7.2: Properties, Names, and Formulas page 268 Acids
advertisement

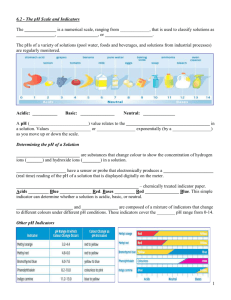
7.2: Properties, Names, and Formulas page 268 Acids and bases are two classes of chemicals that play an important role in many consumer products and environmental problems. Acids and bases can be classified by their properties. An acid is a compound that dissolves in water to give a solution that does all of the following: conducts electricity (release H+ ions) tastes sour changes the colour of litmus from blue to red reacts with active metals such as zinc, releasing hydrogen gas reacts with carbonates, releasing carbon dioxide gas In general, when acids and metals react, For example, 2 HCl(aq) + Mg(s) H2(g) + MgCl2(aq) In general, when acids and carbonates react, For example, 2 HCl(aq) + CaCO3(s) CO2(g) + H2O(l) + CaCl2(aq Table 1: Common Binary Acids Table 2: Common Oxyacids and Their Related Polyatomic Ions A base is a compound that dissolves in water to give a solution that does all of the following: conducts electricity (releases OH- ions) tastes bitter changes the colour of litmus from red to blue feels slippery on the skin reacts with an acid to destroy its properties acid + base → salt + water In general, in neutralization reactions, acid + base ionic compound + water For example, HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) Table 4: Common Bases and Their Uses Formulas for Acids and Bases Common acids are easily recognizable because of their formulas begin with hydrogen. Sulfuric acid Carbonic acid H2SO4 H2CO3 To determine if a substance is basic from its formula is a little more difficult. Most bases are compounds that contain the hydroxide ion (OH-). Sodium hydroxide NaOH Others may not contain the hydroxide ion. Hydrogen carbonate HCO3Sodium bicarbonate NaHCO3 But when they react with water, they form OH- ions. Check Your Learning: questions 1 – 8, page 271 Colour with Acid-Base Indicators page 270 Acids and bases are common chemical compounds that can be grouped according to their physical and chemical properties. Acid-Base Indicators – a substance that turns a different colour in acids and bases. Litmus – is an indicator that determines whether a substance is an acid or base. RED litmus paper turns blue in the presence of n base, but stays red in the presence of an acid. BLUE litmus paper turns red in the presence of an acid, but stays blue in the presence of a base. Other indicators can be prepared from many sources including plants. Flowers, fruits, roots, leaves, and other parts of plants include teal leaves, black currants, beet roots, rose pedals, red cabbage, tumeric, and blackberries. Synthetic indicators are often used in chemistry laboratories rather than plant extracts. This is because most natural indicators lose their colours and sensitivities when stored for long periods of time. Synthetic indicators can be prepared in large quantities as powders, and stored, without losing their properties. Table 3: Colours of common Synthetic Acid-Base Indicators 7.3: The pH Scale page 272 Chemist use a pH Scale to represent how acidic or basic a solution is. Most acids and bases can be ranked on this scale. The pH scale has a range of 0 – 14. pH Scale – a numerical scale ranging from 0 to 14 that is used to compare the acidity of a solution. pH – a measure of how acidic or basic a solution is Neutral – neither acidic nor basic, with a pH of 7 The range from 0 – 6.9 represents acids, a pH of 1 is more acidic then a pH of 5. Low value, the more acidic. The range form 7.1 – 14 represents bases, a pH of 8 is less basic then a pH of 12. High value, the more basic (Alkaline). Figure 2 the pH scale is used to compare the hydrogen ion concentration of a broad range of substances. Consumer products that are on opposite ends of the scale are corrosive ad have Household Hazardous Product symbols indicating this on their labels. pH: A Logarithmic Scale A logarithmic scale means that every change of one unit on the scale represents a tenfold effect on the concentration of the solution. (10, 102, 103, etc.) As the pH decreases, solutions become more acidic, a pH of 3 is 10x more acidic then a pH of 4. As the pH increases, solutions become more basic, a pH of 13 is 10x more basic then pH of 12. The pH is defined according to the following formula: pH = -log10 [H+] Check Your Learning: questions 1 – 10, page 275 7.5: Neutralization Reactions page 278 Neutralization reactions are a special case of double displacement reactions. → AB + CD Acid + Base → AD + CB Water + Ionic Compound The reaction between hydrochloric acid and sodium hydroxide involves an exchange of ions. HCl (aq) + NaOH (aq) → H2O (l) + NaCl (aq) Sulfuric acid and potassium hydroxide yields…. H2SO4(aq) + 2KOH(aq) → 2H2O(l) + K2SO4(aq) During the neutralization reaction, the hydrogen ion from the acid reacts with the hydroxide ion from the base to form water. H+ + OH- → H2O Applications of neutralization reactions include: a) treating chemical spills b) using antacids to provide relief of heartburn by neutralizing stomach acid c) restoring lakes affected by acid precipitation Check Your Learning: questions 1 – 10, page 281 7.8: Acid Precipitation page 285 Acid precipitation (rain, snow, fog) forms when certain pollutants combine with water in the atmosphere before falling to Earth. Figure 1: Emissions of sulfur dioxide and nitrogen oxides combine with water in the atmosphere to form acids that fall back to Earth as acid precipitation (rain, snow, and sleet). Acid-forming pollutants can also fall to Earth directly as dry deposition (particles or gases). Two pollutants of special concern are sulfur dioxide (SO2) and nitrogen oxides (NOx). SO2 produces sulfuric acid through the following series of reactions: S(s) + O2(g) SO2(g) 2 SO2(g) + O2(g) 2 SO3(g) SO3(g) + H2O(l) H2SO4(aq) NOx produces nitric acid through the following reactions: 2 NO(g) + O2(g) 2 NO2(g) 3 NO2(g) + H2O(l) 2 HNO3(aq) + NO(g) These pollutants are produced in Canada from the following sources: Acid precipitation has serious effects on aquatic ecosystems soil forests These effects result in poor growing conditions for lumber and crops a reduction in fish stocks damage to steel structures, limestone buildings and stone monuments Possible remedies for acid precipitation include switching to low-sulfur fossil fuels to generate electricity scrubbers to remove sulfur from gas emissions improved pollution control on vehicles stricter vehicle emissions standards Check Your Learning: questions 1 – 14, page 290 Chapter 7 Review: questions 1 – 22, page 294 – 295