Regulating Axon Growth within the Postnatal Central Nervous System
advertisement

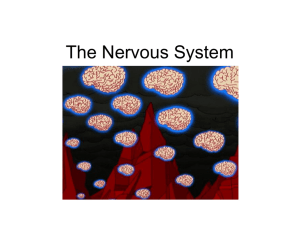
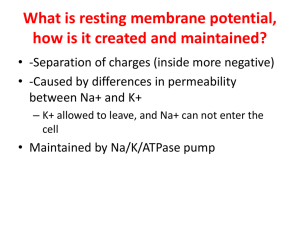
Regulating Axon Growth within the Postnatal Central Nervous System Fenghua, Hu, and Stephen M. Strittmatter As neuronal development enters its final stages, axonal growth is restricted. Recent work indicates that several myelin-derived proteins, Nogo, MAG and OMgp, play a critical role in restricting axonal growth in the mature central nervous system (CNS). These proteins function by binding to an axonal NgR protein that limits axonal growth by activating Rho in neurons. Hypoxic conditions during the later stages of neuronal development have a prominent effect on oligodendrocytes and hence on the expression of these axon growth inhibitors. Reduced expression of these proteins caused by the developmental hypoxia, or direct blockade of the myelin inhibitor pathways in the adult CNS leads to axonal sprouting and the formation of new neuronal connections. The regulation of axonal growth, sprouting and connections in the postnatal brain by myelin proteins is an area of important investigation and potential therapeutic intervention. Semin Perinatol 28:371-378 © 2004 Elsevier Inc. All rights reserved. D uring the early prenatal development of the nervous system, neurons project axons over long distances to reach their final targets. Successful targeting of axons depends both on the supply of neurotrophic factors and the directed steering of axons by the extracellular distribution of guidance factors. At the leading edge of a growing axon is the growth cone, a structure capable of sensing and rapidly responding to its environment. Attractive and repellent axon guidance cues are sensed by growth cones and affect growth cone dynamics and motility. Several families of guidance molecules and their receptors have been identified and studied during the past few years, including RGMs/Neogenin, netrins/DCC, slits/Robos, semaphorins/neuropilins/plexins, ephrins/Eph tyrosine kinase receptors, and cell adhesion receptors.1-4 Axons progressively stop growing as neuronal networks are formed during the final stages of development. If there is injury to the nervous system in the later stages of development or in the adult, the axonal growth cones formed after the resealing of the injured axons interact with secreted factors, extracellular matrix and neighboring cells, all of which affect growth cone dynamics and the extent of the axon regeneration process. Neurons in the mammalian peripheral nervous system (PNS) can regrow functional axons after injury but neurons from adult mammalian central nervous sysDepartment of Neurology, Yale University School of Medicine, New Haven, CT. Address reprint requests to: Dr. Stephen M. Strittmatter, Department of Neurology, Yale University School of Medicine, P.O. Box 208018, New Haven, CT 06520. E-mail: stephen.strittmatter@yale.edu 0146-0005/04/$-see front matter © 2004 Elsevier Inc. All rights reserved. doi:10.1053/j.semperi.2004.10.001 tem (CNS) cannot. Experiments have demonstrated that the inability of regeneration in the CNS is not solely an intrinsic deficit of the CNS neurons but depends at least in part on an unfavorable environment for axon regeneration in the CNS.5-7 When provided with a permissive milieu, damaged CNS neurons can regenerate axons. Several inhibitory factors prevent axon outgrowth at the site of a CNS lesion, including CNS myelin produced by oligodendrocytes and a glial scar formed primarily by astrocytes. Recently, many inhibitory molecules have been identified and characterized in the CNS myelin and this review will update some of recent findings. Apart from their role as inhibitors of CNS regeneration after injury, it is hypothesized that CNS myelin proteins might also help preserve an appropriate CNS neuronal network, by preventing over-exuberant axonal sprouting with aberrant misconnections. An example of the correlation between a downregulation of myelin protein and increased axonal sprouting occurs in a rodent model for periventricular leukomalacia (PVL), which is a common brain lesion seen in premature human infants.8 CNS Myelin Derived Inhibitors The importance of CNS myelin derived proteins for inhibiting CNS axon growth has been demonstrated by several studies. In mice immunized with CNS myelin, significant regeneration of injured corticospinal tract (CST) fibers is observed while virtually no axon regeneration is seen in control mice.9 Other studies including sciatic nerve transplant studies, tissue culture experiments and olfactory ensheathing cell trans371 F. Hu and S.M. Strittmatter 372 Figure 1 Inhibition of neurite outgrowth by CNS myelin proteins. The myelin protein components, Nogo, MAG and OMGP, inhibit axon regeneration by binding to a common receptor, NgR. NgR lacks a transmembrane domain and is thought to form a functional receptor complex with p75NTR and Lingo-1 to transduce intracellular signals. RhoA and PKC are activated in response to myelin ligands to induce growth cone collapse and inhibit outgrowth. Upregulation of cAMP attenuates the inhibitory effect of myelin. Nogo-A may exist in an alternative topology with amino Nogo extracellular. Amino Nogo might inhibit axon outgrowth and fibroblast spreading through a distinct receptor. plant studies have also confirmed the inhibitory effect of CNS myelin. Multiple inhibitors have been identified in the CNS myelin, including Nogo, myelin-associated glycoprotein (MAG), oligodendrocyte-myelin glycoprotein (OMGP) and chondroitin sulfate proteoglycan (CSPG) (Fig. 1). Although each of these molecules have been shown to inhibit neurite outgrowth in vitro, the relative inhibitory activity of these molecules in the CNS myelin is not fully defined. Nogo A monoclonal antibody (IN-1) raised against a fraction of myelin enriched for inhibitory activity has been shown to enhance regeneration of corticospinal tract (CST), corticorubral and corticopontine fibers after spinal cord injury. Furthermore, this axon growth is correlated with improved functional recovery following injury.10,11 The antigen for the IN-1 antibody is a protein of 250 kDa (NI-250). With partial protein sequence data derived from a proteolytic digest of bovine NI-250, three groups identified the cDNA for this major inhibitory protein, termed Nogo.12-14 Alternative promoter usage and differential splicing of the Nogo gene give rise to three major transcript (Nogo-A, -B and –C). Three isoforms of Nogo have overlapping expression patterns15: Nogo-A is expressed by CNS myelin forming oligodendrocytes and some neuronal subpopulations, heart and testis, but not by peripheral myelin forming Schwann cells. Nogo-B has a widespread expression pattern while Nogo-C is present in CNS neurons and is particularly enriched in skeletal muscles. The three isoforms of Nogo share a common carboxylterminal of 188 amino acids, which is homologous to the reticulon protein family and contains two transmembrane domains. The loop between the two transmembrane domains of Nogo (Nogo66) has been shown to be extracellular and has an axon growth inhibitory effect on its own.12 Nogo-A contains a unique sequence (amino-Nogo) that has been shown to inhibit both axon growth and fibroblast spreading in vitro.16,17 The topology of Nogo (and reticulons) remains controversial. There are two proposed topologies of Nogo, one with amino-Nogo being intracellular and the other with amino-Nogo facing extracellular. The majority of Nogo in the cell has been found to associate with endoplasmic reticulum (ER), just like other members of the reticulon family.15 Nogo lacks a conventional signal peptide at its amino-termini and translocation into the ER is assumed to be directed by the two hydrophobic domains. The two putative transmembrane domains in reticulons are very large and could span the membrane either once or twice. Antibodies against Nogo66 and amino-Nogo can stain surface of differentiated oligodendrocyte in culture, suggesting that, at least in these cells, both Nogo66 and amino-Nogo can be extracellular.17 In transfected Cos7 cells, only low levels of surface staining for Nogo66, and none for amino-Nogo, can be detected.12 It is possible that Nogo can assume different topologies in differ- Regulating axon growth ent cell types or its topology might be regulated in response to signals, such as contact with axons or in response to axon damage. Further studies on Nogo topology and factors that might regulate its topology and intracellular distribution will be very interesting. The importance of Nogo in inhibiting axon regeneration in the CNS has been addressed through genetic studies. Transgenic mice expressing Nogo-A or Nogo-C in peripheral Schwann cells show delayed axonal regeneration and decreased recovery rate after sciatic nerve injury,18,19 demonstrating that Nogo is sufficient to inhibit axon regeneration in vivo. Three groups independently generated Nogo knockout mice and determined axon regeneration and functional recovery after spinal cord injury in these mice.20-22 All Nogo mutant mice exhibit normal brain histology and behavior. Myelin appears to be normal and there are no obvious defects in axons and oligodendrocytes, suggesting that Nogo does not play an essential role in CNS development or the maintenance of CNS integrity in the absence of injury. In vitro assays demonstrate that myelin lacking Nogo-A is significantly less inhibitory for axon growth. After spinal injury, one gene-trap derived strain of NogoA/B deficient mice exhibits regeneration of CST fibers and improved functional recovery of locomotion.20 A lesser degree of CST fiber growth is detected in mice selectively lacking Nogo-A; this might be explained by increased Nogo-B expression in oligodendrocytes.21 On the other hand, no CST spouting or regeneration is apparent in additional strains of Nogo-A/B or Nogo-A/B/C deficient mice.22 The basis for the variable phenotypes in the mutant mice requires further investigation to determine the exact role that Nogo-A plays in CNS regeneration. Despite variable phenotypes in Nogo gene deletion mice, several other experiments demonstrate a role for Nogo in limiting CNS axon regeneration in vivo. Treatment with IN-1 antibodies or implantation of mAb IN-1-secreting hybridoma cells,23,24 or direct administration of a partially humanized, recombinant Fab fragment (rIN-1 Fab) derived from the original mAb IN-125 improve axon regeneration and functional recovery after spinal cord injury, as judged by the BBB locomotor score. A 40-residue peptide (NEP1-40) from the Nogo66 region behaves as a Nogo66 antagonist and also promotes CST axon growth and functional recovery in vivo following spinal cord injury.26 Nogo has been implicated in physiological functions other inhibition of axon growth. Recently, Nogo has also been reported to interact with the Caspr-F3 complex at paranodes and this interaction might have a role in modulating axoglial architecture and possibly potassium channel localization during development.27 Nogo-B might have a function as proapoptotic protein.28 A recent report demonstrates that Nogo-B has a nonneuronal role in regulating vascular homeostasis and remodeling.29 MAG Myelin associated glycoprotein (MAG) contains five Ig-like domains and is a member of the immunoglobin superfamily. 373 MAG is present in both PNS and CNS myelin and is a potent inhibitor of neurite outgrowth from a variety of neurons in vitro.30-32 It appears that MAG has a dual function in regulating axon growth: it stimulates neurite outgrowth from immature neurons but inhibits outgrowth from mature neurons.31-34 MAG is thought to contribute significantly to the inhibitory activity of myelin in vitro.30 However, in one study, myelin from the MAG-deficient mouse behaves similarly to myelin prepared from wild type mice in axon outgrowth assays and the extent of axonal regeneration after spinal cord injury is similar in MAG-deficient and wild-type mice.35 It is not clear if there are other inhibitory molecules up-regulated in the absence of MAG or if MAG is not essential for the inhibition of CNS axon regeneration. In mutant backgrounds with delayed Wallerian degeneration, MAG does appear to have some role in limiting axon regeneration rate.36 The physiologic function of MAG might be more involved in axoglial interactions necessary for myelin formation and integrity.37 OMGP Oligodendrocyte-myelin glycoprotein (OMGP) is a glycosylphosphatidylinositol (GPI)-anchored CNS myelin protein and belongs to the leucine rich repeat protein family. OMGP is expressed on the surface of mature oligodendrocyte and it is a potent inhibitor of neurite outgrowth from cultured neurons.38,39 OMGP is also highly expressed by some neurons, but its neuronal functions are ill-defined.40 OMGP gene deletion studies should help reveal the relative significance of OMGP in inhibiting CNS regeneration. CSPG The scar that forms at the site of CNS lesion is comprised largely of reactive astrocytes and contributes to the failure of axon regeneration in the CNS. Chondroitin sulfate proteoglycans (CSPGs) are thought to be major inhibitory components of the glial scar. Inhibitory CSPGs have also been isolated from CNS myelin.41 Specific CSPGs, including NG2, versican, neurocan and phosphocan, are expressed at high levels at the sites of CNS injury and have been shown to inhibit neurite outgrowth.42,43 Removal of the chondroitin sulfate glycosaminoglycan chains from the core proteins by chondroitinase ABC treatment neutralizes the inhibitory effect of many CSPGs and promotes axon regeneration in vivo.44,45 However, chondroitinase ABC treatment does not appear to reduce the inhibitory activity of NG2 proteoglycan, suggesting that the inhibitory activity of NG2 also resides the protein core.46-48 The mechanisms by which CSPGs inhibit neurite outgrowth are still unknown. Nogo Receptor (NgR) Nogo receptor was identified as a protein that interacts with Nogo66 in an expression cloning screen.16 NgR expression is confined to neurons in the adult nervous system. It binds Nogo66 with high affinity. Release of NgR from membrane with phosphatidylinositol-specific phos- 374 pholipase C (PI-PLC) treatment abolishes Nogo66 responsiveness and transfection of NgR confers a Nogo66 response in otherwise nonresponsive neurons, demonstrating that NgR is the receptor for Nogo66. NgR contains a leucine rich repeat (LRR) structure and the carboxylterminal (CT) domain, which does not have sequence homology to known protein sequences. The LRR domain of NgR is comprised of eight typical LRR segments flanked by cysteine-rich LRR amino-terminal (LRRNT) and the LRR carboxyl-terminal (LRRCT) domains. The crystal structure of the NgR LRR domain has been determined.49,50 The NgR LRR domain is most closely related to that of platelet glycoprotein-1b-␣ (GP1b-␣). LRR segments in NgR are arranged in a parallel way as -sheet segments, creating a banana-shaped structure that has a concave and a convex surface capable of protein-protein interaction. NgR lacks a transmembrane domain and it is tethered to the plasma membrane through a glycosylphosphatidylinositol (GPI) moiety, suggesting that NgR might associate with a transmembrane coreceptor to transduce intracellular signals. Interestingly, two other inhibitory components of myelin, MAG and OMGP also interact with NgR and require NgR for their inhibitory activity in vitro.39,51,52 All three ligands, Nogo66, MAG and OMGP bind to the leucine rich repeat domains of NGR. It is interesting that NgR can interact with three ligands that share no homology to each other: Nogo 66 does not have a defined domain structure; MAG is a protein with IgG domain and OMGP contains leucine rich repeat domains. How these various ligands interact with NgR is still unclear. Detailed mutagenesis studies of NgR and cocrystallization of NgR with its ligands will help define the ligand binding regions in NgR and give us more insight into the molecular basis of NgR interaction with three structurally distinct ligands. Blockade of NgR signaling in mice using a soluble version of NgR (NgR-Ecto) which blocks NgR function and the inhibitory effects of NgR ligands in vitro has been shown to improve axon growth and/or recovery after optic nerve injury and after stroke, suggesting that signaling through NgR plays an important role in limiting CNS axon growth.53,54 Recently, two homologs of NgR (now termed NgR1) have been identified, termed NgR2 (also known as NgRH1) and NgR3 (also known as NgRH2).49,55 NgR2 and NgR3 do not appear to interact with known NgR ligands, at least in the affinity range for NgR. The expression pattern of NgR2 and NgR3 are quite similar to NgR1 and the functions of these two homologs are waiting to be explored. The inhibitory effect of amino-Nogo on fibroblast spreading and neurite outgrowth does not seem to be dependent on NgR, suggesting that there might exist a distinct receptor for amino-Nogo.17 Work by Schwab’s group suggest that there exist two distinct inhibitory regions in amino-Nogo: an amino-terminal region common to Nogo-A and B which can inhibit fibroblast spreading and a Nogo-A specific stretch that inhibits neurite outgrowth and cell spreading (NiG-⌬20).17 Amino-Nogo binds to the cell surface of responsive cells and to rat brain cortical F. Hu and S.M. Strittmatter membranes, suggesting the existence of specific binding receptors.17 Amino-Nogo does not bind to NgR2 or NgR3 (Hu and Strittmatter, unpublished data). It will be very interesting to identify the receptor for amino-Nogo and to explore the mechanisms that amino-Nogo inhibits fibroblast spreading and neurite outgrowth. Coreceptors: p75, Lingo, Others? NgR lacks a transmembrane domain, suggesting that it might need a coreceptor or coreceptors for signaling. Recent studies have identified two possible coreceptors for NgR: p75NTR and Lingo-1.56-58 p75NTR interacts with a variety of ligands and is well known to function as a neurotrophin receptor in concert with the Trk receptor tyrosine kinase family.59,60 The first hint that p75NTR might function in the NgR pathway came from the observation that MAG-dependent inhibition of neurite outgrowth and RhoA activation is impaired in neurons from p75NTR-deficient mice.61 Two groups explored the possibility that p75NTR might be the coreceptor for p75 and they showed that these two proteins bind to each other and p75NTR is required for the inhibitory activity of Nogo, MAG and OMGP.56,57 In a yeast two hybrid screen, the cytoplasmic domain of p75NTR was found to interact with Rho, a small GTPase implicated in mediating growth inhibition by myelin. Later, the p75-Rho interaction was described as being indirect and mediated via the Rho-guanine dissociation inhibitor (GDI).62 p75NTR is postulated to activate Rho by acting as a displacement factor that releases Rho from Rho-GDI.62 However, there are several indications that p75-NTR does not constitute the sole mediator of NgR signaling.63,64 Immunohistochemical data show that p75NTR is expressed in only a very small subset of neurons.63 Furthermore, the depletion of the functional p75NTR or local administration of a dominant negative p75NTR -Fc molecule does not promote axon regeneration after spinal cord injury, suggesting that p75NTR may not be a critical molecule mediating the function of myelinassociated inhibitory factors in vivo.63 Recently, a nervous system specific leucine-rich repeat protein named Lingo-1 (LRR and Ig domain-containing, Nogo receptor-interacting protein) was found to be a third component of the NgR signaling complex.58 Lingo-1 interacts with NgR1 and p75 physically. Coexpression of NgR, p75NTR and Lingo-1 in nonneuronal cells conferred cellular activation of RhoA in response to OMGP treatment, while coexpression of NgR and p75 did not. Interfering Lingo-1 function using a dominant-negative construct or exogenously added soluble Lingo-1-Fc fusion protein attenuated the inhibitory effect of myelin and myelin ligands to neurons. These data suggest that Lingo-1 is a functional component of the NgR-p75 signaling complex. It remains an open question whether there are additional or alternative components in NgR receptor complexes. There might be other transmembrane proteins interacting with Regulating axon growth 375 NgR-p75-Lingo-1 complex and required for RhoA activation. Alternatively, different neurons might utilize different coreceptors to transduce signals from myelin ligands. p75NTR is only expressed by a subset of neurons and it is possible that there are other coreceptors functioning together with NgR in p75-negative neurons. Intracellular Signaling: Rho, cAMP, PKA, PKC The Rho family of small guanine triphosphatases (GTPase), including Rho, Rac and Cdc42, regulates actin cytoskeleton and growth cone dynamics. Myelin and individual NgR ligands have been shown to activate RhoA. Blockade of RhoA by C3 transferase mediated ADP-ribosylation can attenuate myelin and NgR ligand-mediated axon growth inhibition.65-67 C3 treatment can promote axon regeneration and functional recovery in vivo.67,68 Some studies have demonstrated that Rho-associated kinase (ROCK) might play a prominent role in downstream signaling of Rho in response to NgR activation. The ROCK inhibitor Y-27632 reverses myelin inhibition in vitro and promotes axon regeneration and locomotor recovery in vivo.66,67 The level of cytosolic nucleotides can modulate the neuronal response to a number of factors involved in neurite attraction and repulsion. Recently the relative ratio of cAMP to cGMP has been shown to play an important role in determining growth cone turning behavior.69 MAG/myelin dependent inhibition can be overcome by priming neurons with neurotrophins and this effect is mediated by the cAMP-PKA pathway.70 cAMP level is dramatically higher in young neurons than in the same types of older neurons and only older neuron are inhibited by MAG. Elevating cAMP in older neurons blocks MAG inhibition of neurite outgrowth.33 Elevation of cAMP by direct injection into dorsal root ganglion (DRG) overcomes inhibition by MAG and myelin and results in extensive regeneration of dorsal column axons lesioned one week later.71 Rolipram elevates cAMP in the brain by inhibiting phosphodiesterase 4, and enhances SCI recovery in combination with Schwann cell transplants.72 Arginase I and polyamines are likely to act downstream of cAMP in overcoming inhibition of axonal growth by MAG and myelin in vitro.73 Thus, modulation of cAMP levels and downstream signaling might be important for axon regeneration. Recently, protein kinase C (PKC) was also found to play an important role in mediating inhibitory effects of myelin components and CSPGs.74 Both the myelin inhibitors and CSPGs induce PKC activation. Blocking PKC activity pharmacologically and genetically attenuates the ability of CNS myelin and CSPGs to activate Rho and inhibit neurite outgrowth. Intrathecal infusion of a PKC inhibitor into the site of dorsal hemisection promotes regeneration of dorsal column axons across and beyond the lesion site in adult rats. Since PKC is involved in many cellular events, whether the activation of PKC is a direct effect of myelin ligands or occurs through interference with other pathways remains to be determined. Figure 2 Changes in axonal sprouting and myelin axon growth inhibitors in PVL. In response to hypoxia induced damage, Nogo-A and MAG are selectively downregulated in oligodendrocytes. The decreased expression of Nogo-A and MAG in developing CNS myelin might contribute to the axon sprouting observed in animals subjected to chronic sublethal hypoxia (CSH) and in humans with PVL. Suppression of Oligodendrocyte Nogo-A Expression in Response to Neonatal Hypoxia Recently, in a rodent model for periventricular leukomalacia (PVL), Nogo-A expression was found to be selectively lost from oligodendrocytes8 (Fig. 2). As discussed elsewhere in this volume, PVL is characterized a reduction of central myelinated tracts and is a common brain white matter lesion in infants born with very low birth weight (VLBW).75 Both mental retardation and attention deficit hyperactivity disorder occur with increased frequency in VLBW infants. Hypoxia occurs after birth in most VLBW infants and is believed to be the primary cause of PVL.75,76 Hypoxia both causes direct neuronal damage and modifies the environment for axonal re-growth. Oligodendrocytes precursors are the CNS cells most sensitive to hypoxic damage in premature infants.77 Rodent chronic sublethal hypoxia (CSH) from P3 to 33 (postnatal day 3-33) provides a model for PVL. Rats exposed to these conditions experience changes comparable to premature humans including failure of brain growth, progressive cerebral ventriculomegaly, decreased subcortical white F. Hu and S.M. Strittmatter 376 matter, decreased corpus callosum size and decreased cortical volume.78 Mice exposed to CSH from P3-P33 followed by normoxia from P33-P75 continue to exhibit a locomotor hyperactivity and anxiety that resembles behavioral changes observed in some human children born with VLBW, confirming the validity of CSH as a model for PVL.8 In microarray studies, many oligodendrocyte-specific proteins are reduced in abundance in the hypoxic neonatal brain.8,79 Both MAG and myelin basic protein (MBP) protein levels from P12 hypoxic brain are decreased and return to normal after exposure to normoxic conditions from P33 to P75.8 There is more dramatic oligodendrocyte-selective loss of Nogo-A during the P3-P12 period in response to hypoxia compared with MAG.8 Oligodendrocyte selective Nogo-A loss is also transient during hypoxia and resolves by adulthood (P75). In contrast, axonal NgR expression levels are indistinguishable between normoxic and hypoxic animals.8 Although myelin protein expression returns to normal by maturity (P75), persistent abnormalities in axonal trajectories are detectable.8 Anterograde axonal tracing from motor cortex demonstrates ectopic corticofugal fibers in the CST, corpus callosum and caudate nucleus of adult animals reared in CSH. Clinically, misrouted axonal trajectories have been documented in VLBW infants.80 The loss of myelin inhibitors in combination with the mild hypoxic insult to the neuron itself is likely to cause the observed fiber misrouting since absence or blockade of myelin-derived inhibitors alone do not result in CST fibers sprouting in uninjured animals.20,26 It is still not clear whether ectopic corticofugal fibers ameliorate or exacerbate behavioral deficits in CSH animals. In the presence of NgR blockade, spinal-injured animals exhibit enhanced functional recovery despite many ectopic fibers. By analogy, increased axonal sprouting in the absence of myelin inhibitors induced by hypoxia might ameliorate deficits in CSH mice and PVL humans. Alternatively, supratentorial sprouting and axonal misconnections may contribute to the hyperactivity and anxiety observed in the CSH mice. Conclusion and Future Directions Identification of Nogo as an inhibitory component of myelin and the cloning of its receptor (NgR) not only shed light on the inhibitory mechanisms of the CNS myelin but also provides therapeutic approaches to modulate the inhibitory effect of CNS myelin on axon growth. Animals treated with a Nogo antibody or a peptide antagonist of Nogo 66 (NEP1-40) showed enhanced axon regeneration and improved functional recovery after spinal cord injury.23-26,81 Nogo, MAG and OMGP bind to a common receptor NgR to inhibit neurite outgrowth and blockade of NgR with NgR-Ecto attenuates the inhibitory effects of all three ligands16,39,51,52,82 (Fig. 1). Animals treated with NgR-Ecto showed improved axon growth and/or recovery after optic nerve injury and after stroke, raising the hope that NgR-Ecto or other possible NgR antagonists might be used as a drug to treat spinal cord injury.53,54 The organization of the NgR receptor complex and the downstream signaling mechanisms need to be studied in more details. p75NTR and Lingo-1 appear to be functional components of the NgR receptor complex56-58 (Fig. 1). However, it is still not clear whether there are other necessary components. RhoA and PKC seem to signal downstream of NgR,64,74 but how Rho and PKC become activated on NgR ligand binding needs to determined (Fig. 1). It is very interesting that in rodent models of PVL, oligodendrocyte Nogo-A is selectively downregulated.8 The mechanisms of altered Nogo-A expression are still not clear, and may occur at the mRNA level, the protein level or both. Nogo-A and MAG downregulation correlates with increased axon sprouting and hyperactivity in animals subjected to hypoxia neonatally. Enhanced axon sprouting is part of the pathology of PVL, and may have simultaneous adaptive and maladaptive consequences. Acknowledgments This work was supported by grants to S.M.S. from the NIH. References 1. Huber AB, Kolodkin AL, Ginty DD, et al: Signaling at the growth cone: Ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci 26:509-563, 2003 2. Dickson BJ: Molecular mechanisms of axon guidance. Science 298: 1959-1964, 2002 3. Monnier PP, Sierra A, Macchi P, et al: RGM is a repulsive guidance molecule for retinal axons. Nature 419:392-395, 2002 4. Rajagopalan S, Deitinghoff L, Davis D, et al: Neogenin mediates the action of repulsive guidance molecule. Nat Cell Biol 6:756-762, 2004 5. Richardson PM, McGuinness UM, Aguayo AJ: Axons from CNS neurons regenerate into PNS grafts. Nature 284:264-265, 1980 6. David S, Aguayo AJ: Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214: 931-933, 1981 7. Benfey M, Aguayo AJ: Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature 296:150-152, 1982 8. Weiss J, Takizawa B, McGee A, et al: Neonatal hypoxia suppresses oligodendrocyte Nogo-A and increases axonal sprouting in a rodent model for human prematurity. Exp Neurol 189:141-149, 2004 9. Huang DW, McKerracher L, Braun PE, et al: A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron 24:639-647, 1999 10. von Meyenburg J, Brosamle C, Metz GA, et al: Regeneration and sprouting of chronically injured corticospinal tract fibers in adult rats promoted by NT-3 and the mAb IN-1, which neutralizes myelin-associated neurite growth inhibitors. Exp Neurol 154:583-594, 1998 11. Z’Graggen WJ, Metz GA, Kartje GL, et al: Functional recovery and enhanced corticofugal plasticity after unilateral pyramidal tract lesion and blockade of myelin-associated neurite growth inhibitors in adult rats. J Neurosci 18:4744-4757, 1998 12. GrandPre T, Nakamura F, Vartanian T, et al: Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403:439444, 2000 13. Chen MS, Huber AB, van der Haar ME, et al: Nogo-A is a myelinassociated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403:434-439, 2000 14. Prinjha R, Moore SE, Vinson M, et al: Inhibitor of neurite outgrowth in humans. Nature 403:383-384, 2000 15. Oertle T, Schwab ME: Nogo and its paRTNers. Trends Cell Biol 13:187194, 2003 16. Fournier AE, GrandPre T, Strittmatter SM: Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409:341346, 2001 17. Oertle T, van der Haar ME, Bandtlow CE, et al: Nogo-A inhibits neurite Regulating axon growth 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. outgrowth and cell spreading with three discrete regions. J Neurosci 23:5393-5406, 2003 Kim JE, Bonilla IE, Qiu D, et al: Nogo-C is sufficient to delay nerve regeneration. Mol Cell Neurosci 23:451-459, 2003 Pot C, Simonen M, Weinmann O, et al: Nogo-A expressed in Schwann cells impairs axonal regeneration after peripheral nerve injury. J Cell Biol 159:29-35, 2002 Kim JE, Li S, GrandPre T, et al: Axon regeneration in young adult mice lacking Nogo-A/B. Neuron 38:187-199, 2003 Simonen M, Pedersen V, Weinmann O, et al: Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 38:201-211, 2003 Zheng B, Ho C, Li S, et al: Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron 38:213-224, 2003 Bregman BS, Kunkel-Bagden E, Schnell L, et al: Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 378:498-501, 1995 Merkler D, Metz GA, Raineteau O, et al: Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelinassociated neurite growth inhibitor Nogo-A. J Neurosci 21:3665-3673, 2001 Brosamle C, Huber AB, Fiedler M, et al: Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J Neurosci 20:8061-8068, 2000 GrandPre T, Li S, Strittmatter SM: Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 417:547-551, 2002 Nie DY, Zhou ZH, Ang BT, et al: Nogo-A at CNS paranodes is a ligand of Caspr: Possible regulation of K(⫹) channel localization. EMBO J 22:5666-5678, 2003 Watari A, Yutsudo M: Multi-functional gene ASY/Nogo/RTN-X/RTN4: Apoptosis, tumor suppression, and inhibition of neuronal regeneration. Apoptosis 8:5-9, 2003 Acevedo L, Yu J, Erdjument-Bromage H, et al: A new role for Nogo as a regulator of vascular remodeling. Nat Med 10:382-388, 2004 McKerracher L, David S, Jackson DL, et al: Identification of myelinassociated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 13:805-811, 1994 Mukhopadhyay G, Doherty P, Walsh FS, et al: A novel role for myelinassociated glycoprotein as an inhibitor of axonal regeneration. Neuron 13:757-767, 1994 DeBellard ME, Tang S, Mukhopadhyay G, et al: Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol Cell Neurosci 7:89-101, 1996 Cai D, Qiu J, Cao Z, et al: Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci 21:4731-4739, 2001 Johnson PW, Abramow-Newerly W, Seilheimer B, et al: Recombinant myelin-associated glycoprotein confers neural adhesion and neurite outgrowth function. Neuron 3:377-385, 1989 Bartsch U, Bandtlow CE, Schnell L, et al: Lack of evidence that myelinassociated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron 15:1375-1381, 1995 Schafer M, Fruttiger M, Montag D, et al: Disruption of the gene for the myelin-associated glycoprotein improves axonal regrowth along myelin in C57BL/Wlds mice. Neuron 16:1107-1113, 1996 Li C, Trapp B, Ludwin S, et al: Myelin associated glycoprotein modulates glia-axon contact in vivo. J Neurosci Res 51:210-217, 1998 Kottis V, Thibault P, Mikol D, et al: Oligodendrocyte-myelin glycoprotein (OMGP) is an inhibitor of neurite outgrowth. J Neurochem 82: 1566-1569, 2002 Wang KC, Koprivica V, Kim JA, et al: Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417:941-944, 2002 Habib AA, Marton LS, Allwardt B, et al: Expression of the oligodendrocyte-myelin glycoprotein by neurons in the mouse central nervous system. J Neurochem 70:1704-1711, 1998 Niederost BP, Zimmermann DR, Schwab ME, et al: Bovine CNS myelin 377 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. contains neurite growth-inhibitory activity associated with chondroitin sulfate proteoglycans. J Neurosci 19:8979-8989, 1999 Morgenstern DA, Asher RA, Fawcett JW: Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res 137:313-332, 2002 Properzi F, Asher RA, Fawcett JW: Chondroitin sulphate proteoglycans in the central nervous system: Changes and synthesis after injury. Biochem Soc Trans 31:335-336, 2003 Bradbury EJ, Moon LD, Popat RJ, et al: Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636-640, 2002 Moon LD, Asher RA, Rhodes KE, et al: Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci 4:465-466, 2001 Dou CL, Levine JM: Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci 14:7616-7628, 1994 Chen ZJ, Ughrin Y, Levine JM: Inhibition of axon growth by oligodendrocyte precursor cells. Mol Cell Neurosci 20:125-139, 2002 Ughrin YM, Chen ZJ, Levine JM: Multiple regions of the NG2 proteoglycan inhibit neurite growth and induce growth cone collapse. J Neurosci 23:175-186, 2003 Barton WA, Liu BP, Tzvetkova D, et al: Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J 22:3291-3302, 2003 He XL, Bazan JF, McDermott G, et al: Structure of the Nogo receptor ectodomain: A recognition module implicated in myelin inhibition. Neuron 38:177-185, 2003 Liu BP, Fournier A, GrandPre T, et al: Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science 297:11901193, 2002 Domeniconi M, Cao Z, Spencer T, et al: Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron 35:283-290, 2002 Fischer D, He Z, Benowitz LI: Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci 24:1646-1651, 2004 Lee JK, Kim JE, Sivula M, et al: Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci 24:62096217, 2004 Pignot V, Hein AE, Barske C, et al: Characterization of two novel proteins, NgRH1 and NgRH2, structurally and biochemically homologous to the Nogo-66 receptor. J Neurochem 85:717-728, 2003 Wong ST, Henley JR, Kanning KC, et al: A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci 5:1302-1308, 2002 Wang KC, Kim JA, Sivasankaran R, et al: P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420:7478, 2002 Mi S, Lee X, Shao Z, et al: LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci 7:221-228, 2004 Dechant G, Barde YA: The neurotrophin receptor p75(NTR): Novel functions and implications for diseases of the nervous system. Nat Neurosci 5:1131-1136, 2002 Roux PP, Barker PA: Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 67:203-233, 2002 Yamashita T, Higuchi H, Tohyama M: The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol 157:565570, 2002 Yamashita T, Tohyama M: The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci 6:461-467, 2003 Song XY, Zhong JH, Wang X, et al: Suppression of p75NTR does not promote regeneration of injured spinal cord in mice. J Neurosci 24: 542-546, 2004 McGee AW, Strittmatter SM: The Nogo-66 receptor: Focusing myelin inhibition of axon regeneration. Trends Neurosci 26:193-198, 2003 Niederost B, Oertle T, Fritsche J, et al: Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci 22:10368-10376, 2002 Fournier AE, Takizawa BT, Strittmatter SM: Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci 23:14161423, 2003 378 67. Dergham P, Ellezam B, Essagian C, et al: Rho signaling pathway targeted to promote spinal cord repair. J Neurosci 22:6570-6577, 2002 68. Lehmann M, Fournier A, Selles-Navarro I, et al: Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci 19: 7537-7547, 1999 69. Nishiyama M, Hoshino A, Tsai L, et al: Cyclic AMP/GMP-dependent modulation of Ca2⫹ channels sets the polarity of nerve growth-cone turning. Nature 423:990-995, 2003 70. Cai D, Shen Y, De Bellard M, et al: Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron 22:89-101, 1999 71. Qiu J, Cai D, Dai H, et al: Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34:895-903, 2002 72. Pearse DD, Pereira FC, Marcillo AE, et al: cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med 10:610-616, 2004 73. Cai D, Deng K, Mellado W, et al: Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron 35:711-719, 2002 74. Sivasankaran R, Pei J, Wang KC, et al: PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci 7:261-268, 2004 F. Hu and S.M. Strittmatter 75. Rezaie P, Dean A: Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology 22:106-132, 2002 76. Volpe JJ: Perinatal brain injury: From pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev 7:56-64, 2001 77. Back SA, Luo NL, Borenstein NS, et al: Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21:1302-1312, 2001 78. Ment LR, Schwartz M, Makuch RW, et al: Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res Dev Brain Res 111:197-203, 1998 79. Curristin SM, Cao A, Stewart WB, et al: Disrupted synaptic development in the hypoxic newborn brain. Proc Natl Acad Sci U S A 99: 15729-15734, 2002 80. Huppi PS, Murphy B, Maier SE, et al: Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics 107:455-460, 2001 81. Li S, Strittmatter SM: Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci 23:4219-4227, 2003 82. Fournier AE, Gould GC, Liu BP, et al: Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci 22:8876-8883, 2002