El Paso Community College Discipline - Medical Laboratory Technology (SLO's)
advertisement
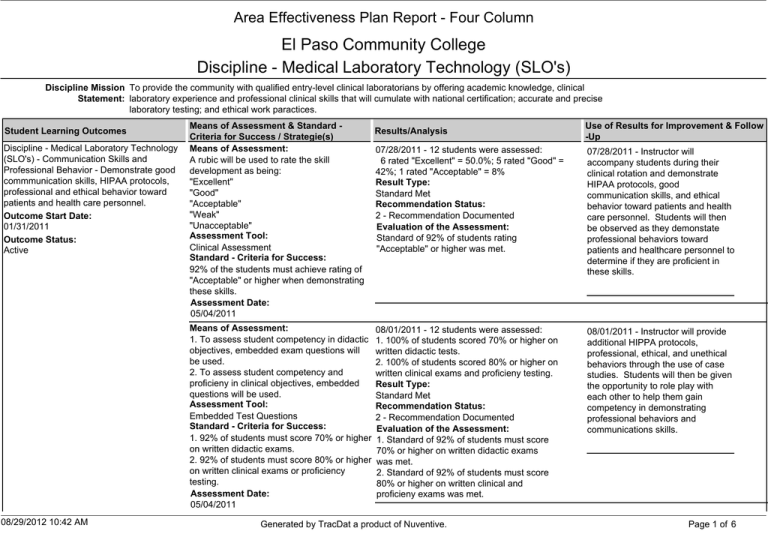
Area Effectiveness Plan Report - Four Column El Paso Community College Discipline - Medical Laboratory Technology (SLO's) Discipline Mission To provide the community with qualified entry-level clinical laboratorians by offering academic knowledge, clinical Statement: laboratory experience and professional clinical skills that will cumulate with national certification; accurate and precise laboratory testing; and ethical work paractices. Student Learning Outcomes Discipline - Medical Laboratory Technology (SLO's) - Communication Skills and Professional Behavior - Demonstrate good commmunication skills, HIPAA protocols, professional and ethical behavior toward patients and health care personnel. Outcome Start Date: 01/31/2011 Outcome Status: Active Means of Assessment & Standard Criteria for Success / Strategie(s) Means of Assessment: A rubic will be used to rate the skill development as being: "Excellent" "Good" "Acceptable" "Weak" "Unacceptable" Assessment Tool: Clinical Assessment Standard - Criteria for Success: 92% of the students must achieve rating of "Acceptable" or higher when demonstrating these skills. Assessment Date: 05/04/2011 Means of Assessment: 1. To assess student competency in didactic objectives, embedded exam questions will be used. 2. To assess student competency and proficieny in clinical objectives, embedded questions will be used. Assessment Tool: Embedded Test Questions Standard - Criteria for Success: 1. 92% of students must score 70% or higher on written didactic exams. 2. 92% of students must score 80% or higher on written clinical exams or proficiency testing. Assessment Date: 05/04/2011 08/29/2012 10:42 AM Results/Analysis Use of Results for Improvement & Follow -Up 07/28/2011 - 12 students were assessed: 6 rated "Excellent" = 50.0%; 5 rated "Good" = 42%; 1 rated "Acceptable" = 8% Result Type: Standard Met Recommendation Status: 2 - Recommendation Documented Evaluation of the Assessment: Standard of 92% of students rating "Acceptable" or higher was met. 07/28/2011 - Instructor will accompany students during their clinical rotation and demonstrate HIPAA protocols, good communication skills, and ethical behavior toward patients and health care personnel. Students will then be observed as they demonstate professional behaviors toward patients and healthcare personnel to determine if they are proficient in these skills. 08/01/2011 - 12 students were assessed: 1. 100% of students scored 70% or higher on written didactic tests. 2. 100% of students scored 80% or higher on written clinical exams and proficieny testing. Result Type: Standard Met Recommendation Status: 2 - Recommendation Documented Evaluation of the Assessment: 1. Standard of 92% of students must score 70% or higher on written didactic exams was met. 2. Standard of 92% of students must score 80% or higher on written clinical and proficieny exams was met. 08/01/2011 - Instructor will provide additional HIPPA protocols, professional, ethical, and unethical behaviors through the use of case studies. Students will then be given the opportunity to role play with each other to help them gain competency in demonstrating professional behaviors and communications skills. Generated by TracDat a product of Nuventive. Page 1 of 6 Student Learning Outcomes Discipline - Medical Laboratory Technology (SLO's) - Health and Bio-Safety Compliance - Demonstrate compliance with government, state, and organizational safety and biohazard regulations. Outcome Start Date: 01/31/2011 Outcome Status: Active Means of Assessment & Standard Criteria for Success / Strategie(s) Means of Assessment: A rubric will be used to rate the skill development as being: "Exellent" "Good" "Acceptable" "Weak" "Unacceptable" Assessment Tool: Clinical Assessment Standard - Criteria for Success: 92% of students must achieve no less than "Acceptable" or higher rating when demonstrating these skills. Results/Analysis 07/28/2011 - 12 students were assessed Rubic results: 9 rated "Excellent" = 75%; 3 rated "Good" = 25% Result Type: Standard Met Recommendation Status: 2 - Recommendation Documented Evaluation of the Assessment: Standard of 92% of students rating "Acceptable" or higher was met. Use of Results for Improvement & Follow -Up 07/28/2011 - Continue to demonstrate biohazard regulations and use case studies to demonstrate the dangers of noncomplicance. Students will demonstrate biohazard complicance, protocols and procedures as they perform medical laboratory procedures in the clinical/classroom lab. A rubic will continue to be used to determine that skills are improving. Assessment Date: 05/04/2011 Discipline - Medical Laboratory Technology (SLO's) - Avoiding Analytical Errors - Avoid 08/29/2012 10:42 AM Means of Assessment: 1. To assess student competency in didactic objectives, embedded exam questions will be used. 2. To assess student competency and proficieny in clinical objectives, embedded exam questions will be used. Assessment Tool: Embedded Test Questions Standard - Criteria for Success: 1. 92% of students must score 70% or higher on written didactic exams. 2. 92% of students must score 80% or higher on written clinical exams or proficiency testing. Assessment Date: 05/04/2011 08/01/2011 - 1. 100% of students scored 70% or higher on didactic exams. 2. 100% of students scored 80% or higher on clinical exams and proficiency testing. Result Type: Standard Met Recommendation Status: 2 - Recommendation Documented Evaluation of the Assessment: 1. Didactic: Standard of 92% of students to score 70% or higher on written didactic exams was met. 3. Clinical: Standard of 92% of students to score 80% or higher on written clinical and proficiency exams was met. Means of Assessment: A rubic will be used to rate the skill 07/28/2011 - 12 student were assessed. 8 rated "Excellent" = 67%; 4 rated "Good" = 33% Generated by TracDat a product of Nuventive. 08/01/2011 - 1. To include demonstrations or case studies envolving Chemical, Fire, Electrical, Radiation, Physical, Biohazard and Laboratory Safety protocols to maintain a safe lab. 2. To include hospital emergency codes. Page 2 of 6 Student Learning Outcomes Means of Assessment & Standard Criteria for Success / Strategie(s) Results/Analysis Use of Results for Improvement & Follow -Up pre-analytical, analytical, and post-analytical errors when collecting, transporting, and processing patient samples. Outcome Start Date: 01/31/2011 Outcome Status: Active development as being: "Excellent" "Good" "Acceptable" "Weak" " Unacceptable" Result Type: Standard Met Recommendation Status: 2 - Recommendation Documented Evaluation of the Assessment: Rubric: standard of 92% of students to achieve "Accepatable" or higher rating was met. 07/28/2011 - 1. Continue to use rubric. 2. Increase number of patient draws. 3. Reinforce patient ID, speciment collections procedures and protocols 4. Reinforce rejection/acceptance specimen policies. Means of Assessment: 1. To assess student competency in didatic objectives, embedded exam questions will be used. 2. To assess student competency and proficiency in clinical didatic objectives, embedded questions will be used. Assessment Tool: Embedded Test Questions Standard - Criteria for Success: 1. 92% of students will score 70% or higher on written didactic exams. 2. 92% of students will score 80% or higher on written clinical exams or proficency testing. Assessment Date: 05/04/2011 08/01/2011 - 1. 100% of students scored 70% or higher on written didactic exams. 2. 100% of students scored 80% or higher on written clinical exams and proficiency testing. Result Type: Standard Met Recommendation Status: 2 - Recommendation Documented Evaluation of the Assessment: 1. Didactic: Standard of 92% of students to score 70% or higher on wirtten didactic exam was met. 2. Clinical: Standard of 92% of students to score 80% or higher on written clinical exams and proficiency testing was met. 08/01/2011 - Have student compare results from a properly collected patient sample against a hemolyzed sample; day-old sample, refrigerated sample, and samples collected in wrong tube, wrong temperature, and wrong additive. Comparisons will demonstrate to the students analytical errors resulting in inaccurate test results. Means of Assessment: Students will perform Quality Assurance and Quality Control procedures including Proficiency Testing activities in their respective clinical sites and clinical test 07/28/2011 - 1.100% of students are performing QC and Proficiency testing with results falling within the 25% established Reference Ranges. 2. 100% of students are scoring 80% or higher on clinical exams and proficiency testing. 07/28/2011 - 1. Include "Low", 'Normal" and "Abnormal" manufactures controls for clinical testing. 2. Incude manufacture product Assessment Tool: Clinical Assessment Standard - Criteria for Success: 92% of students must achieve no less than an "Acceptable" or higher rating when demonstrating these skills. Assessment Date: 05/04/2011 Discipline - Medical Laboratory Technology (SLO's) - Quality Assurance, Quality Control, and Proficiency Testing Participate in Quality Assurance, Quality Control, and Proficiency Testing protocols 08/29/2012 10:42 AM Generated by TracDat a product of Nuventive. Page 3 of 6 Student Learning Outcomes to determine acceptability of clinical test results. Outcome Start Date: 01/31/2011 Outcome Status: Active Discipline - Medical Laboratory Technology (SLO's) - Performing Laboratory Testing Demonstrate knowledge, skills, and abilities to perform, interpret, and report-out patient clinical laboratory test results as a Medical Laboratory Technician. Outcome Start Date: 01/31/2011 Outcome Status: Active 08/29/2012 10:42 AM Means of Assessment & Standard Criteria for Success / Strategie(s) results must fall within established Reference Ranges. Assessment Tool: Clinical Assessment Standard - Criteria for Success: 1. 92% of students must be able to reproduce QC and Proficieny test results within 25% of established Reference Ranges. 2. 92% of students must score 80% or higher in clinical exams. Assessment Date: 05/04/2011 Results/Analysis Result Type: Standard Met Recommendation Status: 2 - Recommendation Documented Evaluation of the Assessment: 1. Standard of 92% of the students performing QC and Proficiency testing with results falling within 25% of established ranges was met. 2. Standard of 92% of student scoring 80% or higher on clinical exams and proficieny testing was met. Means of Assessment: 1. Students will participate in Quality Assurance, Quality Control and Proficieny testing protocols. 2. Students will perform clinical laboratory testing on patient specimens to determine precision and accuracy in the ability to reproduce results. 3. Student will interpret clinical laboratory test results to determine if results are normal, abnormal, or critical for a patient. 07/28/2011 - 1. 100% of student performed QA, QC and Proficiency testing with test results within 25% or less from the established Reference Values. 2. 100% of student reproduced patient test results that were within 25% or less from the patients actual value. 3. 100% of students scored 80% or higher on clinical and proficiency exams. Result Type: Standard Met Recommendation Status: Assessment Tool: 2 - Recommendation Documented Clinical Assessment Evaluation of the Assessment: Standard - Criteria for Success: 1. Standard of 92% of students performing 1. 92% of students performing Quality QA, QC and Proficiency testing with test Assurance, Quality Control, and Proficency results falling within 25% or less was met. testing protocols must have test results fall 2. Standard of 92% of students reproducing within 25% or less of established Reference patient test results that were 25% or less Ranges. from the patients actual value was met. 2. 92% of students performing clinical 3. 100% of students scored 80% or higher laboratory testing on patient samples must on clinical and proficiency exams was met. be able to reproduce testing results within 25% or less of original patient results. 3. 92% of students must correctly interpret Generated by TracDat a product of Nuventive. Use of Results for Improvement & Follow -Up inserts for additional student reference. 3. Provide additional analyte and cell patient Reference Ranges for comparison. 07/28/2011 - 1. Include "Low", "Normal", and "Abnormal" controls to be tested. 2. Include manufactures inserts for additional reference. 3. Provide additional patient Reference Ranges for analytes, and cell counts. 4. Provide case studies. Page 4 of 6 Student Learning Outcomes Means of Assessment & Standard Criteria for Success / Strategie(s) clinical laboratory test results as being normal, abnormal, or critical for a patient. Results/Analysis Use of Results for Improvement & Follow -Up Assessment Date: 05/04/2011 Discipline - Medical Laboratory Technology (SLO's) - Certification as an MLT - Provide option to take National Medical Laboratory Technician certification/licensure examination. Outcome Start Date: 06/01/2010 Outcome Status: Active 08/29/2012 10:42 AM Means of Assessment: 1. To assess student competency in didactic objectives, embedded exam questions will be used. 2. To assess student competency and proficiency in clinical objectives, embedded questions will be used. Assessment Tool: Embedded Test Questions Standard - Criteria for Success: 1. 92% of students must score 70% or higher on written didactic exams. 2. 92% of students must score 80% or higher on written clinical exams or proficiency testing. Assessment Date: 05/04/2011 08/01/2011 - 1. 100% of students scored 70% or higher on written didactic exams. 2. 100% of students scored 80% or higher on written clinical exams and proficiency testing. Result Type: Standard Met Recommendation Status: 2 - Recommendation Documented Evaluation of the Assessment: 1. Didatic: Standard 92% of students to score 70% of higher on written didactic exams was met. 2. Clinical: Standard of 92% of students scoring 80% or higher on written clinical exams or proficieny testing was met. 08/01/2011 - 1. Include manufactures inserts for additional reference. 2. Provide additional patient Reference Ranges for analytes, and cell counts. 3. Provide case studies. Means of Assessment: National Medical Laboratory Technician certification examination offered by The American Society for Clinical Pathology Board of Certification. Assessment Tool: Test Licensure Standard - Criteria for Success: Have 80% of students taking the American Society of Clinical Pathology Board of Certification examination pass the examination becoming professional Medical Laboratory Technicians. Assessment Date: 06/02/2010 07/28/2011 - Students take National Certification Examination with American Socienty for Clinical Pathology Board of Certification to become professional Medical Laboratory Technicians. Exam: Year 2010, 7 tested with 6 passing = 85.7% Result Type: Standard Met Recommendation Status: 2 - Recommendation Documented Evaluation of the Assessment: 1. Standard of program pass rate of 80% was met. 2. Scores were compared to National Average 3. Student scores in Clinical Chemistry, Hematology, Lab Operations, Microbiology, 08/01/2011 - 1. Incorporate class textbook change 2. Include more varied patient testing samples. 3. Instructor to create new student handouts for better comprehension of immunohematology testing concepts and visualization. 4. Additional case studies to be applied Generated by TracDat a product of Nuventive. Page 5 of 6 Student Learning Outcomes Means of Assessment & Standard Criteria for Success / Strategie(s) Results/Analysis Use of Results for Improvement & Follow -Up and Urinalysis were higher than the national average. 4. Student scores in Immunohematology and Immunology were lower than the national average. 08/29/2012 10:42 AM Generated by TracDat a product of Nuventive. Page 6 of 6





