Section 6.6: The Composition of Unknown Compounds
advertisement
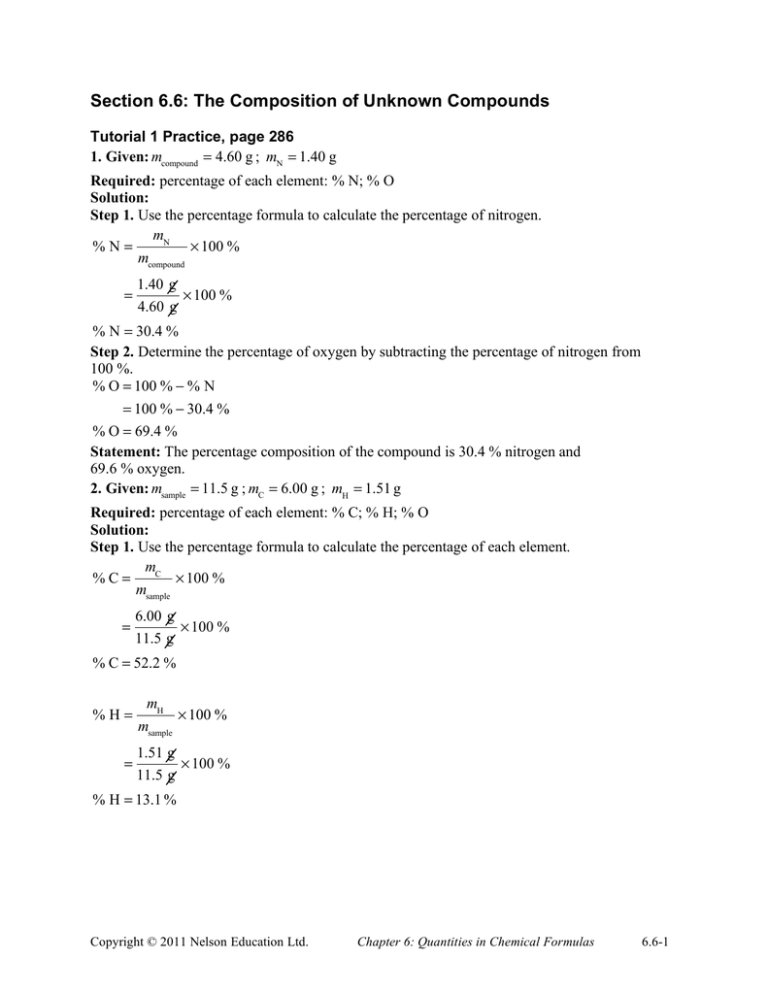
Section 6.6: The Composition of Unknown Compounds Tutorial 1 Practice, page 286 1. Given: mcompound = 4.60 g ; mN = 1.40 g Required: percentage of each element: % N; % O Solution: Step 1. Use the percentage formula to calculate the percentage of nitrogen. mN %N= ! 100 % mcompound = 1.40 g ! 100 % 4.60 g % N = 30.4 % Step 2. Determine the percentage of oxygen by subtracting the percentage of nitrogen from 100 %. % O = 100 % ! % N = 100 % ! 30.4 % % O = 69.4 % Statement: The percentage composition of the compound is 30.4 % nitrogen and 69.6 % oxygen. 2. Given: msample = 11.5 g ; mC = 6.00 g ; mH = 1.51 g Required: percentage of each element: % C; % H; % O Solution: Step 1. Use the percentage formula to calculate the percentage of each element. mC %C= ! 100 % msample = 6.00 g 11.5 g ! 100 % % C = 52.2 % %H= = mH msample ! 100 % 1.51 g ! 100 % 11.5 g % H = 13.1 % Copyright © 2011 Nelson Education Ltd. Chapter 6: Quantities in Chemical Formulas 6.6-1 Step 2. Determine the percentage of oxygen by subtracting the sum of the percentages of the other elements from 100 %. % O = 100 % ! (% C + % H) = 100 % ! (52.2 % + 13.1 %) = 100 % ! 65.3 % % O = 34.7 % Statement: The percentage composition of the compound is 52.2 % carbon, 13.1 % hydrogen, and 34.7 % oxygen. 3. (a) Given: sodium sulfate, Na2SO4 Required: percentage composition of Na2SO4 Solution: Step 1. Calculate the molecular mass of the compound. M Na SO = (2 ! 22.99 u) + (32.07 u) + (4 ! 16.00 u) 2 4 M Na SO = 142.05 u 2 4 Step 2. Calculate the percentage of each of the elements sodium, Na, and sulfur, S. 45.98 u % Na = ! 100 % 142.05 u % Na = 32.37 % 32.07 u ! 100 % 142.05 u % S = 22.58 % Step 3. Determine the percentage of oxygen by subtracting the sum of the percentages of the other elements from 100 %. % O = 100 % ! (% Na + % S) %S= = 100 % ! (32.37 % + 22.58 %) = 100 % ! 54.95 % % O = 45.05 % Statement: The percentage composition of sodium sulfate is 32.37 % sodium, 22.58 % sulfur, and 45.05 % oxygen. (b) Given: ammonium nitrate, NH4NO3 Required: percentage composition of NH4NO3 Solution: Step 1. Calculate the molecular mass of the compound. M NH NO = (2 ! 14.01 u) + (4 ! 1.01 u) + (3 ! 16.00 u) 4 M NH 3 4 NO3 = 80.06 u Copyright © 2011 Nelson Education Ltd. Chapter 6: Quantities in Chemical Formulas 6.6-2 Step 2. Calculate the percentage of each of the elements N and H. 28.02 u %N= ! 100 % 80.06 u % N = 35.00 % 4.04 u ! 100 % 80.06 u % H = 5.05 % Step 3. Determine the percentage of oxygen by subtracting the sum of the percentages of the other elements from 100 %. % O = 100 % ! (% N + % H) %H= = 100 % ! (35.00 % + 5.05 %) = 100 % ! 40.05 % % O = 59.95 % Statement: The percentage composition of ammonium nitrate is 35.00 % nitrogen, 5.05 % hydrogen, and 59.95 % oxygen. Mini Investigation: Determining Percentage Composition of a Compound, page 288 Answers are determined from experimental data. Sample answer: A. The paperclip compound uses 3 small paperclips (assigned “chemical symbol”, Sp) and one large paper clip (assigned “chemical symbol”, Lp). Therefore, the formula of the compound is LpSp3. B. One mole of the paperclip compound contains 6.02 × 1023 “molecules” rather than just one. Therefore, grams per mole is not an appropriate unit for the mass of one molecule. C. The mass of one Sp is 1.4 g. The mass of one Lp is 3.6 g. The mass of one molecule is 7.8 g. % Lp = 3.6 g 7.8 g ! 100 % % Lp = 46 % % Sp = 100 % ! % Lp = 100 % ! 46 % % Sp = 54 % Therefore, the percentage composition of the compound is 46 % Lp and 54 % Sp. D. Increasing the number of molecules increases the mass of the sample. The percentage composition, by mass, of each element in the compound is a constant. Therefore, adding more compounds with the same “chemical formula” has no effect on the percentage composition of each element in the sample. Copyright © 2011 Nelson Education Ltd. Chapter 6: Quantities in Chemical Formulas 6.6-3 Section 6.6 Questions, page 288 1. Given: mCa = 20.0 g ; mC = 6.0 g ; mO = 24.0 g Required: percentage of each element: % Ca; % C; % O Solution: Step 1. Calculate the mass of compound. mcompound = 20.0 g + 6.0 g + 24.0 g = 50.0 g Step 2. Calculate the percentage of each element. mCa % Ca = ! 100 % mcompound = 20.0 g ! 100 % 50.0 g % Ca = 40.0 % %C= = mC mcompound ! 100 % 6.0 g ! 100 % 50.0 g % C = 12 % %O= = mO mcompound ! 100 % 24.0 g ! 100 % 50.0 g % O = 48.0 % Statement: The percentage composition of the compound is 40.0 % calcium, 12 % carbon, and 48.0 % oxygen. 2. Given: methanol = 5.00 g ; mC = 2.61 g ; mH = 0.66 g Required: percentage of each element: % C; % H; % O Solution: Step 1. Determine the mass of oxygen by subtracting the sum of the masses of the other elements from 5.00 g. mO = 5.00 g ! (2.61 g + 0.66 g) = 1.73 g Copyright © 2011 Nelson Education Ltd. Chapter 6: Quantities in Chemical Formulas 6.6-4 Step 2. Calculate the percentage of each element. mC %C= ! 100 % msample = 2.61 g ! 100 % 5.00 g % C = 52.2 % %H= = mH ! 100 % msample 0.66 g ! 100 % 5.00 g % H = 13 % %O= = mO msample ! 100 % 1.73 g ! 100 % 5.00 g % O = 34.6 % Statement: The percentage composition of ethanol is 52.2 % carbon, 13 % hydrogen, and 34.6 % oxygen. 3. According to the law of definite proportions, a compound always contains the same proportion of elements by mass. If the quantity of hydrogen peroxide is doubled, twice the quantity of oxygen atoms will be present. As a result, twice the quantity of oxygen is produced. 4. (a) The mass of hydrogen in the sample is determined by subtracting the mass of carbon from the mass of benzene. mH = 10.00 g ! 9.20 g = 0.80 g The mass of hydrogen in the sample is 0.80 g. (b) Given: mbenzene = 10.00 g ; mC = 9.20 g ; mH = 0.80 g Required: percentage composition of benzene Solution: Calculate the percentage of each element. mC %C= ! 100 % msample 9.20 g ! 100 % 10.00 g % C = 92.0 % = Copyright © 2011 Nelson Education Ltd. Chapter 6: Quantities in Chemical Formulas 6.6-5 %H= = mH msample ! 100 % 0.80 g ! 100 % 10.00 g % H = 8.0 % Statement: The percentage composition of benzene is 92.0 % carbon and 8.0 % hydrogen. 5. (a) C3H4 has the greater percentage of carbon by mass than C2H4 since it contains one more carbon atom but the same number of hydrogen atoms. Therefore, carbon contributes more to its molecular mass than it does in C2H4. The percentage of carbon in C3H4 is 89.9 % as compared to 85.6 % in C2H4. (b) Since the ratio of carbon to hydrogen in both compounds are the same, the percentage of carbon is the same in both compounds. The percentage of carbon is 85.6 % in C2H4 and in C3H6. (c) C2H4 has the greater percentage of carbon by mass since it contains two less hydrogen atoms than C2H6, but the same number of carbon atoms. Therefore, carbon contributes more to its molecular mass than it does in C2H6. The percentage of carbon in C2H6 is 79.9 % as compared to 85.6 % in C2H4. 6. (a) The total mass of solids in the furnace should decrease because carbon dioxide gas is released from the compound. (b) Heating has no effect on the amount of calcium present in the compound. Over time, the percentage of carbon and oxygen decreases. Therefore, the percentage of calcium increases. 7. (a) Given: phosphoric acid, H3PO4 Required: percentage composition of H3PO4 Solution: Step 1. Calculate the molecular mass of the compound. M H PO = (3 ! 1.01 u) + (30.97 u) + (4 ! 16.00 u) 3 4 M H PO = 98.00 u 3 4 Step 2. Calculate the percentage of each element. 3.03 u %H= ! 100 % 98.00 u % H = 3.09 % 30.97 u ! 100 % 98.00 u % P = 31.60 % %P= 64.00 u ! 100 % 98.00 u % O = 65.31 % Statement: The percentage composition of phosphoric acid is 3.09 % hydrogen, 31.60 % phosphorus, and 65.31 % oxygen. %O= Copyright © 2011 Nelson Education Ltd. Chapter 6: Quantities in Chemical Formulas 6.6-6 (b) Given: copper(I) sulfide, Cu2S Required: percentage composition of Cu2S Solution: Step 1. Calculate the molecular mass of the compound. M Cu S = (2 ! 63.55 u) + (32.07 u) 2 M Cu S = 159.17 u 2 Step 2. Calculate the percentage of each element. 127.10 u % Cu = ! 100 % 159.17 u % Cu = 79.85 % %S= 32.07 u ! 100 % 159.17 u % S = 20.15 % Statement: The percentage composition of copper(I) sulfide is 79.85 % copper and 20.15 % sulfur. (c) Given: iron(III) oxide, Fe2O3 Required: percentage composition of Fe2O3 Solution: Step 1. Calculate the molecular mass of the compound. M Fe O = (2 ! 55.85 u) + (3 ! 16.00 u) 2 3 M Fe O = 159.70 u 2 3 Step 2. Calculate the percentage of each element. 111.7 u % Fe = ! 100 % 159.70 u % Fe = 69.94 % 48.00 u ! 100 % 159.70 u % O = 30.06 % Statement: The percentage composition of iron(III) oxide is 69.94 % iron and 30.06 % oxygen. (d) Given: boric acid, B(OH)3 Required: percentage composition of B(OH)3 Solution: Step 1. Calculate the molecular mass of the compound. M B(OH) = (10.81 u) + (3 ! 16.00 u) + (3 ! 1.01 u) %O= 3 M B(OH) = 61.84 u 3 Copyright © 2011 Nelson Education Ltd. Chapter 6: Quantities in Chemical Formulas 6.6-7 Step 2. Calculate the percentage of each of the elements B and O. 10.81 u % B= ! 100 % 61.84 u % B = 17.48 % 48.00 u ! 100 % 61.84 u % O = 77.62 % Step 3. Determine the percentage of hydrogen by subtracting the sum of the percentages of the other elements from 100 %. % H = 100 % ! (% B + % O) %O= = 100 % ! (17.48 % + 77.62 %) = 100 % ! 95.10 % % H = 4.90 % Statement: The percentage composition of boric acid is 17.48 % boron, 77.62 % oxygen, and 4.90 % hydrogen. 8. Ammonium nitrate has the greater percentage of nitrogen since it makes up more of the total mass of the compound than it does in ammonium phosphate. Given: ammonium phosphate, (NH4)3PO4; ammonium nitrate, NH4NO3 Required: percentage of nitrogen in (NH4)3PO4; percentage of nitrogen in NH4NO3 Solution: Step 1. Calculate the molecular mass of each compound. M (NH ) PO = (3 ! 14.01 u) + (12 ! 1.01 u) + (30.97 u) + (4 ! 16.00 u) 4 3 M (NH 4 4 )3 PO 4 M NH 4 NO3 M NH 4 NO3 = 149.12 u = (2 ! 14.01 u) + (4 ! 1.01 u) + (3 ! 16.00 u) = 80.06 u Step 2. Calculate the percentage of nitrogen in each compound. In (NH4)3PO4: 42.03 u %N= ! 100 % 149.12 u % N = 28.19 % In NH4NO3: 28.02 u %N= ! 100 % 80.06 u % N = 35.00 % Statement: The percentage of nitrogen in ammonium phosphate and ammonium nitrate are 28.19 % and 35.00 % respectively. Therefore, the prediction is correct. Copyright © 2011 Nelson Education Ltd. Chapter 6: Quantities in Chemical Formulas 6.6-8 9. Answers may vary. Sample answer: Gold fingerprinting is based on the idea that each gold deposit contains a unique mixture of other elements. As a result, the composition of the mixture is a “fingerprint” of the site where the gold was mined. Copyright © 2011 Nelson Education Ltd. Chapter 6: Quantities in Chemical Formulas 6.6-9