Quality Management Determined from Risk Assessment Bruce Thomadsen
advertisement

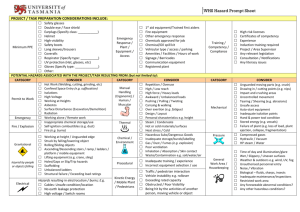
Quality Management Determined from Risk Assessment Bruce Thomadsen University of Wisconsin and The Center for the Assessment of Radiological Sciences Disclosure I am the President of the Center for the Assessment of Radiological Sciences, a nonprofit Patient Safety Organization listed with the Agency for Heathcare Research and Quality. The Center is dedicated to improving patient safety in radiotherapy and radiology. Learning Objectives To understand how to approach developing a QM program from a risk analysis: 1. Redesign to eliminate potential failures, 2. Ensure resources and key core components, 3. Fix environment and technical problems 4. Commission well and add QC and QA So, What to Do After making the fault tree. What now? n Address the potential failures. n What to Do? Start with the branches of the fault tree with either highest PRN or S. n Wherever you start, you will consider all the possible failure modes until prevention is not worth the resources. n So, if you are off in your values for the FMEA, not a big deal. n Pay particular attention to common causes. n Patient misidentified Human error: Omission – Timeout not performed Or Training – patient identified incorrectly US images inadequate Or Human Failure: Inattention/Poor performance Temp/ate not seated properly US probe misaligned Incorrect dose, dose distribution, location Human Failure: Inattention/Poor performance Probe cover not correctly filled US images inadequate – QA failure Or RO fails to match the volume study images Fault Tree for Prostate Implants with Loaded Needles Training – Probe cover not correctly filled Or Or Human Failure: Poor performance Changes in prostate Training failure RO fails to align images along the range Or Human Failure: Inattention/Poor performance US images inadequate – QA failure RO fails to image adequately Or Training failure: US images inadequate – Needle order Human failure: US images inadequate – Needle order Human Failure: Inattention/Poor performance MP fails to hand the correct needle to RO Or Confusion between packages Poor demarcation of needles Poor room layout MP drops needle Or Human failure: MP slips Human Failure: Inattention RO fails to insert into correct hole RO fails to insert needle properly Or Bad viewing conditions Confusion between holes Or Training Failure Sources placed in wrong location RO fails to insert needle to correct depth Or RO fails to hold stylet stable during retraction Or US images inadequate – QA failure Human failure: RO fails to hold stylet Or Confusion between planes Human failure: RO pushes on stylet RO selects next needle that obscures subsequent needles RO erroneously modifies source distribution Human Failure: Inattention/Poor performance Training Failure Or Human Failure: Inattention/Poor performance RO adds unnecessary sources Or RO fails to add necessary additional sources Or US images inadequate – QA failure Training Failure Or US images inadequate – QA failure Training Failure Don’t worry about reading it, this is for scale. Generalization about Fixes The prevention of events can be by: n Eliminating progenitor causes, OR n By interrupting the propagation. Patient Misidentified X Human error: Omission - Time out not performed Or X Training - patient identified incorrectly Example of a Common Progenitor Cause Incorrect activity calculation Failure selecting date and time for treatment Or Misread spreadsheet Incorrect activity taken to procedure Or Incorrect activity received AND AND Incorrect activity shipped Or Incorrect activity ordered Vendor error Failure to check order verification Or Transcription error from spreadsheet to ordered activity Or MP error reading spreadsheet Misunderstanding of spreadsheet. Poor training And Physicist/dosimetrist check images failure Post-procedure CT imaging error Or Or Non-Positional Failure Modes Poor quality/ incomplete images Channel numbering error: marking or recording 25 Catheter indicators not inserted fully 26 Sounding measurement error 26 Wrong catheter position Marked 25 23,24 51 Distal-most dwell location inaccurately digitized Dwell position construction failure Or Unit step size inaccurate Applicator in wrong location 52 53 Treatment length incorrect (wrong transfer tube length, wrong sounding information, wrong dwell spacing) Physicist check plan failure Or 50 Or Channel Mismatch Units length distance is incorrect Or Or Treatment planning Error Catheter trajectory inaccurately localized Or Single or multiple catheter failure Adequate QM program for planning and afterloader systems Inadequately trained personal Poor images Default distances used Equipment failure 52 And Dose in wrong location due to source position And Intricate Common Cause Catheter localization failure Assisting therapist misses errors 46 Systematic offset Commissioning failure Wrong catheter slice images Or Inadequately trained personal Or Incorrect catheter number asssigned Or 48 Poor labeling on photographs Poor image quality 49 Operator check failure: imported parameters vs. plan Initial treatment failure And Wrong data file imported Program treatment unit failure Or Inconsistency between treatment program and default operating parameters 72, 70, 71 Or 73 And Non-Positional Failure Modes Or Connect transfer tubes to applicator failure Tree from Jeff Williamson Failure: Physicist & MD final setup check Or Wrong length transfer tube 75 Channel and applicator numbers not matching when connecting transfer tubes to applicator 76 Incorrect Catheter Polar rotation Non-Positional Failure Modes 86 Software failure Innatention Which of the following is true about common cause? 20% 1. 20% 20% 2. 20% 3. 20% 4. 5. It is a cause for a potential failure that is common in everyday life. It is a left-wing political action group. It is a cause that produces a common potential failure. It is a cause that is not very interesting or difficult to fix. It is a cause that can produce potential failures along more than one pathway. 10 Which of the following is true about common cause? 20% 1. 20% 20% 2. 20% 3. 20% 4. 5. It is a cause for a potential failure that is common in everyday life. It is a left-wing political action group. It is a cause that produces a common potential failure. It is a cause that is not very interesting or difficult to fix. It is a cause that can produce potential failures along more than 10 one pathway. Reference Slides 8 and 9. n B. Thomadsen. Elements of Quality and Safety in Healthcare. Cognella Academic Publishing (2015). n Redesign n The best way to avoid potential errors at some step is to redesign the procedure so that error is not possible (i.e., what leads to it no longer exists). Redesign The best way to avoid potential errors at some step is to redesign the procedure so that error is not possible. n Re-evaluate after a redesign because new possible errors may have been produced. n Possible Interventions • • • First correct any environmental problems. Fix technical problems. Both usually are relatively inexpensive but effective operations that managers understand. Possible Interventions 2 Then consider the key core components identified by AAPM TG 100: § Standardized procedures § Adequate staff, physical and IT resources § Adequate training of staff § Maintenance of hardware and software resources § Clear lines of communication among staff Possible Interventions 3 n As you start with the highly ranked potential failures, it is useful to consider all the given branch of the fault tree at once. n It is also efficient to work though all the branch of the process tree at once. n Work down through the rankings until you get to potential failures that you don’t care if they happen given your resources. Commissioning n Identify those potential failures that can be eliminated through commissioning. n This is likely to be many. n Commissioning is when the limits of reliability for equipment operation are found and all data needed for its use gathered. n BUT, commissioning is for more than equipment. Commissioning 2 Commissioning is also for procedures. n Walk though the procedure with all principles. n Make sure all equipment and instruments are where they need to be and operators know how they work. n Establish the lines of communication: n Who passes what information to whom and how. n Everyone understands what everyone else does when. n Start slowly and work up to clinical speed. Inadequate or lack of procedures / practices Defective materials/tools/ equipment (2.1, 2.2, 2.3) Commissioning >3*sigma error contouring errors: wrong organ, wrong site, wrong expansions (1) Don’t try to read! User Error (2.5, 2.6) 366 Or 250 Inadequate design specification (3.2) Inadequate assessment of operational capabilities (3.5) Inadequate programming (3.6) Inattention, Rushed process, lack of time or staff, fatigue, failure to review work Inadequate or lack of procedures / practices Taken care of by the generally complete training, establishing clear communication modalities (possibly forms) and establishing protocols, policies and procedures 255 Availability of defective materials/tools/ equipment (2.1, 2.2) Materials/tools/ equipment used incorrectly or inadequate assessment of materials/tools/ equipment for task (2.5, 2.6) Excessive delineation errors resulting in <3* sigma segmentation Errors (2) Key item for commissing 326 Inadequate design specification (3.2) Or Inadequate assessment of operational capabilities (3.5) Inadequate programming (3.6) 260 Inadequate training / orientation (6.1) Key item for facility managerial changes Inattention, Rushed process, lack of time or staff, fatigue, failure to review work Inadequate or lack of procedures / practices Availability of defective materials/tools/ equipment (2.1, 2.2) Taken from TG 100 Error in delineating GTV/CTV (MD) and other structures for planning and optimization Materials/tools/ equipment used incorrectly or inadequate assessment of materials/tools/ equipment for task (2.5, 2.6) Or Poorly drawn contours (spikes, sloppy, etc) (3) 265 Inadequate design specification (3.2) 212 Or Inadequate assessment of operational capabilities (3.5) Inadequate programming (3.6) Inadequate training / orientation (6.1) Inattention, Rushed process, lack of time or staff, fatigue, failure to review work 270 After Checking Resources Identify those potential failures that can be eliminated through commissioning. n This is likely to be many. n For the remaining, consider QC and QA. n Quality Management Quality Management – All activities designed to achieve the desired quality in treatments. Quality Control – Activities that force specific quality on a process. Quality Assurance – Activities that demonstrate the level of quality of a process. Organiza(onal Difference between QC and QA Input 1 Input 2 Input 3 Input 4 QC QC Process QA QC QC After Checking Resources Identify those potential failures that can be eliminated through commissioning. n This is likely to be many. n For the remaining, consider QC and QA. n All fault tree branches eventually need to be covered somewhere before the far left box. n Let’s consider some examples. n Which of the following is true about commissioning? 20% 1. 20% 2. 20% 3. 20% 20% 4. 5. It is the preparations for a procedure. It is only for equipment. It is where the operation of equipment is compared with the order specifications. It doesn’t need to be done if there is only one person who performs a procedure. It is when the values for the FMEA are determined. 10 Which of the following is true about commissioning? 20% 1. 20% 2. 20% 3. 20% 20% 4. 5. It is the preparations for a procedure. It is only for equipment. It is where the operation of equipment is compared with the order specifications. It doesn’t need to be done if there is only one person who performs a procedure. It is when the values for the FMEA are determined. 10 Reference Slide 19. n The Report of Task Group 100 of the AAPM: Application of Risk Analysis Methods to Radiation Therapy Quality Management. Medical Physics In press (2015). n B. Thomadsen. Elements of Quality and Safety in Healthcare. Cognella Academic Publishing (2015). n One Example Patient Misidentified Human error: Omission - Time out not performed Or Training - patient identified incorrectly Systemic corrections One Example Quality assurance Quality control Managerial changes Human error: Omission – Timeout not performed Procedural changes AND Patient misidentified Or Training – patient identified incorrectly QC failure: Time out form Another Example Human failure: Inattention/Poor performance MP fails to hand correct needle to RO Confusion between packages Or Poor demarcation of needles Poor room layout MP drops needle Or Human failure: MP slips Systemic corrections Another Example Quality assurance Quality control AND Managerial changes Procedural changes MP fails to hand the correct needle to RO Or Human Failure: Inattention/Poor performance Failure of call and response Confusion between packages Poor demarcation of needles Poor room layout MP drops needle Or Human failure: MP slips Another Example Training Failure RO fails to align images along the range Or Human Failure: Inattention/Poor performance US images inadequate Systemic corrections Quality assurance Another Example Quality control Managerial changes Procedural changes Or Human Failure: Inattention/Poor performance AND RO fails to align images along the range Training failure US images inadequate US QA failure Some Thoughts on Human Errors You can never eliminate human error except by eliminating the humans. n You need to design the system to be resilient to human error. There are ways to address some factors that increase the likelihood of human error. n Protect it downstream with interventions. n Best if these are automatic. n Or Training failure QC failure: Prostate image time out RO fails to align images along the range Add some QC down stream Or Human Failure: Inattention/Poor performance AND AND No Preventing Human Error US images inadequate US QA failure A Note on Equipment Failure Equipment failure is not entirely under your control because sometimes equipment just fails. You cannot eliminate that possibility. n You can do things to influence equipment failure: n n Thorough commissioning n PMI, a resource and procedural issue n QA Ranking of QM Tools The strength of actions varies: 1. Forcing functions and constraints 2. Automation and computerization 3. Protocols and standard order forms 4. Independent check systems and other redundancies 5. Rules and policies 6. Education and Information From the Institute for Safe Medical Practices toolbox (ISMP, 1999) Which of the following is the weakest intervention? 20% 1. 20% 20% 2. 20% 3. 20% 4. 5. Policies because they may not be followed. Automation because it might not work. Forcing functions because an action may not be anticipated. Education because it can be forgotten. Checklist because it might be omitted. 10 Which of the following is the weakest intervention? 20% 1. 20% 20% 2. 20% 3. 20% 4. 5. Policies because they may not be followed. Automation because it might not work. Forcing functions because an action may not be anticipated. Education because it can be forgotten. Checklist because it might be omitted. 10 Reference Slide 37. n Institute for Safe Medical Practices toolbox (ISMP, 1999). n The Report of Task Group 100 of the AAPM: Application of Risk Analysis Methods to Radiation Therapy Quality Management. Medical Physics In press (2015). n B. Thomadsen. Elements of Quality and Safety in Healthcare. Cognella Academic Publishing (2015). n Summary To prevent the effect of a failure requires either preventing the progenitor cause OR interrupting the propagation. n First, look at redesign and reassess. n Ensure resources, environment and key core components. n Commission well. n Organize the QM steps by QC and QA. n Often it is most efficient and effective to consider complete branches of the fault tree and process tree at the same time. n