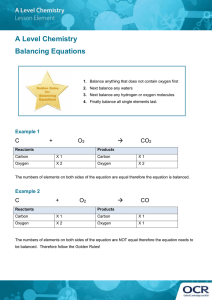
Chemical Reactions & Equations Review Name _____________________________
advertisement

Chemical Reactions & Equations Review Name _____________________________ Date ___________________ Period ____ Directions: Complete each blank with the appropriate word, phrase or symbol using your knowledge of chemical reactions. A chemical reaction can be concisely represented in symbols by a chemical _________________. The substances that undergo a chemical change are the __________________ and are found on the __________________ side of the sign. The new substance(s) formed in a chemical reaction are the ____________________. In accordance to the law of conservation of ______________, a chemical equation must be ________________. In balancing an equation, ____________________ are placed in front of the reactants and products so that the same number of atoms of each _________________ are on each side of the equation. An equation must NEVER be balanced by changing the __________________ in the chemical formulas of the substances. Special symbols are used to show the physical states of a substance in a reaction. The symbol for a liquid is _______; for a solid _______; and for a gas ________. A substance dissolved in water is designated by _______ (which stands for _______________.) If ____________ is used to increase the speed of a chemical reaction, a is written above the . The separates the __________________ from the ________________. It is read as ______________ and is equivalent to an _______ sign in a mathematical equation. A ______ sign is the symbol used to separate different products or reactants from each other. It is possible to _______________ the products of some chemical reactions. In order to do this, you must be able to recognize at least five general types of reactions. For example, in a _______________ reaction, the reactants are two or more __________________ and or compounds and there is always a ____________ product. In a ____________________ reaction, a single compound is broken down into two or more simpler substances. In a _____________ __________________ reaction, the reactants and products are an element and a compound. . An insoluble solid substance produced in this type of reaction is called a ____________________. A ______________ ____________________ reaction involves the exchange of the positive parts between two compounds. The reaction generally takes place between two ionic compounds in _______________ solution. One of the reactants in a combustion reaction is the element, ________________, which must be written as O2 as it is a _________________ element. The products of the complete combustion reaction of a hydrocarbon are _____________________ and ____________. Identify the type, predict the products if it occurs, and balance the following reactions: → 1. _____ Ba + O2 2. _____ Zn + CuSO4 → 3. _____ Ag + FeSO4 → 4. _____ BaCl2 + Na3PO4 → 5. _____ C6H12O6 + O2 6. _____ Zn + Pb(NO3)2 → 7. _____ Cu + MgSO4 8. _____ KClO3 → 9. _____ C4H8 + O2 → → →