trial,12 a 5-site multicenter trial of a
screening and educational program for pregnant women conducted over 5 years. The goal
was to identify women at risk for preterm birth
through a screening program with the Creasy
Score.13 High-risk women were randomly assigned to a control group that received standard
205
care, or to a study group that received frequent
visits and education about how to recognize the
earliest signs and symptoms of preterm labor.
Once educated to note the symptoms and to
self-palpate to detect contractions, the March of
Dimes investigators hypothesized that women
assigned randomly to the education and selfpalpation group would present earlier to the
health care system for care, so that tocolytic
medication would be more effective. More than
3,000 women were enrolled. The results were
negative, ie, there was no difference in the rate
of preterm delivery between the study and control groups.12
The response of many to these negative results was again based on Explanation No. 3 as
the operating hypothesis: Tocolysis as used in
the March of Dimes Trial was still too late to be
beneficial. Newman et al14 reported that women
taught to self-palpate their contractions did not
identify the majority of contractions recorded by
a sensitive tocodynamometer. These observations were the basis for the next effort to detect
the earliest signs and symptoms of preterm labor
in women with commonly recognized risk factors for preterm birth. Technological advances
in uterine contraction detection that used a
“guard-ring” tocodynamometer, and in electronic transmission of contraction data via the
telephone allowed pregnant women to use a
contraction monitor at home several times each
day, and to transmit their contraction data to a
monitoring center where it could be reviewed by
specially trained nurses.15 This approach was
based on the belief that 1) uterine activity was
higher in women destined for preterm birth,
and 2) uterine activity was increased 24 to 48
hours before an episode of preterm labor.15,16
Some reports of pregnancy outcomes in high
risk women who used the HUAM device and
service were promising,16-18 but other studies did
not show any difference in the rate of preterm
birth when the monitor was used compared to
controls.19-21 Ultimately, 2 large randomized trials2,3 were conducted that both found no difference in the frequency of preterm birth in
women who received a contraction monitor
compared to those who did not. The study by
Dyson et al3 enrolled more than 2,400 women
with risk factors for preterm birth who were
randomly assigned to 1 of 3 groups: Daily out-
206
Jay D. Iams
patient contraction monitoring accompanied by
daily nurse contact, daily nurse contact without a
contraction monitor, or weekly nurse contact
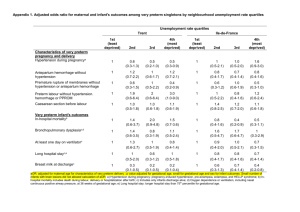
alone. Results are shown in Table 1. There was
no benefit to either daily monitoring and contact or daily contact when compared with weekly
contact. Women in both intervention groups
had more visits and more drug treatment but to
no advantage.
The CHUMS2 and Dyson3 trials effectively
demonstrated that the contraction surveillance
and early treatment approach to prematurity
prevention was ineffective. Nevertheless, the reasons for the failure of this approach to reduce
the occurrence of preterm birth were not completely explained, and the connection of increased uterine activity to preterm birth risk remained anecdotally strong. As Dyson et al3 were
conducting their trial within the Kaiser health
care system in northern California, the NICHD
MFMU Network was considering a randomized
trial of similar design. Ultimately, the Network
Steering Committee and NICHD Program Officers decided on a different approach, for both
practical reasons (the Dyson trial was enrolling
successfully and the cost of a randomized trial
within the Network exceeded available funds)
and for scientific reasons described in the following paragraphs.
Rationale for the HUAM Prediction Study
When the Network Steering Committee reviewed the available literature that related uterine contraction frequency to preterm birth risk
in singleton pregnancy, several important unanswered questions were identified:
1. Is the frequency of uterine activity greater in
women destined for preterm birth than in
women who will deliver at term?
2. Is the frequency of uterine activity greater in
women with historical risk factors for preterm
birth (eg, a prior preterm birth) greater than
in women who have no risk factors?
3. Does uterine activity differ according to gestational age in women destined for preterm
birth?
4. Does uterine activity differ according to time
of day in women destined for preterm birth?
5. Is the measure of uterine activity an efficient
test to: screen for risk of preterm birth; predict
preterm birth; or detect preterm labor before
it is detected by clinical signs and symptoms?
Although small studies had addressed each of
these questions, there were no large blinded
observational studies to establish reference
values for uterine activity by gestational age,
time of day, fetal number, or obstetrical history. Without a firm knowledge of the range of
Table 1. Outcomes of Pregnancy in the Weekly-Contact, Daily-Contact, and Home-monitoring Groups
All Women (N ⫽ 2422)
Outcome
Preterm birth (%)
⬍37 wk
⬍35 wk
⬍32 wk
Birth weight
⬍1500 g
⬍2500 g
No. of unscheduled
visits*†
Prophylactic to colyticdrug therapy (%)†
Preterm labor ⬍35 wk (%)
Women with Twin Pregnancies (N ⫽ 844)
Weekly
Contact
(N ⫽ 798)
Daily
Contact
(N ⫽ 796)
Home
Monitoring
(N ⫽ 828)
Weekly
Contact
(N ⫽ 280)
Daily
Contact
(N ⫽ 277)
Home
Monitoring
(N ⫽ 287)
30
14
4
31
13
5
30
14
4
49
22
7
54
24
9
51
24
6
4
26
4
26
4
28
6
52
8
55
9
59
1.2 ⫾ 1.5
1.8 ⫾ 2.0
2.3 ⫾ 2.3
1.3 ⫾ 1.5
1.9 ⫾ 2.0
2.5 ⫾ 2.4
12‡
23
14‡
22
19‡
27
8‡
35
11
34
16‡
40
*Plus-minus values are means ⫾ SD. P ⬍ .002 for all comparisons between treatment groups.
†Therapy was given after symptoms appeared but before the criteria for preterm labor were met.
‡P ⬍ .01 for the comparisons between the weekly-contact or the daily-contact and the home-mon-itoring groups for all the
women and between the weekly-contact and home-monitoring groups for the women with twin pregnancies.
Reprinted with permission.3
Uterine Contractions and Preterm Birth
uterine activity in normal pregnancy, it was
possible that the uterine activity thresholds
used in clinical and research use of HUAM
were incorrect.
Moore et al22 found that uterine activity (UA)
in normal women studied for 24 hours a day
twice weekly from 24 weeks’ through delivery
was significantly influenced by the following:
1. Gestational age–UA increases as gestational
age advances, especially after 28 weeks.
2. Time of day –UA is greater in the afternoon
and evening than in the morning.
3. Rest – UA decreases for 1 to 2 hours after an
hour of recumbence.
4. Coitus– UA increases for 1 to 2 hours after
coitus.
Similarly, Germain et al23 studied patterns of
uterine activity in 3 groups of women: 1) Lowrisk women who delivered at term, 2) Women
with a prior preterm birth who delivered at term
in the current pregnancy, and 3) Women with a
prior preterm birth who delivered prematurely
in the current pregnancy. Those in the first 2
groups maintained a normal pattern of uterine
activity with fewer contractions in the morning
and more in the evening, while high-risk women
who delivered preterm had no variation in uterine contractions with time of day.
The Network investigators thus hypothesized
that failure to account for these influences on
uterine activity could explain the clinical failure
of HUAM-based protocols to reduce preterm
birth because of an inability to correctly identify
women with an increased risk of preterm birth.
A protocol was therefore developed to study
uterine activity as a screening test for preterm
birth risk and as a diagnostic test for early preterm labor. The study protocol was patterned
after the Preterm Prediction Study as described
by Goldenberg et al in this issue,24 except that
the women enrolled would be chosen to create a
population with increased risk of preterm birth,
and would in addition to cervical examinations
also collect uterine activity data.
The HUAM Prediction Study
Methods
Women with singleton gestations with a prior
spontaneous preterm birth between 20 and 36
207
weeks or second trimester bleeding in the current pregnancy were recruited. A limited number of women with no risk factors were enrolled
to allow comparison of contraction frequency in
low- and high-risk women. The sample size was
chosen to create an 80% power to detect a difference of 0.5 standard deviations {eg, a difference of one contraction per hour if the standard
deviation was no more than 2 contractions per
hour.22 By enriching the study population with
high-risk patients, a sample size of 300 women
was appropriate for an endpoint of preterm delivery before 35 weeks’, in which neonatal morbidity is greater. We planned to enroll 50 to 70
low-risk women and 230 to 250 high-risk women.
Eligibility was determined before 22 weeks’
gestation. An ultrasound examination was performed before enrollment to determine gestational age and exclude major fetal anomalies
and placenta previa. Women treated before
screening with tocolytic medication or a cerclage
were excluded. Study visits were scheduled to
collect data at 22-24 weeks’ (visit 1), 25-26 weeks’
(visit 2), 27-28 weeks’ (visit 3), 29-30 weeks’ (visit
4), 31-32 weeks’ (visit 5), and ⱖ33 weeks of
gestation (visit 6).
Research nurses trained to use a home contraction monitor (Healthdyne System 37; Matria
Inc, Marietta, GA) visited each woman at home
to instruct her to use the monitor and transmit
data. Uterine activity was recorded for a minimum of 1 hour at least twice daily in 2 sessions at
least 2 hours apart, 1 between 0400 and 1559
and the other between 1600 and 0359, on 2 or
more days per week from enrollment to 28
weeks. After 28 weeks, 2 additional monitoring
sessions per week were required. Data were
transmitted immediately after collection to the
Data Coordinating Center at The George Washington University Biostatistics Center. Protocol
compliance for time, date, and quality of the
recordings was followed weekly for each woman.
All other study data were transmitted weekly to
the center.
The duration of pregnancy and reason(s) for
preterm birth were recorded. Providers, investigators, and patients were blinded to results of
testing. None of the tests being evaluated were
performed outside of the study.
Uterine activity monitor recordings were an-
208
Jay D. Iams
alyzed according to a standard protocol by four
research nurses who were unaware of pregnancy
outcome. A contraction was a defined as a deflection from baseline with a rounded peak that
lasted 40 to 120 seconds. Inverted, “doublepeak” and “camel back” contractions were included. “Possible” contractions that were subtle,
had a variable baseline, a flat peak, or were
accompanied by artifact were not considered
contractions. Regular audits of contraction recordings were conducted throughout the study
to assure consistent interpretation. Discordant
interpretation was not greater than one contraction per hour, and was evenly distributed between increased and decreased contractions.
Analyses of uterine activity were performed to
identify relationships with gestational age, time
of day [0400-1559 (designated AM) v 1600-0359
(designated PM)], risk status (high v low), and
term versus spontaneous preterm delivery ⬍35
weeks’ gestation (after preterm labor or preterm
ruptured membranes). Contraction rates per
hour were calculated for each patient and each
gestational week, and analyzed using a repeated
measures random effects model. Because each
woman could contribute data only as long as her
pregnancy continued, more data were collected
for women who remained undelivered. Contraction frequency data from women who delivered
after 35 weeks’ was therefore compared with
data collected between 240/7 and 286/7 weeks’
for women who delivered preterm between
290/7 and 326/7 weeks’, and with data collected
between 240/7 and 326/7 weeks’ for women who
delivered preterm between 330/7 and 346/7
weeks’. Maximum a.m. and p.m. contraction
rate per hour for each 2-week interval (22-24,
25-26, 27-28, 29-30, 31-32, and ⱖ 33 weeks’) were
evaluated as predictors of preterm birth with
logistic regression.
We determined the sensitivity, specificity, and
predictive values of mean and maximum a.m.
and p.m. contraction frequency at each gestational age interval to predict spontaneous preterm birth before 35 weeks’ gestation. Contraction frequency was dichotomized as ⬍4 v ⱖ4 per
hour when tested as a categorical variable. Receiver operating characteristic curves were constructed for mean and maximum contraction
frequency per 2-week gestational age interval.
Results
Among 454 women with singleton pregnancies
who met inclusion criteria and consented to
enrollment, there were 146 (32.2%) who were
not compliant with monitoring, and 2 who delivered within a week of enrollment. The remaining 306 women constitute the study population. Of 274 women at increased risk for
preterm birth, 194 (76.4%) had 1 prior preterm
birth, 57 (22.4%) had a history of 2 or more
preterm births, and 8 (3.1%) had second trimester bleeding. Some had more than 1 risk factor.
There were 106 women (34.6%) who delivered
before 37 weeks’ gestation, 48 (15.7%) before 35
weeks’, and 18 (5.9%) before 32 weeks’.
The 106 enrolled women recorded 34,908
hours of contraction data, of which 20.8%
(7,268 hours) had contractions. Contraction frequency was unrelated to maternal risk status
(P ⫽ 0.219). Data from low- and high-risk
women were therefore combined for analysis.
Mean contraction frequency increased significantly with gestational age and was increased
during p.m. (1600 – 0359) hours regardless of
gestational age at delivery (Fig 1).
Separate analyses were performed for a.m.
and p.m. contraction data. After controlling for
gestational age at the time of monitoring,
women who delivered before 35 weeks’ had
more contractions as measured by both a.m.
contraction frequency (P ⫽ .09 for 240/7-286/7
weeks’ and P ⫽ .03 for 240/7-326/7 weeks’) and
p.m. contraction frequency (P ⬍ .001 for 240/7286/7 weeks’ and P ⫽ .02 for 240/7-326/7 weeks’)
Figure 1. Relation of the week of gestation, time of
day, and timing of delivery (before 35 weeks of gestation or at 35 weeks or more of gestation) to the
frequency of contractions between 24 and 32 weeks.
(Reprinted with permission.1)
209
Uterine Contractions and Preterm Birth
than did women who delivered after 35 weeks’
gestation.
In univariate analyses, maximum contraction
frequency was inconsistently related to spontaneous birth before 35 weeks’ gestation (Table
2). Because the logistic regression models for
maximum a.m. and p.m. contraction frequency
had slightly better fit than that for mean contraction frequency, maximum frequency was
used in subsequent analyses. Change from baseline contraction frequency was also evaluated
but performed less well than either mean or
maximum frequency as a screening test for preterm birth. In multivariate analyses, (Table 3)
maximum p.m. contraction frequency was significantly related to preterm birth only at 27 to 28
weeks’. Sensitivity, specificity, and predictive values for maximum a.m. and p.m. contraction
frequency are shown in Table 4. Maximum contraction frequency of 4 or more per hour was a
poor screening test for preterm birth. At 22 to 24
weeks’, the sensitivity of contraction frequency
to predict birth ⬍35 weeks’ was below 10%. The
sensitivity of maximum p.m. contraction frequency improved at later gestational age intervals, but continued to have low sensitivity and
positive predictive value to detect women with
increased risk of preterm birth.
We also performed analyses of contraction
data to look for a relationship between contraction frequency and an impending episode of
preterm labor. Preterm labor was diagnosed as
admission to the hospital and treatment for preterm labor in response to contraction frequency
Table 2. Odds Ratio for Spontaneous Delivery at
Less Than 35 Weeks, According to the Maximal
Daytime and Nighttime Frequency of Contractions
Odds Ratio (95% CI)*
Week of No. of
Daytime
Nighttime
Gestation Women (4 a.m.-3:59 p.m.) (4 p.m.-3:59 a.m.)
22-24
25-26
27-28
29-30
31-32
⬎33
270
301
294
288
281
266
0.9 (0.6-1.3)
1.2 (1.0-1.5)‡
1.0 (0.8-1.2)
1.1 (0.9-1.2)
1.0 (0.8-1.3)
0.8 (0.6-1.2)
*CI denotes confidence interval.
†P ⫽ .02.
‡P ⫽ .03.
§P ⫽ .003.
Reprinted with permission.1
1.3 (1.0-1.6)†
1.2 (1.0-1.4)‡
1.2 (1.1-1.4)§
1.1 (1.0-1.2)
1.1 (0.9-1.3)
1.1 (0.9-1.4)
Table 3. Relation of Contraction Frequency to
Occurrence of Spontaneous Birth ⬍35 Weeks
Maximum evening ⱖ4 contractions/hr
Gestational Age 22-24
27-28
weeks
weeks
Odds Ratio
3.0
3.0
95% CI
0.6-14.6
1.0-8.7
P value
0.18
0.04
31-32
weeks
1.3
0.3-5.2
0.74
Maximum morning ⱖ4 contractions/hr
Gestational Age 22-24
27-28
weeks
weeks
Odds Ratio
3.2
1.6
95% CI
*0.3-33.6
0.4-6.2
P value
0.34
0.54
31-32
weeks
0.5
0.1-3.2
0.49
Modified and reprinted with permission.1
accompanied by documented cervical change.
There were 108 women with an episode of preterm labor. Mean and maximum contraction
frequency for the 1- and 2-week periods before
an episode of preterm labor were compared to
mean and maximum contraction frequency for
the week in which preterm labor was diagnosed
to determine a “Delta CPH” or change in contractions per hour. Neither Delta CPH for mean
nor maximum contraction frequency was significantly related to a diagnosis of preterm labor
(P ⫽ .19 and .77, respectively).
The results of the study may be summarized
as follows:
1. Is the frequency of uterine activity greater in
women destined for preterm birth than in
women who will deliver at term? Yes.
Table 4. Uterine Contraction Frequency to Predict
Spontaneous Birth ⬍35 Weeks
Maximum evening contraction frequency ⱖ4
Gestational Age
22-24
27-28
31-32
weeks
weeks
weeks
Sensitivity %
8.6
28.1
27.3
Specificity %
96.4
88.7
82.0
Positive PV %
25.0
23.1
11.3
Negative PV %
88.3
91.1
93.0
Maximum morning contraction frequency ⱖ4
Gestational Age
22-24
27-28
31-32
weeks
weeks
weeks
Sensitivity %
0
12.9
13.6
Specificity %
98.4
93.9
84.9
Positive PV %
0
20.0
7.1
Negative PV %
87.0
90.2
92.1
Modified and reprinted with permission.1
210
Jay D. Iams
2. Is the frequency of uterine activity greater in
women with historical risk factors for preterm
birth greater than in women who have no risk
factors? No.
3. Does uterine activity differ according to gestational age in women destined for preterm
birth? Yes.
4. Does uterine activity differ according to time
of day in women destined for preterm birth?
Yes.
5. Is there a measure of uterine activity that is an
efficient test to: Screen for risk of preterm
birth? No. Predict preterm birth? No. Detect
preterm labor before it is detected by clinical
signs and symptoms? No.
Discussion
The distinction between association and prediction is a frequent source of confusion in medicine. Our findings highlight the importance of
the difference. We found that contraction frequency is significantly increased in women who
will deliver before 35 weeks’ gestation, but the
magnitude of that association was too small to be
clinically useful. The results of this study serve to
explain in part the failure of HUAM-based
screening programs to yield a decrease in the
rate of preterm birth. If the assumptions of contraction-based screening are presumed, HUAM
must function as a reasonably sensitive screening
test in order to apply interventions to reduce
preterm birth. Our data indicate that even in a
population with an increased risk of preterm
delivery, the majority of women destined for
preterm birth do not have a contraction frequency that distinguishes them from low-risk
women. Similarly, increased contraction frequency in any individual woman is more likely to
reflect advancing gestational age or diurnal variation than occult preterm labor. Given these
observations, it is not surprising that clinical trials of uterine contraction surveillance of at-risk
pregnancies do not show any advantage of such
monitoring. The results of other MFMU Network studies summarized in this issue24 also
reveal another reason for the failure of contraction-based prevention programs: their grounding in an incomplete understanding of how preterm labor occurs. There is growing evidence
that subacute or even chronic pathophysiologic
changes precede an eventual clinical diagnosis
of preterm labor or preterm rupture of the
membranes. Findings of inflammatory cytokines
in second-trimester amniotic fluid,25 fetal fibronectin expression in cervico-vaginal mucus,26
cervical shortening as seen on ultrasonography,27 and increased concentrations of maternal
salivary estriol,28 all detected weeks to months
before a preterm birth, provide evidence that
spontaneous preterm birth is the result of a
long-term process that culminates rather than
begins with uterine contractions.
More than two decades of clinical research
into the appropriate role of uterine contraction
frequency have followed a familiar if not altogether ideal path: An association is noted between an adverse outcome (eg, preterm labor
and birth) and a clinical marker – a symptom,
sign, or test result (eg, uterine contractions detected by education, self-palpation, and/or tocodynamometry). The association is clinically obvious, and leads to an equally obvious
intervention, in this case tocolysis, in the expectation that the adverse endpoint will occur less
often if the associated clinical marker is suppressed. Clinical trials are conducted but do not
produce the expected result, directing the attention of the research community back to the original association, which when examined in detail
proves to be much more complex than previously thought. This has been the path for contraction frequency monitoring, a path accompanied by great controversy in large part because
of the presence of private companies as research
sponsors and advocates in the process. It is appropriate to remember that the very same path
has been and is now being followed for other
markers associated with preterm birth such as
nutritional and social support, vaginal infections, cervical length, and salivary estriol. What
happened with uterine contraction monitoring
is typical of clinical research. Explanation No. 2,
also known as Dr. Eastman’s dictum, must always
be kept in mind: “Only when the factors underlying prematurity are completely understood
can any intelligent attempt at prevention be
made.”4 Meanwhile, however, there are patients
who need care before “the factors . . . are completely understood.” Our care for them must of
necessity be based on the best understanding we
have at present tempered by the humility of
knowing that we do not really understand, yet.
Uterine Contractions and Preterm Birth
References
1. Iams JD, Newman RB, Thom EA, et al for The National
Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units: Frequency of
uterine contractions and the risk of spontaneous preterm delivery. N Engl J Med 346:250-255, 2002
2. Collaborative Home Uterine Monitoring Study
(CHUMS) Group: A multicenter, randomized controlled trial of home uterine monitoring: Active versus
sham device. Am J Obstet Gynecol 173:1120-1127, 1995
3. Dyson DC, Danbe KH, Bamber JA, et al: Monitoring
women at risk for preterm labor. N Engl J Med 338:1519, 1998
4. Eastman NT: Prematurity from the viewpoint of the
obstetrician. Am Pract 1:343, 1947
5. Taylor ES, Main DM, Gabbe SG, et al: Can preterm
deliveries be prevented? Am J Obstet Gynecol 151:892898, 1985
6. Papiernik E, Bouyer J, Dreyfus J, et al: Prevention of
premature births: A perinatal study in Haguenau France.
Pediatrics 76:154-159, 1985
7. Heron M, Katz M, Creasy RK: Evaluation of a preterm
birth prevention program: Preliminary report. Obstet
Gynecol 59:542-546, 1982
8. Iams JD: Obstetric inertia: An obstacle to the prevention
of prematurity. Am J Obstet Gynecol 159:796-799, 1988
9. Merkatz IR, Peter JB, Barden TP: Ritodrine hydrochloride: A betamimetic agent for use in preterm labor. II.
Evidence of efficacy. Obstet Gynecol 56:7-12, 1980
10. Nathanielsz PW: A time to be born: Implications of
animal studies in maternal-fetal medicine. Birth 21:163169, 1994
11. Zlatnik FJ: Applicability of tocolytic therapy. Am J Perinatol 9:494-496, 1992
12. Collaborative Group on Preterm Birth Prevention: Multicenter, randomized controlled trial of a preterm birth
prevention program. Am J Obstet Gynecol 169:352-366,
1999
13. Creasy RK, Gummer BA, Liggins GC: A system for predicting spontaneous preterm birth. Obstet Gynecol 55:
692-696, 1980
14. Newman RB, Gill PJ, Wittreich P, et al: Maternal perception of prelabor uterine activity. Obstet Gynecol 68:765769, 1986
15. Katz M, Gill PJ: Initial evaluation of an ambulatory system for home monitoring and transmission of uterine
activity data. Obstet Gynecol 66:273-277, 1985
211
16. Katz M, Gill PJ, Newman RB: Detection of preterm labor
by ambulatory monitoring of uterine activity: A preliminary report. Obstet Gynecol 68:773-777, 1986
17. Morrison JC, Martin JM, Martin RW, et al: Prevention of
preterm birth by ambulatory assessment of uterine activity: A randomized study. Am J Obstet Gynecol 156:536,
1987
18. Corwin MJ, Mou SM, Sunderji SG, et al: Multicenter
randomized clinical trial of home uterine activity monitoring: Pregnancy outcomes for all women randomized.
Am J Obstet Gynecol 175:1281-1285, 1996
19. Iams JD, Johnson FF, O’Shaughnessy RW: A prospective
random trial of home uterine activity monitoring in
pregnancies at increased risk of preterm labor. Part II.
Am J Obstet Gynecol 159:595-603, 1988
20. Nagey DA, Bailey-Jones C, Herman AA: Randomized
comparison of home uterine activity monitoring and
routine care in patients discharged after treatment for
preterm labor. Obstet Gynecol 82:319-323, 1993
21. Brown HL, Britton KA, Brizendine EJ, et al: A randomized comparison of home uterine activity monitoring in
the outpatient management of women treated for preterm labor. Am J Obstet Gynecol 180:798-805, 1999
22. Moore TR, Iams JD, Creasy RK, et al and the Uterine
Activity in Pregnancy Working Group. Diurnal and gestational patterns of uterine activity in normal human
pregnancy. Obstet Gynecol 83:517-523, 1994
23. Germain AM, Valenzuela GJ, Ivankovic M, et al: Relationship of circadian rhythms of uterine activity with
term and preterm delivery. Am J Obstet Gynecol 168:
1271-1277, 1993
24. Goldenberg RL, Iams JD, Mercer BM, et al: The preterm
prediction study. Sem Perinatol 27:185-193, 2003
25. Ghidini A, Eglinton GS, Spong CY, et al: Elevated midtrimester amniotic fluid tumor necrosis alpha levels: A
predictor of preterm delivery. Am J Obstet Gynecol 174:
307, 1996 (abstr)
26. Goldenberg RL, Mercer BM, Meis PJ, et al: The Preterm
Prediction Study: Fetal fibronectin testing and spontaneous preterm birth. Obstet Gynecol 87:643-648, 1996
27. Iams JD, Goldenberg RL, Meis PJ, et al: The length of
the cervix and the risk of spontaneous premature delivery. N Engl J Med 334:567-572, 1996
28. McGregor JA, Jackson GM, Lachelin GC, et al: Salivary
estriol as risk assessment for preterm labor: A prospective trial. Am J Obstet Gynecol 173:1337-1342, 1995