Ascension of Our Lord Secondary School Chemistry, Grade 12, College Preparation
advertisement

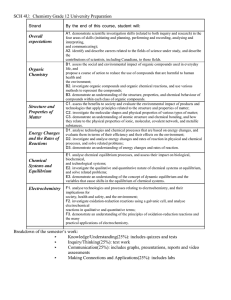
Ascension of Our Lord Secondary School Chemistry, Grade 12, College Preparation SCH 4C Course Outline MINISTRY GUIDELINE: PREREQUISTE: CREDIT VALUE: DEPARTMENT HEAD: The Ontario Curriculum, Grades 11 and 12, Science Science, Grade 10, Academic or Applied 1.0 Mr. B. Carolan TEACHER: Mr. H. Vu Student Name: __________________ Textbook #: ________ Course Description: This course enables students to develop an understanding of chemistry through the study of matter and qualitative analysis, organic chemistry, electrochemistry, chemical calculations, and chemistry as it relates to the quality of the environment. Students will use a variety of laboratory techniques, develop skills in data collection and scientific analysis, and communicate scientific information using appropriate terminology. Emphasis will be placed on the role of chemistry in daily life and the effects of technological applications and processes on society and the environment. Overall Catholic Graduate Expectations: 1. be an effective communicator who responds critically in light of gospel values. 2. be a reflective, creative and holistic thinker who solves problems and makes responsible decisions with an informed moral conscience for the common good. 3. be a self-directed, responsible, lifelong learner who develops and demonstrates their God-given potential. 4. be a collaborative contributor who finds meaning, dignity and vocation in work which respects the rights of all and contributes to the common good. 5. be a responsible citizen who gives witness to Catholic social teaching by promoting peace, justice and the sacredness of human life. Overall Course Expectations for each Strand: Matter and Qualitative Analysis evaluate the effects of chemical substances on the environment, and analyse practical applications of qualitative analysis of matter; investigate matter, using various methods of qualitative analysis; demonstrate an understanding of the basic principles of qualitative analysis of matter. Chemical Calculations analyse processes in the home, the workplace, or the environment sector that use chemical quantities and calculations, and assess the importance of accuracy in chemical calculations; investigate chemical compounds and chemical reactions using appropriate techniques of quantitative analysis, and solve related problems; demonstrate an understanding of the mole concept and its quantitative relationships in chemical reactions. Organic Chemistry evaluate the impact on society, human health, and the environment of products made using organic compounds; investigate the physical and chemical properties of organic compounds, and analyse some common organic chemical reactions; demonstrate an understanding of the structure and the physical and chemical properties of organic compounds. Electrochemistry analyse technological applications or processes relating to oxidation-reduction reactions, and assess their impact on the environment; investigate the oxidation-reduction reaction that occurs in a galvanic cell; demonstrate an understanding of the concepts of oxidation and reduction, and the principles of oxidation-reduction reactions. Chemistry in the Environment evaluate the importance of government regulations, scientific analyses, and individual actions in improving air and water quality, and propose a personal plan of action to support these efforts; investigate chemical reactions, using appropriate techniques of quantitative analysis; demonstrate an understanding of chemical reactions that occur in the environment as a result of both natural processes and human activities. SCIENTIFIC INVESTIGATION SKILLS AND CAREER EXPLORATION A1. A2. demonstrate scientific investigation skills (related to both inquiry and research) in the four areas of skills (initiating and planning, performing and recording, analysing and interpreting, and communicating); identify and describe careers related to the fields of science under study, and describe the contributions of scientists, including Canadians, to those fields. Efforts will be made to meet the individual learning needs of students to promote student success with respect to meeting the expectations of this course. Resources: The course will use a variety of resources which will be distributed to students throughout the course. The text and all other resources assigned to each student are the responsibility of the student. Science trips and activities, some of which may, require supplemental fees. Textbook: Chemistry 12: College Preparation (Nelson) or substitute textbook. Any damage incurred will result in payment for replacement. The cost for textbook replacement will be $90 Evaluation Policies 1. Student marks will be determined by evaluating process & product according to 4 categories (see below) & 4 levels of the Achievement Chart as found in the Ministry Policy document for Science. Evaluation Structure: Knowledge & Understanding Thinking & Investigation Communication Application 25% 35% 15% 25% Term Evaluations = 70% of the final mark. Final Evaluation = 30% of the final mark. Final Evaluations will include: Final Written Exam = 15% CPT Lab Performance Exam = 15% 2. Feedback will also be provided for student learning skills. Working independently, teamwork, organization, work habits/homework, and initiative are assessed apart from student achievement in the four categories outlined above and will conform to the coding: E – Excellent G – Good S – Satisfactory N - Needs Improvement 3. Assignments submitted after the due date established by the teacher will receive a penalty in accord with our Board Assessment & Evaluation Policy Document as outlined in the student agenda. 4. Should a student miss an evaluation due to a legitimate absence, in accord with our Board A&E Policy Document, the student and teacher will make arrangements to address the missed evaluation in a timely manner. In the cases of extended vacation or prolonged absence, consultation with the appropriate administrator is required. 5. In the event that the student does not make up the missed evaluation(s), a zero may be assigned. If it is determined that the evaluation(s) has/have been missed as a result of a skip/truancy or has/have been plagarized, a zero may be assigned. 6. For all other cases of absence and/or missed evaluations (including absence during the final examination period), please refer to our Board A&E Policy as outlined in the student agenda. May God bless your efforts this semester! My signature below indicates that I have read the Course Outline handout, and I am in agreement with its contents. Parent’s/Guardian’s Signature: _________________________ Date: _______________ Student’s Signature: ________________________________ Date: _______________