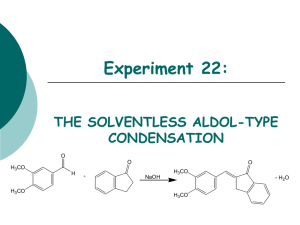
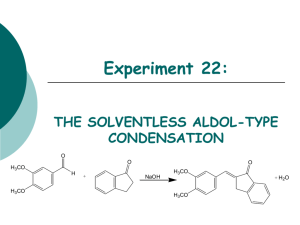
Facing the Challenges of Formulating
advertisement

Facing the Challenges of Formulating Christopher Johnson Christopher Christopher Johnson ‐ Johnson ‐ Kinetik Technologies Kinetik Technologies SCC Ontario Chapter SCC Ontario Chapter –– 2008 Education Day September 18, 2008 good is to: black hot bad white cold organic i i (A i lt ) non‐organic (Agriculture) non‐ inorganic (Chemistry) unnatural chemical synthetic y supernatural Adj ti Adjective 1. existing in or formed by (opposed to artificial) 2. based on the state of things in ; constituted by 3. in a state of in a state of ; uncultivated, as land ; uncultivated, as land Physical world, including living Physical world, including living organisms excluding organisms excluding manufactured objects from human interaction manufactured objects from human interaction unnatural Coming from nature >> harvest, collection Synthetic: Plant Animal Soil Olive Oil Milk Petroleum Saponification Fermentation Cracking Glycerin Cheese Gasoline • Growing concerns regarding the fate of our planet (Global Warming, Pollution, Fuel Crisis, etc.) • Increased demand for alternative fuel sources, organic foods and sustainable business practices (reduced ‘carbon footprint’) • Increased consumer awareness is resulting in rapid growth (15% in 2005) of h( ) f personal care l • Signifies major shift in consumer culture • No official definition for personal care y g • Limited formulatory access to ingredients or unethical behavior leading to products with conventional ingredients • Limited knowledge of consumers makes it easier to make claims as a marketing tool without corresponding formulating effort d f l ff • Consumers believe ingredients used in personal care are: personal care are: – Safer to use on themselves and their families – Promoting Promoting overall health & well‐ Promoting overall health & well overall health & well‐ well‐being – Better for the environment • The reality is… The reality is ingredients are not always safe – Finished products are costly to produce Finished products are costly to produce – Often inferior in performance – Challenging to formulate Ch ll i t f l t – Problems with large scale manufacturing • aa chemical philosophy encouraging the design chemical philosophy encouraging the design of products and processes that reduce or eliminate the use and generation of hazardous eliminate the use and generation of hazardous substances • Raw materials considered 2. 3. 4 4. 5. should be: made up of building blocks and be produced produced using environmentally sound processes using environmentally sound processes Efficient: Run chemical reactions at ambient Efficient: Run chemical reactions at ambient temperature and pressure to reduce energy waste Safe: Safe: should not be suspected of causing human should not be suspected of causing human health or environmental risk S t i bl biodegradable and derived from Sustainable: biodegradable and derived from Sustainable: bi d d bl dd i df renewal vegetal feed stocks Responsible: p no animal testing in its development g p (unique to cosmetics)* (unique to cosmetics)* a set of guidelines to help define the term the term as as it applies to it applies to personal care • All All products that are labeled or branded products that are labeled or branded must: – Be made with at least 95% all Be made with at least 95% all ingredients – Contain only synthetic ingredients specifically allowed under this standard and environmentally allowed under this standard and environmentally environmentally‐‐ friendly products that are nurturing to us and as harmless as possible to the earth • What is ? – Ingredients that come or are made from a I di t th t d f renewable resource found in (flora, fauna, mineral) mineral), with absolutely no petroleum with absolutely no petroleum compounds. • Ingredients that are prohibited: – Ingredients that have suspected human health risks as indicated by peer indicated by peer‐‐reviewed third reviewed third‐‐party scientific literature – Incorporate synthetic silicone or petroleum compounds p y p p • Parabens Parabens –– Synthetic preservatives that are potential endocrine disrupters endocrine disrupters • Sodium Sodium Lauryl Lauryl Sulfate Sulfate –– Harsh cleansing agent • Petrolatum/Mineral Oil/Paraffin Petrolatum/Mineral Oil/Paraffin –– Non Non‐‐renewable byproducts of crude oil • Chemical Sunscreens (Avobenzone Chemical Sunscreens (Avobenzone//Oxybenzone Oxybenzone) ) –– Synthetic sunscreens Synthetic sunscreens • Glycols Glycols –– Petroleum derived synthetic chemicals • Phthalates – Phthalates – Synthetic fragrance components that are Phthalates Synthetic fragrance components that are potential toxins • Ethoxylated Ingredients ‐ Ethoxylated Ingredients ‐ Ingredients that are made in part with ethylene oxide, • Ethanolamines – Foam and viscosity boosting ingredients that can interact with other ingredients to form that can interact with other ingredients to form nitrosamines, a known carcinogen(37) • Synthetic polymers (PVP/ Synthetic polymers (PVP/Acrylates Acrylates) ) ‐‐ may contain residual hydrocarbons ‐ widespread hydrocarbons ‐ hydrocarbons widespread organic pollutants organic pollutants • Formaldehyde Donors (DMDM Hydantoin Formaldehyde Donors (DMDM Hydantoin/ / Diazolidinyl Diazolidinyl Urea/ Methylisothiazolinone Urea/ Methylisothiazolinone) ) –– Preservatives that work by releasing formaldehyde • Allowed synthetic ingredients Allowed synthetic ingredients –– those temporarily allowed in the initial phase of this standard: – Non Non‐‐paraben, non paraben, non‐‐formaldehyde formaldehyde‐‐donating synthetic preservatives – Non N ‐phthalate, non Non‐ phthalate, non‐ hh l ‐irritating synthetic fragrances i i i h i f – Quaternary anti Quaternary anti‐‐static hair conditioners – Coco Coco Betaine Betaine i • P hibit d i Prohibited ingredients: di t – – – – – – – – – – – – – – – – – – – – Ammonium Lauryl Sulfate Amodimethicone Behentrimonium Methosulfate Butylene glycol Carbomer Ceteareth‐20 Cetrimonium Chloride Coco DEA Cocoamidopropyl Betaine Cyclopentasiloxane Diazolidinyl Urea Dimethicone Disodium Cocoamphodiacetate EDTA EthylHexylGlycerin Glycereth‐7 Cocoate Isoceteth 20 Isopropyl Palmitate Lauramide MEA Lauryl DEA • P hibit d i Prohibited ingredients: di t – – – – – – – – – – – – – – – – – – – Methoxycinnamate Olefin Sulfonate Oleyl Betaine Parabens (methyl, propyl, butyl, etc.) PEG‐150 Distearate PEG‐7 Glyceryl Cocoate Polyquaternium 10 Propylene Glycol Sodium Cocoyl Sarcosinate Sodium Hydroxymethylglycinate Sodium Laureth Sulfate Sodium Lauroyl Sarcosinate Sodium Lauryl Carboxylate Sodium Lauryl Sulfate Sodium Lauryl Sulfoacetate Sodium Myreth Sulfate Sodium PCA or Na PCA (pyrrolidone carbonic acid) Soyamidopropalkonium Chloride Stearamidopropyl d l Dimethyl h l Amine INCI: Purified Water, Aloe Barbadensis INCI: Purified Water Aloe Barbadensis Leaf Leaf Juice, Sodium Lauryglucosides Hydroxypropylsulfonate, Cocamidopropyl Cocamidopropyl Betaine, Decyl Glucoside, Glycerin, Guar Betaine Guar Hydroxypropyltrimonium Chloride, Hydroxypropyltrimonium Chloride Panthenol, Allantoin, Simmondsia Chinensis (Jojoba) Seed Oil(2), Helianthus Annuus (Sunflower) Seed Oil(3) Olea Europaea (Sunflower) Seed Oil(3), Olea (Olive) Fruit Oil(3), Linum Usitatissimum (Linseed*) Seed Oil(4), Borago Officinalis Seed Oil(3), Vitis Vinifera (Grape) Seed Oil(4), Rosa Canina l( ) Fruit Oil(4), Citric Acid, l( ) d Polysorbate 20 20, Sodium Benzoate, Potassium Sorbate, Ethylhexylglycerin. • List of Allowed Ecological Processes – Saponification of vegetable oils to make soap – Hydrolysis of Proteins into Amino Acids Hydrolysis of Proteins into Amino Acids – Fat Fat‐‐Splitting of vegetable oils to produce glycerin and fatty acids – Hydrogenation of oils Hydrogenation of oils – Hydrogenolysis of methyl esters to products fatty alcohols – Glucosidation of fatty Alcohols and glucose – Sulfation of fatty alcohol – Protein fragment Protein fragment acylation acylation – Etherificiation of glycerin making of glycerin making polyglycerol gy gp polyglycerol yg y – Esterification or or Transesterification Transesterification to produce esters • Polyglyceryl esters – 100% vegetable 100% vegetable‐‐based – Based on Chemistry – Esterification of of polyglycerol polyglycerol with fatty acids – Functional emulsifiers & surfactants – Wide range of HLBs for O/W & W/O emulsions g / / – PEG PEG‐‐Free, biodegradable & free of toxic impurities INCI Function Polyglyceryl‐‐3 Stearate Polyglyceryl O/W O/W‐‐Emulsifier Polyglyceryl‐‐3 Polyricinoleate Polyglyceryl W/O W/O‐‐Emulsifier Polyglyceryl‐‐10 Laurate Polyglyceryl 10 Laurate Solubilization Polyglyceryl‐‐5 Oleate Polyglyceryl Emulsifier (Oil gel) Polyglyceryl‐10 Laurate (Solubilizer) 20 18 16 14 Polyglyceryl‐5 Oleate (O/W‐Emulsifier) 12 10 Polyglyceryl‐3 Stearate P l l l 3 St t (O/W‐Emulsifier) 8 6 4 2 0 Polyglyceryl‐3 Polyricinoleate (W/O‐Emulsifier) 10 C‐8 Fatty acid 9 C‐10 C‐12 8 7 C‐14 6 C‐16 C‐18 2 x C‐18 2 C 18 2 Polyricinoleate 5 4 3 g y Degree of Polymerization HLB-Value 20 18 Polyglyceryl 3 Stearate Polyglyceryl‐3 Stearate (O/W Emulsifier) 16 14 12 10 8 6 4 2 0 10 C‐8 Fatty acid 9 C‐10 C‐12 8 7 C‐14 6 C‐16 C‐18 2 x C‐18 2 C 18 2 Polyricinoleate 5 4 3 g y Degree of Polymerization HLB-Value • • • • • • non‐ionic O/W Emulsifier non‐ vegetable origin vegetable origin HLB: ~ 10 – HLB: ~ 10 – 12 use level: 2 ‐ use level: 2 l l 2 ‐ 4 % 4% optimal pH: 5 – optimal pH: 5 – 8 oilphase 15 ‐ oilphase 15 ‐ 30 % • Formulating Tips: Formulating Tips: – Melt in oil phase 65 – Melt in oil phase 65 – 80 80 °°C – Increased viscosity with increased emulsifier Increased viscosity with increased emulsifier level, slight viscosity increase during storage – Poor compatibility with electrolytes Poor compatibility with electrolytes – Typical combination with Sodium Stearoyl Lactylate Lactylate* – Support stability with Glyceryl Stearate, fatty alcohol, fatty acids and/or Xanthan Gum alcohol, fatty acids and/or Xanthan Gum • • • • • • anionic O/W Co anionic O/W Co‐‐Emulsifier vegetable origin vegetable origin HLB: > 10 (depending on pH) use level: up to 3% l l t 3% optimal pH: 5 – optimal pH: 5 – 7 oil phase 15 – oil phase 15 – 30% • Formulating Tips: – Melt in oil phase 65 – Melt in oil phase 65 p – 80 80 °°C – Support stability with Glyceryl Stearate, fatty alcohol, fatty acids and / or Xanthan Gum – Slight downshift of pH may be observed after emulsification Certain molecules in a formulation (e.g. essential oils) may interact with the interphase of an emulsion droplet Oil Phase Oil Phase Water Phase Water Phase That can potentially result in a destabilization of the emulsion That can potentially result in a destabilization of the emulsion Oil Phase Oil Phase Water Phase Water Phase Using mixtures of non U Using mixtures of non‐ i i f ‐ionic and anionic emulsifiers can i i ionic and anionic emulsifiers can improve d i i l ifi i improve stability of the emulsion stability of the emulsion Polyglyceryl‐‐3 Stearate Polyglyceryl ‐ Na+ Sodium Stearoyl Lactylate Sodium Stearoyl Lactylate Ionic co‐ Ionic co‐emulsifiers create a layer of organized water molecules to protect the interphase Oil Phase ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ Na+ ‐ N + Na ‐ Na+ ‐ Na+ ‐ Na+ ‐ Na+ Water Phase Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Page 41 ∙Biesterfeld product training seminar27‐28.08.2008 Phase Ingredient INCI A Deionised Water B C D Supplier % 68.00 Sodium Phytate Dr.Straetmans 0.10 Glycerin y Various 3.00 Glyceryl Caprylate Dr. Straetmans 0.70 p‐Anisic Acid Dr. Straetmans 0.20 Xanthan Gum Kelco 0.25 M Magnesium Aluminium Silicate i Al i i Sili t R T V d bilt R.T. Vanderbilt 05 0.5 Polyglyceryl‐3 Stearate Dr. Straetmans 3.00 Sodium Stearoyl Lactylate Dr.Straetmans 2.00 Glyceryl Stearate Cognis 3.00 Cetyl Alcohol Cognis 2.00 Butyrospermum Parkii (Shea Butter) Kinetik 2.00 Decyl Cocoate Evonik 6.00 Caprylic/Capric Triglyceride py / p gy Various 5.00 Squalane Various 3.00 Tocopherol, Helianthus Annuus (Sunflower) Seed Oil Dr. Straetmans 0.05 Tocopheryl Acetate Dr. Straetmans 1.00 Fragrance (Natural) Fragrance (Natural) Various 0 20 0.20 Citric Acid (20% sol.) Various q. s. Formula # L016‐05‐1007 100.00 • Emulsion properties: l – white viscous cream – pH: 5.0 pH: 5.0 –– 5.5 – viscosity: ~ 35,000 cps – stability: > 3 months @ 40, 20 & 4 stability: > 3 months @ 40, 20 & 4°°C – passes micro challenge 20 18 Polyglyceryl‐3 Polyricinoleate P l l l 3P l i i l t (W/O‐Emulsifier) 16 14 12 10 8 6 4 2 0 10 C‐8 Fatty acid 9 C‐10 C‐12 8 7 C‐14 6 C‐16 C‐18 2 x C‐18 2 C 18 2 Polyricinoleate 5 4 3 g y Degree of Polymerization HLB-Value • • • • • • non‐ionic W/O Emulsifier non‐ vegetable origin HLB: ~ 4 HLB: ~ 4 –– 5 use level: 3 – 6 % use level: 3 6% wide range of viscosity from creme to lotion up to 80 % waterphase • Suitable stabilizing / thickening systems – ZnSO4 or MgSO4 – Mg Stearate or Zn Stearate – Bentonite – Hydrogenated Castor Oil – Beeswax – Carnauba Wax Page 46 ∙Biesterfeld product training seminar27‐28.08.2008 • Formulating Formulating Tips: Tips: – Viscosity can be increased by: • llowering oil concentration i il t ti • lowering polarity of oil • increasing amount of W/O increasing amount of W/O‐ / ‐emulsifier – Additional factors: • emulsification temperature > 75 emulsification temperature > 75°°C • slow stirring during cooling • homogenization at < 45° homogenization at < 45°C Phase Ingredient INCI g A Deionised Water B B1 C Supplier pp % 55.25 Glycerin Various 7.00 Zinc Sulfate Various 1.00 Glyceryl Caprylate Dr. Straetmans 0.70 p‐Anisic Dr. Straetmans 0.20 Citric Acid (20% sol) Various q. s. P l l Polyglyceryl‐3‐Polyricinoleate l3P l i i l t D St t Dr. Straetmans 5 00 5.00 Cera Alba Kahl & Co 4.00 Helianthus Annuus (Sunflower) Seed Oil Various 16.00 Tocopherol, Helianthus Annuus (Sunflower) Seed Oil Dr. Straetmans 0.20 Magnesium Stearate Various 0.50 Caprylic/Capric Triglyceride Sasol 10.00 Fragrance (Natural) Formula # L019‐30B‐108 0.15 100.00 • Emulsion properties: l – thick white cream – viscosity: ~ 60,000 – viscosity: ~ 60,000 – 100,000 cps – stability: > 3 months @ 40 stability: > 3 months @ 40, 20 & 4 , 20 & 4°°C – passes micro challenge 20 18 Polyglyceryl 5 Oleate Polyglyceryl‐5 Oleate (O/W‐Emulsifier) 16 14 12 10 8 6 4 2 0 10 C‐8 Fatty acid 9 C‐10 C‐12 8 7 C‐14 6 C‐16 C‐18 2 x C‐18 2 C 18 2 Polyricinoleate 5 4 3 g y Degree of Polymerization HLB-Value • • • • • non‐ionic O/W Emulsifier for oil gels non‐ vegetable origin HLB: 11.5 (calculated) use level: 5 ‐ 10 % use level: 5 ‐ use level: 5 10 % oil phase: >75% Phase Ingredient INCI Supplier % A Deionized Water B Glycerin Various 15,00 Polyglyceryl‐5 Oleate Dr. Straetmans 6,00 Caprylic/Capric Triglycerides Various 31,50 Olea Europaea (Olive) Fruit Oil Various 45,87 Tocopherol, Helianthus Annuus (Sunflower) Seed Oil Dr. Straetmans 0,20 C Formula # q.s. L004‐01‐11‐707 100,00 • Oil Gel properties: – clear yellow viscous gel – viscosity: ~ 60,000 cps – stability: > 3 months @ stability: > 3 months @ 20 & 4 20 & 4°°C, some turbidity at 40° some turbidity at 40°C after 6 weeks • Formulating Tips: Formulating Tips: – Combine Polyglyceryl Combine Polyglyceryl‐‐5 Oleate with Glycerin – Add oil in small portions under virgorous stirring Add oil in small portions under virgorous stirring to form homogenious gel, viscosity will increase – Add increasingly larger portions of oil, form gel Add increasingly larger portions of oil form gel before each seperate addition step – Add water in the end to adjust the refractive index Add water in the end to adjust the refractive index – Remove air by vacuum • Potential applications: – cleansing gels – massage gels – self self‐‐emulsifying bath gels – exfoliating gels – lip treatment gels p g 20 18 Polyglyceryl 10 Laurate Polyglyceryl‐10 Laurate (Solubilizer) 16 14 12 10 8 6 4 2 0 10 C‐8 Fatty acid 9 C‐10 C‐12 8 7 C‐14 6 C‐16 C‐18 2 x C‐18 2 C 18 2 Polyricinoleate 5 4 3 g y Degree of Polymerization HLB-Value • • • • non ionic surfactant vegetable based HLB approximately HLB: i t l 15 use level: 3:1 (solubilizer : oil) Transparency Test Transparency Test Water 98.8 % 88.8 % 78.8 % Polyglycery‐10 Laurate 1.0 % 1.0 % 1.0 % Fragrance 0.2 % 0.2 % 0.2 % Ethanol 0.0 % 10.0 % 20.0 % clear solubilisation up to 1 % essential oil Phase Ingredient INCI A Deionized Water B B1 Supplier % 67,65 Glycerin Various 7,00 Sodium Phytate Dr.Straetmans 0,10 Ethanol Various 20,00 Glyceryl Caprylate Dr.Straetmans 1,00 Bisabolol Kinetik 0,05 Polyglyceryl‐10 Laurate Dr.Straetmans 2,00 Fragrance (Natural) Various 0,20 Formula # L010‐03‐107 100,00 • Toner properties: – clear light yellow liquid – pH: ~ 5.5 – stability: > 3 months @ stability: > 3 months @ 20 & 4 20 & 4°°C • Formulating Tip: – Combine fragrance oils directly into Polyglyceryl‐‐10 Laurate Polyglyceryl ‐ Without Without parabens parabens ‐ Without preservatives ih i ‐ Natural Background: ‐ Parabens have been found Parabens have been found in in breast cancer cells breast cancer breast cancer cells (Darbre, J. et al (Darbre, J. et al Appl. Toxicology, 24, Appl. Toxicology, 24, (2004),1.) (2004) 1.)) (2004),1 ‐ Parabens might Parabens might facilitate facilitate skin ageing (Ishiwatari et al, Proc. IFSCC (Ishiwatari et al, Proc. IFSCC Conf. (2005 Conf . (2005), 129 ), 129‐‐135.) ‐ Without Without parabens parabens ‐ Without preservatives ih i ‐ Natural Background:: Background The consumer relates preservatives to negative effects to negative effects like: like: skin irritation skin irritation allergic reactions cancer causing, etc. cancer causing, etc. ‐ Without Without parabens parabens Background:: Background ‐ Without preservatives ih i ‐ iis considered by is considered by id d b the consumer to be the opposite of opposite of chemical chemical ‐ is associated by the is associated by the consumer with the attributes consumer with the attributes lik safe like safe like f and and gentle d gentle tl organic acids surface active materials surface active materials Product Recommended Performance against Dosage [%] pH range gram+ gram‐ Yeast Mold Levulinic Acid li i id 0 5 1.0 0.5 – 10 <55 < 5.5 Anisic Acid 0.05 – 0.5 < 5.5 A Access to levulinic acid from sustainable starting material l li i id f i bl i i l Mineral Acid Vegetable Starch Levulinic Acid Acid Levulinic Acid pK‐ value Amount of free acid at pH 4.5 5.0 5.5 6.0 4.64 58.0% 30.4% 12.1% 4.2% ‐ Levulinic acid has an infinite water solubility Challenge test: 0.3 % levulinic acid in water Challenge test: 0.3 Challenge test: 0.3 % levulinic acid in water % levulinic acid in water 1000000 100000 10000 1000 100 10 Asp. Ni ger Cand. Al bi c. E.Col i Pseud. Aer ugi n. 1 0 7 14 Staph. Aur eus 21 28 ‐ Levulinic acid has broad antibacterial efficacy organic acids it shows g it shows no weakness no weakness against Pseudomonas against Pseudomonas g ‐ Unlike other Unlike other organic acids Ch Characteristics i i Appearance Clear, colourless liquid Odour mild, typical INCI Sodium Levulinate or Fragrance Optimal pH 4.5 4.5 –– 5.5 Recommended Concentration 0.5 0.5 –– 1.0 % Regulary Status Listed in EU, US, Japan Application Emulsions and rinse Emulsions and rinse‐‐off products O2 / / H2O2 / NaOH trans‐Anethol Natural Anisic Acid p‐anisic acid is accessible in > 99% purity in a patented process by ozonolysis of trans‐anethole which is readily available from star anise oil or basil oil Challenge Test: Challenge Challenge Test: 0.2 Test: 0.2% 0.2% 0.2 % anisic acid as a fungicidal component in a % anisic acid anisic acid as as a fungicidal component in a a fungicidal component in a O/W Emulsion pH = 5.25 O/W Emulsion pH = 5.25 1000000 10000 100 Asp. Niger 1 0 E Coli E.Coli 7 14 St aph. Aureus 21 28 Characteristics Appearance White to slightly yellowish powder INCI p‐Anisic Acid or Fragrance Recommended dosage 0.1 – 0.3% pH ‐ optimum of activity 4.5 – 5.5 Regulatory Status 15/2003/EC IFRA Code Nitro musk CMR ingredients GMO‐status Application Registered in EU, US, Japan no listed allergens full compliance no no CMR Substances non‐GMO Emulsions and rinse‐off products at pH values < 5,5. • Organic Organic acids should be added to the aqueous phase acids should be added to the aqueous phase • When using Anisic Acid, a pre‐ When using Anisic Acid, a pre‐solution with small amounts of sodium hydroxide is often recommended • Levulinic Acid can be added to the aqueous phase or used to adjust the pH of the finished formulation • The pH of the formulation should not exceed 5.5 in order to provide sufficient antimicrobial activity • Specific incompatibilities of the acids with other natural raw materials have not been reported Each organic acid dissociates in an aqueous medium Each organic acid dissociates in an aqueous medium O O + Base H 3C O O HA H 3C O + H H Base+ B O‐ A ‐ Bases can be: water, salts (e.g. citrate, lactate, hydroxide) c ds a d base o a dy a c a d p depe de equ b u Acids and base form a dynamic and pH‐dependent equilibrium pH = pK + log cA‐ / cHA pH = pK + log cA‐ / cHA pH Water Phase O H 3C O O‐ pH = pK O H 3 CO O H After dissociation only the unpolar acid can penetrate the membrane. The The energy consumption and continuously low pH lead to Within the cell the acid dissociates, disturbs the pH‐equilibrium in the The cell struggles to maintain the pH by pumping H+ into the medium with salt death salt remains in the medium and is inactive. remains in and isThis process consumes energy (ATP) inactive This process consumes energy (ATP) the the death of the microorganism. thethe microorganism plasma plasma and inhibts enzyme activity. concurrent concurrent intake of Na andofintake inhibts ofmedium enzyme Na+. activity pH Bacterium O Lipid membrane H 3C O OH M di Medium Oil Phase HH++ H+ H + + + HH O H3CO O H3CO OH O O OH O OH OH OH O H3CO OH O H3CO OH H3CO H3CO O OH OH O H3CO H3CO O OH H3CO O OH OH O H3CO OH O OH H3CO OH H CO 3 O OH O OH H3CO OH O O OH OH O OH H3CO OH H3CO O O H3CO O H3CO OH H3CO H3CO OH OH H3CO H3CO OH O OH OH OH O O O H3CO O H3CO OH O H3CO OH OH O H3CO O H3CO O OH OH O H3CO H3CO O O O H3CO OH O OH O H3CO H3CO O H3CO O H3CO H3CO OH O H3CO OH H3CO O H3CO OH O O H3CO H3CO Na+ Na+ Na+ OH OH O H3CO H3CO O H3CO OH O H3CO OH OH OH H3CO H3CO O O H3CO H3CO OH H3CO O OH O OH Summary: Acids which are capable of penetrating the cell membrane of microorganisms affect the intracellular pH equilibirium and thereby the metabolism of the cell. In order to re In order to re‐‐establish the physiological pH the cell actively pumps out H+ thereby burning cell fuel ATP. Continious efforts to maintain the pH thereby burning cell fuel ATP. Continious efforts to maintain the pH‐‐level of the cytosol finally leads to an exhaustion of cellular energy reserves. y y gy A sufficient efficacy of an organic acid requires: a)) A sufficient number of uncharged acid molecules, which is related A ffi i t b f h d id l l hi h i l t d to the pH and the pK of the organic acid g b)) A sufficient total concentration of the organic acid pH = pK + log cA‐ / cHA Anisic acid: comparison of 0.06 Anisic acid: comparison of 0.06 % at pH % at pH 5.0 5.0 with with 0.5 0.5 % at pH % at pH 6.0 6.0 10000000 1000000 100000 10000 cfu Asp. niger pH 5 Asp. niger pH 6 C. albic. pH 5 C. albic. pH 6 E.coli pH 5 1000 100 E.coli pH 6 10 Ps. aerugin. pH 5 Ps. aerugin. pH 6 1 0 Staph. aureus pH 5 7 14 days Staph. p aureus pH p 6 21 28 • Levulinic Acid and Anisic Acid are two e u c cda d sc cda et o antimicrobially active natural organic acids g p y • These organic acids are accepted for use by several natural organizations including OASIS (USA), BDIH (Germany), Organic Soil Association (UK) and Ecocert (France) (UK) d E t (F ) • The antimicrobial efficacy of these acids is complementary providing broad spectrum complementary, providing broad spectrum protection • Limitation: formulation pH should be <5.5 Limitation: formulation pH should be <5 5* OH OH HO O HO O O O Glyceryl Caprylate O HO Glyceryl Caprate the glyceryl monoesters H OH N HO O O Capryloyl Glycine Ethylhexylglycerin Glyceryl Caprylate Glyceryl Caprylate Glyceryl Caprate Glyceryl Caprate Access to naturally derived glyceryl monoesters Coconut or Palm Oil as a natural source of shorter chain fatty acids y Distillatio Cocofatty acids n Distilled Acids Esterification Coconut Oil Coconut Oil Hydrolysis Glycerol Glyceryl monoester Product Recommended Performance against Dosage [%] pH range gram+ gram‐ Yeast Mold Glyceryl Caprylate l l l 0 3 1.0 0.3 – 10 45 7 4.5 ‐ / Glyceryl Caprate 1.0 – 2.0 4.5 ‐ 7 Challenge Test: 0.7% Glyceryl Caprylate in a g y y py pH = 5.1 O/W Emulsion / 1000000 100000 10000 1000 100 10 Asp. Niger Cand. Albic. E.Coli Pseud. Aerugin. 1 0 7 14 Staph. Aureus 21 28 Glyceryl Caprylate has strong efficacy against bacteria and yeast but only limited activity against fungi Characteristics Glyceryl Caprylate Glyceryl Caprate Appearance Waxy solid Waxy solid INCI y y py Glyceryl Caprylate Glyceryl Caprate y y p Recommended dosage 0.3 – 1.0% 1.0 – 2.0% Optimal pH 4.5 – 7.0 4.5 – 7.0 Regulatory Status g y Registered in EU, US, Japan g , , p Registered in EU, US, Japan g , , p GMO‐status non‐GMO non‐GMO Monoester content > 88 % > 88 % Glyceryl Caprylate is a good basic component for alternative preservation Glyceryl Caprate is a good deodorant active (gram+ bacteria) The active compounds are not used up and remain active The high surface activity and incompatible size of the The active compounds are small amphiphilic molecules, imolecules destabilizes the membrane in the formula. th thlt f lh d l t bili b th li id b that exchange membrane lipids. Oil Phase Cell O O Water Phase Water Phase OH OH 0 min i 1 min 5 min 10 min Lit.: •Bergsson, G., Arnfinnsson, J, Karlsson, S.M., Steingrimsson, Ó, Thormar, H., In Vitro Inactivation of Chlamydia trachomatis by Fatty Acids and Monoglycerides, gy , Antimicrob. Agents g Chemother. 42 (1998) 2290-2294. TIME Treatment of f negatively stained Chlamydia trachomatis with 1 mM of Glyceryl Caprate M f Gl lC Surface active materials, like Glyceryl Caprylate, can penetrate the interphase between oil phase and water phase Oil Phase Oil Phase Water Phase Water Phase At elevated concentrations, the high surface activity of the anti‐ At elevated concentrations, the high surface activity of the anti‐ microbial surfactant may result in a destabilization of the emulsion Oil Phase Oil Phase Water Phase Water Phase Using mixtures of non U Using mixtures of non‐ i i f ‐ionic and anionic emulsifiers can i i d i i l ifi efficiently lead to a stabilization of the emulsion A suitable systems A suitable systems is: is: Polyglyceryl‐‐3 Stearate Polyglyceryl 3 Stearate ‐ Na+ Sodium Stearoyl Lactylate Using ionic emulsifiers leads to an additional layer of organized water molecules at the interphase water molecules at the interphase Oil Phase ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ Na+ ‐ N + Na ‐ ‐ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ Na+ ‐ Na+ Na+ Na+ Na+ Na+ ‐ Water Phase The organic acids are a good choice for alternative preservation without The organic acids are a good choice for alternative preservation without any change of the characteristics of a given formula if pH is suitable. In some cases the performance in emulsions needs to be enhanced. Antimicrobial surfactants can be formulated at a wider pH range but may have an impact on the viscosity or the stability of emulsions. The use of blends of non‐ blends of non non‐ionic and anionic emulsifiers can improve the stability of ionic and anionic emulsifiers can improve the stability of emulsion based products. In rinse‐off products inactivation can sometimes be observed due to the In rinse‐ f formation of micelles. ti f i ll The efficacy of some antimicrobial surfactants alone against fungi is The efficacy of some antimicrobial surfactants alone against fungi is limited limited and additional combinations should be explored p • aa synergy between the (Levulinic Acid, Anisic synergy between the (Levulinic Acid Anisic Acid) organic acids and the surface active materials (Glyceryl Caprylate) has been materials (Glyceryl Caprylate) has been discovered!! • boosts antimicrobial performance boosts antimicrobial performance Phase Ingredient A Deionized Water B C INCI Supplier % ~ 74.10 Sodium Phytate Dr.Straetmans 0.10 Glycerin Various 3.00 Glyceryl Caprylate Glyceryl Caprylate Dr Straetmans Dr.Straetmans p‐Anisic Acid or Fragrance Dr. Straetmans Sodium Hydroxide (10% sol.) Various Xanthan Gum Kelco 0.20 Glyceryl Stearate Citrate Dr. Straetmans 3.50 Decyl Cocoate Goldschmidt 3.00 Olea Europaea (Olive) Fruit Oil Various 2.00 Squalane Various 6 00 6.00 Cetearyl Alcohol Various 2.00 Caprylic/Capric Triglyceride Various 4.00 Tocopherol, Helianthus Annuus (Sunflower) Seed Oil Dr. Straetmans 0.15 Citric acid (20% sol.) Various q.s. Sodium Hydroxide (10% sol.) Various q.s. Formula # D002‐50ff as indicate d q.s. 100.00 European European Pharmacopoeia Criteria Pharmacopoeia Criteria 1000000 100000 10000 1000 100 10 EP Criteria 1 Aspergillus Niger Candida Albicans Escherichia Coli Pseudomonas Aeruginosa St h l Staphylococcus Aureus A Asp .Nig e r 0 Cand .Alb ci . 7 E.Coli 14 Pse ud .Aeru gin . 21 Sta ph .Au re us 28 A A A A A Standard Standard O/W, pH = 5.3 Standard O/W, pH = O/W pH = 5.3 53 Levulinic Acid 0 7% Levulinic Acid 0.7% Levulinic Acid 0.7 7% 1000000 100000 10000 1000 100 10 EP Criteria 1 Aspergillus Niger Candida Albicans Escherichia Coli Pseudomonas Aeruginosa St h l Staphylococcus Aureus A 0 Asp e rg u li sn g i er Can did a alb ci an s 7 Esc h erci hia c oli 14 Pse udo mo na saeru gin osa 21 Sta ph yo l c o c c usau re u s 28 B NF B B A Standard Standard O/W, pH = 5.3 Standard O/W, pH = O/W pH = 5.3 53 Levulinic Acid 0 7% Levulinic Acid 0.7% + Levulinic Acid 0.7 7% + Anisic Acid 0.2 % + Anisic Acid 0.2% Anisic Acid 0 2% 2% 1000000 100000 10000 1000 100 10 EP Criteria 1 Aspergillus Niger Candida Albicans Escherichia Coli Pseudomonas Aeruginosa St h l Staphylococcus Aureus A 0 Asp e rg u li sn g i er Can did a alb ci an s 7 Esc h erci hia c oli 14 Pse udo mo na saeru gin osa 21 Sta ph yo l c o c c usau re u s 28 B F A A A Standard Standard O/W, pH = 5.3 Standard O/W, pH = O/W pH = 5.3 53 Glyceryl Glyceryl Caprylate 0.7% Glyceryl Caprylate 0.7 Caprylate 0 7% 7% 1000000 100000 10000 1000 100 10 EP Criteria 1 Aspergillus Niger Candida Albicans Escherichia Coli Pseudomonas Aeruginosa St h l Staphylococcus Aureus A 0 Asp e rg u li sn g i er Can did a alb ci an s 7 Esc h erci hia c oli 14 Pse udo mo na saeru gin osa 21 Sta ph yo l c o c c usau re u s 28 B A A A A Standard Standard O/W, pH = 5.3 Standard O/W, pH = O/W pH = 5.3 53 Glyceryl Glyceryl Caprylate 0.7% + Glyceryl Caprylate 0.7 Caprylate 0 7% 7% + Anisic Acid 0.2 % + Anisic Acid 0.2% Anisic Acid 0 2% 2% 1000000 100000 10000 1000 100 10 EP Criteria 1 Aspergillus Niger Candida Albicans Escherichia Coli Pseudomonas Aeruginosa St h l Staphylococcus Aureus A 0 Asp e rg u li sn g i er Can did a alb ci an s 7 Esc h erci hia c oli 14 Pse udo mo na saeru gin osa 21 Sta ph yo l c o c c usau re u s 28 A A A A A Standard Standard O/W, pH = 5.3 Standard O/W, pH = O/W pH = 5.3 53 Glyceryl Glyceryl Caprylate 0.5% + Glyceryl Caprylate 0.5 Caprylate 0 5% 5% + Levulinic Acid 0.3 % + Levulinic Acid 0.3% Levulinic Acid 0 3% 3% 1000000 100000 10000 1000 100 10 EP Criteria 1 Aspergillus Niger Candida Albicans Escherichia Coli Pseudomonas Aeruginosa St h l Staphylococcus Aureus A 0 Asp e rg u li sn g i er Can did a alb ci an s 7 Esc h erci hia c oli 14 Pse udo mo na saeru gin osa 21 Sta ph yo l c o c c usau re u s 28 B A A A A Standard Standard O/W, pH = 5.3 Standard O/W, pH = O/W pH = 5.3 53 Glyceryl Glyceryl Caprylate 0.5% + Glyceryl Caprylate 0.5 Caprylate 0 5% 5% + Levulinic Acid 0.3% % + Levulinic Levulinic Acid 0.3% Acid 0 3% + Anisic Acid 0.2 + Anisic Acid 0.2% % 1000000 100000 10000 1000 100 10 EP Criteria 1 Aspergillus Niger Candida Albicans Escherichia Coli Pseudomonas Aeruginosa St h l Staphylococcus Aureus A 0 Asp e rg u li sn g i er Can did a alb ci an s 7 Esc h erci hia c oli 14 Pse udo mo na saeru gin osa 21 Sta ph yo l c o c c usau re u s 28 A A A A A • The antimicrobial efficacy of organic acids at h b l ff f d pH 5.3 could be increased significantly in combination with surface active antimicrobial bi i ih f i i i bi l materials like Glyceyrl Caprylate • Can we use this synergy to extend the scope y gy p of the organic acids to high pH values? O/W T t E l i O/W Test Emulsion, pH 6,5 challenge tests H 6 5 h ll t t 1 Glyceryl Caprylate 2 3 0,3 0,7 Levulinic Acid Levulinic Acid 07 0,7 07 0,7 Anisic Acid 0,4 0,4 0,4 pH‐value 6,5 6,5 6,5 Standard Standard O/W, pH = 6.5 Standard O/W, pH = O/W pH = 6.5 65 Levulinic Acid 0.7% + L li i A id 0 7% Levulinic Acid 0.7 7% + Anisic Acid 0.4 % A Anisic Acid 0.4% i i A id 0 4% 4% % 1000000 100000 10000 1000 100 10 EP Criteria 1 Aspergillus Niger Candida Albicans Escherichia Coli Pseudomonas Aeruginosa St h l Staphylococcus Aureus A 0 Asp e rg u li sn g i er Can did a alb ci an s 7 Esc h erci hia c oli 14 Pse udo mo na saeru gin osa 21 Sta ph yo l c o c c usau re u s 28 A F B A A Standard Standard O/W, pH = 6.5 Standard O/W, pH = O/W pH = 6.5 65 LLevulinic Acid 0.7 Levulinic Acid 0.7% li i A id 0 7% 7% + Anisic Acid 0.4 % + Anisic Acid 0.4% + A i i A id 0 4% 4% + % Glyceryl Caprylate 0.3% Glyceryl Caprylate 0.3% 1000000 100000 10000 1000 100 10 EP Criteria 1 Aspergillus Niger Candida Albicans Escherichia Coli Pseudomonas Aeruginosa St h l Staphylococcus Aureus A 0 Asp e rg u li sn g i er Can did a alb ci an s 7 Esc h erci hia c oli 14 Pse udo mo na saeru gin osa 21 Sta ph yo l c o c c usau re u s 28 A A A A A Standard Standard O/W, pH = 6.5 Standard O/W, pH = O/W pH = 6.5 65 Anisic Acid 0.4% + A i i A id 0 4% Anisic Acid 0.4 4% + Glyceryl Caprylate 0.7 % Gl Glyceryl Caprylate 0.7% lC l 07 7% % % 1000000 100000 10000 1000 100 10 EP Criteria 1 Aspergillus Niger Candida Albicans Escherichia Coli Pseudomonas Aeruginosa St h l Staphylococcus Aureus A 0 Asp e rg u li sn g i er Can did a alb ci an s 7 Esc h erci hia c oli 14 Pse udo mo na saeru gin osa 21 Sta ph yo l c o c c usau re u s 28 A A A A A The surfactants cause a higher permeability by forming pores Surface active species penetrate the cell membrane and The acid molecules can penetrate more easily to unfold their in the membrane. d destabilize the structure of the membrane activity within the cell. h b l hbh h ll f h b O H 3C O OH O O OH O H3CO OH H3CO O H3CO H3CO O H3CO OH OH H3CO OH O H3CO O OH O O OH OH H3CO O OH O OH H3CO O H3CO H3CO OH H3CO O OH O OH O OH H3CO O H3CO OH H3CO H3CO O OH OH O H3CO H3CO OH O OH H3CO O OH O OH Organic acids and surface active substances are versatile options for the alternative preservation of cosmetics. Interesting synergistic effects are observed when organic acids and surface active substances are combined d f b b d By combining with surface active substances the scope of By combining with surface active substances the scope of application for organic acids can be extended to higher pH‐ application for organic acids can be extended to higher pH‐values application for organic acids can be extended to higher pH values (up to 6.5 (up to 6.5) ) Depending on the type of Depending Depending on the type of formulation, the on the type of formulation, the amount of surface formulation the amount of surface amount of surface active substances can be significantly lowered in synergistic combinations to avoid stability problems.