Atomic Structure Applied Chemistry
advertisement

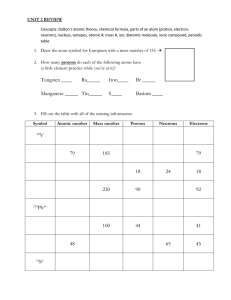
Atomic Structure Applied Chemistry Modern Atomic Theory Three subatomic particles: protons (p+), neutrons (n0), and electrons (e-) Particles found IN the nucleus: protons and neutrons These are called nucleons Particles found OUTSIDE the nucleus: electrons in the electron cloud Relative Mass and Charge of Subatomic Particles Particle Mass (amu) Charge Proton 1 +1 Neutron 1 0 Electron 0 -1 Note: amu = atomic mass unit (1/12 the mass of a carbon atom) Modern Atomic Theory Atomic Number = # of p+1 ALWAYS equals the number of protons (p+) Identifies the element Mass Number = # of p+ + # of n0 Equals the number of protons plus neutrons Neutral atoms: # of p+ = # of e In neutral atoms, the # of protons equals the # of electrons Boxes on the Periodic Table 1 Atomic Number 1.008 Atomic Mass H Hydrogen Element Name Element Symbol For Hydrogen with a Mass Number = 1 # of protons = 1 # of neutrons = 0 # of electrons = 1 Why aren’t atomic masses whole numbers? Neutral atoms of an element have the same number of protons, but the number of neutrons can vary. Isotopes: Atoms of an element with the same number of protons, but different number of neutrons. (Atoms of the same element with different masses.) Atomic Mass is really the average atomic mass of all the isotopes. Mass number and Atomic Mass are NOT the same!!! Isotopes of Hydrogen Isotopic Name – (2 parts) Sodium - 23 Element (Name or symbol can be used) Mass number Example Element Protons Electrons Neutrons Carbon-12 6 6 6 Oxygen -16 8 8 8 Fluorine -19 9 9 10 How are isotopes expressed? Isotopic Symbol – shows symbol for an element, the mass number, and the atomic number. Mass Number 20 Ne 10 Atomic Number Example of Isotopic Symbol 20 Ne 10 Element is Neon Mass number is 20 Atomic Number is 10 # of protons is 10 # of neutrons is 10 # of electrons is 10 Example Chart Isotopic Symbol Isotopic Name Atomic Number Mass Number # of protons 3 7 3 4 3 17 37 17 20 17 56 137 56 81 56 7 3 Li Lithium-7 37 17 Cl Chlorine-37 137 56 Ba Barium-137 # of # of neutrons electrons Are atoms always neutral? A. When atoms have a charge, they are called ions. B. This charge is due to an UNEQUAL number of protons and electrons. C. Charge = protons - electrons Ions (cont.) D. Metals tend to lose electrons and have a positive charge. Positive ions are called cations. The name of the cation is the same name as the element followed by the word ion. Ex. Sodium ion Ions (cont.) E. Nonmetals tend to gain electrons and have a negative charge. Negative ions are called anions. The name of an anion uses the element name but the ending is changed to ide. Example: chloride CATION Ca†ion “cat”ion ANION anion “ant”ion Example of Ions 1. Fluorine-19 becomes an ion by gaining 1 electron. 2. As a result, it will have 9 protons and 10 electrons. IONS Isotopic Symbol 19 9 24 12 F 1 Mg 32 16 59 28 S 2 2 Ni 2 Proton Neutron Electron Mass # Charge Cation or Anion 9 10 10 19 -1 A 12 12 10 24 +2 C 16 16 18 32 -2 A 28 31 26 59 +2 C Where are the electrons in the electron cloud? a) b) + e- nucleus c) Electrons are in energy levels. The outermost electrons are called valence electrons. They are in the highest energy level. The electrons in their lowest energy level are in the ground state. Where are the electrons in the electron cloud? d) e) + e- absorb energy f) g) Electrons can absorb energy often from heat or electricity. When electrons absorb energy, they become excited. They jump up to a higher energy level. They are not stable at the higher energy level. This is called the excited state. Where are the electrons in the electron cloud? h) emitted energy + ei) Because electrons are not stable, the electrons fall back to a lower energy level, called the ground state. It releases (or emits) energy, in the form of electromagnetic radiation when it returns to the ground state. Hydrogen Atom electrons fall to n = 1 and give off ultraviolet light. electrons fall to n = 2 and give off visible light. electrons fall to n = 3 and give off infrared light.