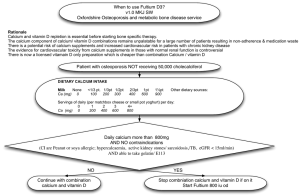
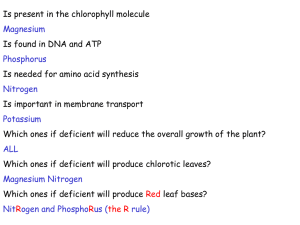
GASTRIC ACID, CALCIUM ABSORPTION, AND THEIR IMPACT ON BONE HEALTH
advertisement

Physiol Rev 93: 189 –268, 2013 doi:10.1152/physrev.00015.2012 GASTRIC ACID, CALCIUM ABSORPTION, AND THEIR IMPACT ON BONE HEALTH Sascha Kopic and John P. Geibel Departments of Surgery and Cellular and Molecular Physiology, Yale School of Medicine, New Haven, Connecticut Kopic S, Geibel JP. Gastric Acid, Calcium Absorption, and Their Impact on Bone Health. Physiol Rev 93:189 –268, 2013; doi:10.1152/physrev.00015.2012.—Calcium balance is essential for a multitude of physiological processes, ranging from cell signaling to maintenance of bone health. Adequate intestinal absorption of calcium is a major factor for maintaining systemic calcium homeostasis. Recent observations indicate that a reduction of gastric acidity may impair effective calcium uptake through the intestine. This article reviews the physiology of gastric acid secretion, intestinal calcium absorption, and their respective neuroendocrine regulation and explores the physiological basis of a potential link between these individual systems. L I. II. III. IV. V. VI. INTRODUCTION GASTRIC ACID SECRETION INTESTINAL CALCIUM ABSORPTION REGULATION OF CALCIUM HOMEOSTASIS THE STOMACH AND CALCIUM CONCLUSIONS 189 189 203 212 231 238 I. INTRODUCTION The average adult human body contains ⬃1.6% calcium, which relates to ⬃1,120 g in a 70-kg individual (743). Ninetynine percent of the calcium is stored in bone and teeth and is therefore inaccessible to most physiological processes (743). Although the amount of the immediately accessible 11 g (1%) of calcium may seem miniscule, this fraction represents a pivotal constituent of our body. It serves a broad diversity of roles, which range from intracellular signaling and maintenance of membrane integrity to muscle contraction and neuronal transmission. To allow for these calcium-dependent processes to function, our body undertakes extensive measures to keep the intracellular and extracellular calcium concentrations and the gradient between these two compartments stable. The extracellular calcium concentration is typically clamped at ⬃1.1 mM, whereas the intracellular environment is kept at a 10,000 times lower concentration. In consequence, relatively small disturbances in calcium homeostasis can lead to severe symptoms, such as cardiac arrhythmias or cognitive dysfunctions. To maintain eucalcemia, our body is therefore tightly regulating the balance between calcium absorption by the intestine and calcium excretion by the kidney. In addition, calcium is deposited in or extracted from bone, which serves as a dynamic calcium reservoir. These three organ systems, i.e., the intestine, the kidney, and bone, are precisely controlled by a complex endocrine network, which primarily consists of the calcitropic hormones: 1,25dihydroxyvitamin D [1,25(OH)2-vitamin D], parathyroid hormone (PTH), and calcitonin. This review mainly focuses on the question as to how calcium enters the body through the intestine and how this mechanism is regulated via the endocrine system. Furthermore, the process of gastric acid secretion as related to calcium homeostasis will be reviewed in detail. This may seem surprising, as gastric acid secretion and intestinal calcium absorption are two distinct physiological processes, which on first examination may not seem to be interdependent. However, recent clinical studies suggest that there may be a relationship between reduced gastric acid secretion and increased risk for sustaining bone fractures, which asks the question whether we need gastric acid to absorb calcium efficiently through the intestine, or whether the stomach exerts endocrine functions that impact bone health. Indeed, it has been put forward several decades ago that gastric acid solubilizes calcium that is then complexed with other dietary constituents, thereby allowing for a more efficient absorption in the intestine (18, 520, 699, 797). Furthermore, it is long known that a partial or complete resection of the stomach results in decreased bone density, also leading to fractures (58, 305, 732, 876). The stomach, the intestine, and bone are therefore functionally more intertwined than one may initially assume. This review will independently analyze the processes of gastric acid secretion, intestinal calcium absorption, and their respective neuroendocrine control and will conclude with a critical attempt at illustrating where these two seemingly independent organ systems intersect in terms of calcium homeostasis and bone health. II. GASTRIC ACID SECRETION The stomach is a unique organ that fulfills multiple roles. The main function of the gastric mucosa is to secrete con- 0031-9333/13 Copyright © 2013 the American Physiological Society 189 SASCHA KOPIC AND JOHN P. GEIBEL centrated hydrochloric acid, which provides a chemical barrier against ingested pathogens and aids in the digestion of foodstuffs. To achieve these functions, the gastric gland contains specialized cells that pump protons into the gastric lumen in an effort to acidify the contents of the stomach. These cells are known as parietal cells, or oxyntic cells. Since concentrated acid is a noxious substance, the gastric mucosa has to undertake extensive measures to protect itself from tissue injury. The protection is accomplished by secreting mucus from mucus neck cells, but also by tightly regulating the secretion of acid (see sect. IIB). A variety of specialized endocrine cells in the gastric mucosa are involved in the regulation of gastric acid secretion. A perturbation of either protective mechanism can lead to severe tissue damage, resulting in gastric ulcers. This section discusses the process of how gastric acid is secreted by reviewing the molecular mechanism underlying acid secretion in the parietal cell and its neuroendocrine regulation. A. Apical Ion Transport in the Parietal Cell The gastric parietal cell is responsible for acidifying the stomach by secreting concentrated acid. Gastric acid secre- tion depends on the apical extrusion of three ions. Protons are pumped into the gastric lumen by a proton pump, the gastric H⫹-K⫹-ATPase, to acidify the gastric content to a pH of as low as 1. Chloride is secreted via apical chloride channels to ensure formation of HCl and to provide the counter-ion conductance to protons. Lastly, potassium leaves the parietal cell apically in a recycling mechanism, thereby fueling reciprocal proton transport by the H⫹-K⫹ATPase (FIGURE 1). It has been demonstrated in numerous investigations that disruption of one of these ion transport mechanism renders the parietal cell incapable of secreting gastric acid (705, 820, 1013, 1029). 1. H⫹-K⫹-ATPase The gastric H⫹-K⫹-ATPase belongs to the family of P2-type ATPases, which also includes the ubiquitous Na⫹-K⫹-ATPase and the sarcoplasmic reticulum Ca2⫹-ATPase (SERCA). As the name implies, it exchanges one intracellular hydrogen ion for one extracellular potassium ion at the expense of ATP. ATP is provided to the pump by a large network of mitochondria, which occupy up to 40% of the cell volume, making the parietal cell one of the most mitochondria-rich cells in the body (292). In the A) STRUCTURE. Apical Basolateral PPIs APAs H+ SSTR SST H2 Hist CCK2 Gast M3 ACh K+ cAMP K+ KCNQ1 Kir Ca2+ Cl– CFTR CIC-2? SLC26A9 Parietal cell FIGURE 1. Parietal cell model. The gastric parietal cell is equipped with apical ion transport mechanisms that allow for the secretion of concentrated hydrochloric acid. Activation of basolateral secretagogue receptors mainly leads to an increase in either cAMP (histamine) or calcium (acetylcholine, gastrin), causing apical insertion and activation of the H⫹-K⫹-ATPase. Somatostatin reduces intracellular cAMP levels. ACh, acetylcholine; APAs, acid pump antagonists; Gast, gastrin; Hist, histamine; PPIs, proton pump inhibitors; SST, somatostatin. 190 Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH process of proton extrusion, the H⫹-K⫹-ATPase can overcome a massive acid gradient of 6 pH units, which is necessary to achieve sufficient gastric acidification. The pump itself is a heterodimer, consisting of a ␣ subunit and a  subunit, while the individual pumps assemble as (␣)4 tetramers on the parietal cell surface (1). The ␣ subunit consists of 10 transmembrane domains and contains the catalytic site, which mediates ion exchange. The  subunit stabilizes the ␣ subunit and is heavily glycosylated (41, 1105). Mutational analysis of the glycosylated asparagine residues suggests that these sites are critical for adequate membrane delivery of the entire pump (41, 1105). Furthermore, the  subunit prevents a reversal of ion transport by a “ratchet”like mechanism, which allows H⫹-K⫹-ATPase to pump against the imposed high proton gradient (4, 294). Both subunits share a significant degree of homology to Na⫹-K⫹ATPase (697, 1012). This close relationship to other P2type ATPase has historically been exploited for homology modeling of H⫹-K⫹-ATPase based on the crystal structure of SERCA, which had been acquired in several conformational states (762, 815, 1092, 1093). Recently, however, direct structural information on H⫹-K⫹-ATPase has been obtained by electron crystallography, also in the presence of the acid pump antagonist SCH28080 (2– 4). In the resting parietal cell, H⫹-K⫹-ATPase is stored in tubulovesicles throughout the cell (292). Following neuronal or hormonal stimulation (see sect. IIB), these vesicles are postulated to fuse with the apical pole, which is characterized by multiple microvilli-lined membrane invaginations, the so-called secretory canaliculi (292). This distinct apical morphology of the parietal cells maximizes cell surface and thereby allows for insertion of a high number of proton pumps per cell following stimulation. The changes in membrane morphology and insertion of H⫹-K⫹ATPase are extremely dynamic to ensure fine regulation of gastric acid secretion (973). H⫹-K⫹-ATPase containing tubulovesicle fusion relies on SNARE complex formation. In particular, the SNARE proteins syntaxin 3/7/12/ 13, VAMP2/8, and SNAP-25 were implicated to be candidates mediating this process (548 –550, 624). The functional significance of these proteins was, for example, demonstrated in primary rabbit parietal cell cultures expressing a SNAP-25 mutation, which was shown to reduce their capacity to secrete gastric acid (548). B) TRAFFICKING. Apart from SNARE proteins, the small GTPases of the rab family (rab2/11a/25/27b) are involved in the regulation of H⫹-K⫹-ATPase vesicle trafficking (147, 293, 386, 387, 1049, 1070). Functional data especially substantiate the importance of rab11a and rab27b. In parallel to SNAP-25 defective cells, parietal cells transfected with a rab11a and rab27b mutant secrete acid less effectively (293, 1049). After stimulation, in the off-phase of gastric acid secretion, H⫹-K⫹-ATPase has to be retrieved from the plasma mem- brane for recycling (336). It is plausible that the initial step of this process relies on the formation of clathrin-coated pits and subsequent vesicle budding. Indeed, clathrin was identified fairly early on H⫹-K⫹-ATPase containing tubulovesicles, although a functional role was not demonstrated (813). One of the multiple clathrin binding proteins is Huntingtin interacting protein 1 related (Hip1r) which aids in vesicle formation and membrane trafficking (309). It is strongly expressed in parietal cells, especially in the vicinity of secretory canaliculi (522). Functionally, Hip1r-deficient animals present with a decreased number of parietal cells, loss of tubulovesicles, and decreased acid output (522, 561). 2. Chloride secretion Apical chloride secretion provides the second component for the formation of concentrated HCl and maintains overall electroneutrality during acid secretion. The importance of chloride efflux for the process of gastric acid secretion has been established in the 1980s. Patch-clamp measurements demonstrated the presence of chloride conductance on the apical pole of the parietal cell in Necturus, the human parietal cell line HGT-1, and rabbit parietal cells (259, 935, 940). All reports demonstrated a sensitivity of the chloride current to cAMP or histamine, which is a common second messenger promoting acid secretion or a direct acid secretagogue, respectively (259, 935, 940). Simple flux measurements in isolated parietal cell vesicles had indicated the presence of a chloride conductance pathway even earlier (232, 895, 1169). In these early experiments, inhibition of chloride flux with chloride channel blockers also abolished proton transport which underlines the necessity of intact chloride secretion for acid secretion to take place (232, 895, 1169). However, the molecular identity of the chloride pathway remained elusive. Today, at least three candidates have been put forward as potential mediators of apical chloride secretion in the parietal cell: the cystic fibrosis conductance regulator (CFTR), chloride channel protein 2 (ClC-2), and solute carrier 26 A 9 (SLC26A9) (FIGURE 1). A) CFTR. CFTR represents a common apical chloride conduc- tance pathway in a broad variety of epithelia, such as the airways, intestine, and pancreas. Its mutation is responsible for the most widespread inherited disease, namely, cystic fibrosis (CF), which results in increased mortality due to secretory defects and concomitant infections. The presence of CFTR has been confirmed in gastric mucosa by in situ hybridization, albeit at low quantities (1044). Nevertheless, functional measurements in isolated gastric glands demonstrated a decreased acid secretory capacity in animals carrying the most common mutation responsible for CF (⌬F508) (1013). Furthermore, acid secretion was reduced in wild-type animals when a specific CFTR inhibitor was applied (1013). Although these observations may suggest a direct involvement of CFTR in the process of chloride secretion, it is plausible that CFTR rather has a regulatory Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 191 SASCHA KOPIC AND JOHN P. GEIBEL effect on H⫹-K⫹-ATPase (1013). In other tissues, CFTR can interact with a variety of ion transport proteins, such as NHE, forming regulatory complexes, making an interaction with H⫹-K⫹-ATPase plausible (1013). B) CLC-2. ClC-2 has been proposed as an alternative chloride secretion pathway to CFTR in other epithelia, such as the lung and intestine (207, 404, 675, 766). ClC-2 has been cloned from rabbit gastric mucosa, which led to the hypothesis that the channel may also be involved in acid secretion (706). However, follow-up investigations revealed that the role of ClC-2 is much less clear. The studies revealed controversial results regarding the channel’s expression in the gastric mucosa (488, 706, 1001). While the initial observations reported mRNA and cDNA expression in rabbit gastric mucosa, no protein could be detected in human and rat gastric glands (488, 706, 1001). The importance of ClC-2 in the stomach has further been severely challenged by the creation of a ClC-2 (⫺/⫺) animal model. Although ClC-2deficient animals present with a distinct phenotype characterized by testicular and retinal abnormalities, no defect in acid secretion was observed (118). C) SLC26A9. Lastly, evidence suggests that chloride may leave the apical pole via SLC26A9, a chloride-bicarbonate antiporter. Both SLC26A9 and an antiporter from the same anion exchanger family (SLC26A6) have been detected in the tubulovesicles of parietal cells (845, 1179, 1180). Concerning the functional involvement, the authors speculate about two potential roles SLC26A9 may play in parietal cell physiology. Being a chloride-bicarbonate exchanger, its activation would entail alkalinization of the gastric lumen by bicarbonate efflux and simultaneous chloride uptake (1180). Since this would neutralize H ⫹ -K ⫹ -ATPasemediated proton extrusion, it has been suggested that SLC26A9 activates in the off-phase of acid secretion to neutralize tubulovesicular pH during vesicle retrieval (1180). Alternatively, SLC26A9 may function as a chloride secretion pathway that contributes to acid secretion. This hypothesis is based on the observation that SLC26A9 can also exhibit the behavior of a bona fide chloride channel, rather than an anion antiporter (88, 281). Undoubtedly, further functional investigations are needed to delineate its exact role in the parietal cell. Its genetic disruption, however, leads to a severely altered parietal cell morphology that is characterized by dilation of gastric glands, loss of tubulovesicles, and decreased acid output (1180). Although these results do not answer whether SLC26A9 serves as an apical chloride efflux pathway, they indicate that it may be necessary for normal parietal cell function. 3. Potassium recycling Even before the identification of H⫹-K⫹-ATPase, it has been observed that potassium is necessary for acid secretion to take place (335). To prevent the luminal depletion of potassium, which would impair proton pumping by H⫹- 192 K⫹-ATPase, potassium has to leak through potassium channels or transporters into the gland lumen to ensure adequate supply to H⫹-K⫹-ATPase (FIGURE 1). This process is referred to as potassium recycling. Early flux measurements in isolated H⫹-K⫹-ATPase containing parietal cell vesicles had already indicated the presence of a large potassium conductance during H⫹-K⫹-ATPase activity (1169). The exact molecular identity of the potassium efflux pathway is, however, under debate. The list of candidates that have been put forward to be responsible for potassium recycling during acid secretion is long and includes KCNQ1 (Kv7.1), KCNJ10 (Kir4.1), KCNJ15 (Kir4.2), KCNJ2 (Kir2.1.), and KCC4. A) KCNQ1. KCNQ1 is a typical “shaker”-like six transmembrane spanning domain voltage-gated potassium channel (1144). It was initially identified in the heart, where its mutation can be responsible for cardiac arrhythmias (1144). Yet, studies in KCNQ1 (⫺/⫺) animals revealed no electrocardiographical abnormalities (641). Rather than suffering from cardiac abnormalities, these animals surprisingly exhibited a distinct gastric phenotype with gastric hyperplasia, dilated gastric glands, vacuolated parietal cells, hypochlorhydria, and hypergastrinemia (641). This observation led to the speculation that KCNQ1 may be the channel responsible for potassium recycling. Subsequently, immunohistochemical studies confirmed a colocalization of the channel with H⫹-K⫹-ATPase, and acid secretion was shown to be inhibited by pharmacological blockade (253, 391). Direct measurement of acid secretion in KCNQ1 (⫺/⫺) mice with modified Ussing chambers (pH stat) later confirmed the initially observed hypochlorhydria (1029). Interestingly, luminal substitution of potassium could rescue the acid secretory deficit, indicating that hypochlorhydria ensued from a true lack of apical potassium secretion rather than a general morphological defect of the KCNQ1 (⫺/⫺) parietal cell (1029). KCNQ1 is a peculiar channel in that it has a low conductance in acidic environments. In the context of the extreme acidic milieu surrounding the parietal cell, this would impede its function as a potassium recycling pathway. To circumvent this limitation, KCNQ1 attaches to a regulatory subunit (KCNE2), which modulates the channel’s gating properties and current amplitude (253, 391, 1087). Coassembly with KCNE2 activates KCNQ1 at acidic pH values and thus facilitates the process of potassium secretion into the gland lumen (391, 436). The importance of KCNE2 for proper channel function is underlined by the observation that KNCE2 (⫺/⫺) animals display a phenotype similar to KCNQ1 (⫺/⫺) mice, i.e., hypochlorhydria, altered parietal cell morphology, and hypergastrinemia (917). B) KIR CHANNELS. Apart from KCNQ1, several members of the inward-rectifier potassium channel (Kir) family have been proposed to be involved in gastric acid secretion, albeit Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH the amount of functional evidence supporting a role of these channels is smaller and the field is divided about the relative contribution of each channel. Kir 2.1, 4.1, 4.2, and 7.1 were all confirmed on an mRNA level in gastric mucosa (353, 431, 707). On a protein level, immunohistochemistry demonstrated colocalization of Kir 2.1, 4.1, and 4.2 with H⫹K⫹-ATPase (353, 431, 556, 707). Cell fractionation experiments further indicated trafficking of Kir 4.1 and 4.2 to the cell surface, following parietal cell stimulation (431, 556). A most recent observation monitored acid secretion in Kir 4.1 (⫺/⫺) mice (1028). Surprisingly, loss of Kir 4.1 results in augmented rather than impaired acid secretion, accompanied by upregulated H⫹-K⫹-ATPase expression (1028). This makes a contribution of Kir 4.1 to potassium recycling highly unlikely. Instead, it has been proposed that the channel may balance excessive potassium loss through KCNQ1 and may be involved in membrane recycling (1028). In summary, more investigations will be necessary to clarify the roles of the individual Kir channels. C) KCC4. Apart from being secreted through channels, potassium and chloride may exit the parietal cell through transporters. This alternative hypothesis is corroborated by a recent observation of Fuji et al. (352). The group reported that the K-2Cl cotransporter KCC4 coimmunoprecipitates with H⫹-K⫹-ATPase in apical membrane fractions of parietal cells (352). Furthermore, flux measurements in H⫹-K⫹ATPase containing vesicles showed decreased chloride and proton transport under pharmacological blockade of KCC4, suggesting a functional coupling of KCC4 to H⫹K⫹-ATPase (352). Although the hypothesis that both potassium and chloride leave the cell via a transporter is intriguing, the observation is, as of now, solitary and needs further experimental validation. B. Control of Acid Secretion Gastric acid secretion is subjected to precise regulation. The complex regulatory machinery that orchestrates the secretion of gastric acid consists of hormonal (gastrin, somatostatin), paracrine (histamine, somatostatin), and neuronal components (FIGURE 2). The need for this tight regulation is highlighted by conditions that lead to a hypersecretion of gastric acid, such as Zollinger-Ellisson syndrome (ZES; gastrinoma). Gastric hypersecretion can overcome the measures our body undertakes to protect itself from the acid and thereby lead to peptic ulcers. A fine on-demand regulation of acid secretion is thus pivotal to ensure the balance between an adequately low intragastric pH and tissue protection. According to the well-established model of acid secretion, the parietal cell is activated by neuronal input from the vagus nerve, endocrine input from gastrin-producing G cells, and paracrine input from histamine-producing enterochromaffin-like (ECL) cells (FIGURES 1 AND 2). The distinct substances released by these cells, i.e., acetylcho- line, histamine, and gastrin, directly or indirectly stimulate the parietal cell by inducing insertion of H⫹-K⫹-ATPase at the apical membrane and are thus commonly referred to as acid secretagogues. The main inhibitor of parietal cell acid secretion is somatostatin, which is secreted by the D-cells of the gastric mucosa (FIGURES 1 AND 2). Because of the complexity of the network that controls the release of acid into the stomach, it has been historically challenging to dissect the relative role of each individual regulatory component. Without a doubt, knockout models have greatly aided us in the last years to gain a more profound understanding of this process, despite their limitations of chronic compensation. The subsequent chapter aims to summarize the key players in our canonical model of acid regulation. 1. Cholinergic stimulation/vagus nerve Since the seminal experiments conducted by Pavlov on dogs, we know that the mere prospect of food ingestion or sham-feeding is sufficient to trigger the secretion of gastric acid (833). This first of three phases of acid secretion is called the cephalic phase and is mostly mediated through the vagus nerve (595, 725, 910). Hence, before the advent of pharmacological inhibitors, vagotomy has been an effective surgical procedure to control acid-related disorders (301). The parietal cell receives neuronal input from the vagus nerve that is relayed via cholinergic postganglionic enteric fibers in the enteric nervous system (ENS) (FIGURES 1 AND 2). In addition, the vagus nerve activates G-cells to release gastrin, resulting in an indirect stimulation of the parietal cell. Direct cholinergic activation occurs mostly via muscarinic M3 receptors, which have been identified on the surface of the parietal cell (507, 541, 846). The M3 receptor is a classic seven-transmembrane domain GPCR. Predictably, knockout of M3 receptors leads to an impairment of gastric acid secretion and compensatory hypergastrinemia due to negative feedback (9). Following acetylcholine binding, M3 receptor activation mostly causes an increase in intracellular calcium concentrations (44, 1163). Calcium rises in response to PLC-mediated IP3 generation and subsequent mobilization from intracellular stores (190). The primary kinases activated by the M3 receptor are protein kinase C (PKC) and calcium/calmodulin-dependent protein kinase II (CaMKII) (136, 196, 314 –316, 773, 774, 1095). While activation of CaMKII has a clear stimulatory effect on acid secretion, PKC has been reported to have dual effects, although reports of an inhibitory role predominate numerically (23, 73, 136, 196, 313, 314, 316, 597, 755, 773, 1095). It has been postulated that the expression of different PKC isoforms may account for this dichotomy (313, 314). Current evidence suggests that the PKC-␣ isoform has a suppressing effect by trans-inhibiting CaMKII activity, whereas PKC-⑀ increases the baseline levels of intracellular calcium, thereby sensitizing the parietal cell to subsequent Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 193 SASCHA KOPIC AND JOHN P. GEIBEL Oxyntic mucosa Lumenal Antrum Basolateral D-cell Lumenal Somatostatin Basolateral D-cell ? VPAC PACAP VIP CCK1 CCK ENS Somatostatin Low lumenal pH ECL-cell SSTR SST G-cell PAC1 PACAP CCK2 Gast SSTR ENS CaSR CaSR ENS Histamine Calcium Amino acids Polyamines PPIs APAs SST Gastrin ACh GRP CaSR H+ H,K-ATPase H2 Hist Circulation K+ CCK2 Gast M3 ACh ENS SSTR SST Parietal cell Somatostatin D-cell FIGURE 2. Neuroendocrine regulation of gastric acid secretion. In addition to direct neuronal regulation, the parietal cell receives paracrine signals from neighboring ECL- and D-cells. Gastrin is produced in the antral mucosa of the stomach and reaches the oxyntic mucosa via the circulation (endocrine regulation). Gastrinmediated histamine release represents one of the major stimulatory pathways leading to the secretion of gastric acid (gastrin-histamine axis). The secretion of gastrin is closely tied to intragastric pH (via somatostatin), thereby creating a negative-feedback loop. ACh, acetylcholine; APAs, acid pump antagonists; ENS, enteric nervous system; Gast, gastrin; Hist, histamine; PPIs, proton pump inhibitors; SST, somatostatin. stimulation (313, 314). Apart from PKC and CaMKII activation, cholinergic signaling activates parietal cell MAPKs, which is partially a downstream effect of PKC activation (771, 1039, 1062, 1063). MAPK activation seems to have a biphasic effect on acid secretion (acute inhibition and chronic augmentation) and also serves as a mediator of trophic responses in the parietal cell. For example, pro- 194 longed MAPK activation (72h) has been shown to serve as a maturation and differentiation signal leading to a transformation of parietal cell morphology in vitro (1039). The change in morphology is accompanied by a downregulation of H⫹-K⫹-ATPase gene expression (1039). As of now, it is challenging to put these findings into a physiological perspective. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH In addition to M3 receptors, M1 receptors have also been implicated to play a role in the process of acid secretion. This hypothesis was derived from the observation that the M1 receptor is expressed in gastric mucosa and that its blocker pirenzepine can inhibit gastric acid secretion (29, 466). Most evidence pointed to an expression of M1 on ECL-cells, where it was speculated to regulate the release of histamine (437, 507). More recent findings somewhat surprisingly report that pirenzepine also suppresses acid secretion in M1-deficient animals. Furthermore, these animals show a normal phenotype in terms of acid output (10). These observations question both the involvement of M1 receptors in acid secretion and the specificity of pirenzepine. Lastly, knockout studies point towards a contribution of the M5 receptor to the regulation of acid secretion, as its deletion correlates with decreased acid output (10). Yet, M5 receptor mRNA could only be detected in whole stomach homogenates, but not in gastric mucosa per se, making its localization to the submucosal enteric plexus more likely (10). 2. Gastrin/G-cell Gastrin has been discovered in 1906 by John S. Edkins, who injected gastric extracts of pig and cat stomachs into the jugular vein of cats and observed a subsequent increase in acid secretion (298). Gastrin is a peptide hormone that is produced in specialized G-cells, located in the antral section of the stomach (FIGURE 2) and endocrine cells in the duodenum, small intestine, colon, pancreas, testis, and pituitary. It is the main mediator of the so-called gastric phase of acid secretion, which initiates when the ingested food enters the stomach. The gastric phase accounts for the majority of the acid secretory response of the stomach. A) SYNTHESIS. The gastrin cDNA encodes a 101-amino acid pre-pro-hormone that undergoes extensive posttranslational processing (113, 519, 551, 552, 1161). In brief, the pre-pro-hormone is first cleaved NH2-terminally to create progastrin and then truncated to two main core proteins, G17 and G34, which can exist in glycine extended (G17Gly; G34-Gly) or terminally amidated (G17-NH2, G34NH2) forms. Furthermore, a fraction of progastrin (⬃47% in humans) is sulfated at Tyr66 in the course of its passage through the Golgi apparatus, thereby giving rise to sulfated and nonsulfated isoforms of gastrin (22). Sulfation has no influence on the acid secretory response, as the affinity to the gastrin receptor remains unchanged (399, 596). G17NH2 is the main circulating form that mediates the secretory effects of gastrin. Although the glycine-extended forms have a low affinity towards the gastrin receptor (CCK2) and thus play no role in gastric acid secretion (they are four to five orders of magnitude less potent in inducing acid secretion), it is still important to acknowledge their existence (178, 722). First, they serve as substrates for the synthesis of amidated gastrin and are cosecreted with gastrin by the G-cells (1040, 1051). Second, they potentiate the acid se- cretory response to amidated gastrin, although they have no intrinsic ability to induce acid secretion (178). Third, progastrin and glycine extended gastrins were shown to act as a proliferative signal, especially in the colon (20, 482, 994, 1145). This is also of pathophysiological relevance as both forms can promote cancer growth by presumably inhibiting apoptosis and inducing angiogenesis (71, 87, 900). For example, it was shown that overexpression of progastrin in mice is a predisposing factor for the development of colorectal or bronchoalveolar cancers (587, 1017). B) REGULATION OF RELEASE. Gastrin is released by the G-cell in response to a variety of stimuli of different origin. Direct neuronal stimulation of the G-cell occurs via ACh and gastrin releasing peptide (GRP), which are released by postganglionic neurons of the enteric nervous system. The postganglionic fibers themselves receive input from the efferent fraction of the vagus nerve (86, 485). On the other hand, food-related signals, such as calcium, amino acids, and amines, can also directly trigger gastrin secretion (FIGURE 2) (257). The secretory stimuli culminate in an increase in intracellular calcium concentrations, leading to vesicle fusion and gastrin secretion. The main inhibitory signal for gastrin secretion is somatostatin, which reaches the G-cells in a paracrine fashion from neighboring D-cells (632, 975). With regard to the neuronal control of gastrin secretion, it is generally thought that vagal stimulation increases the release of gastrin, although some conflicting evidence exists (310, 694). Latest experiments that assessed local gastrin concentrations utilizing microdialysis, however, clearly show an increase in gastrin levels following acute electrical vagal stimulation (310). The vagus nerve then synapses on neurons of the ENS, which are thought to release either the neurotransmitter ACh or GRP on a G-cell, leading to secretion of gastrin (295, 635, 694, 960, 978). It should be noted that a recent investigation failed to observe increased gastrin levels, following exogenous GRP administration in humans (456). Yet, GRP itself serves as a clear acid secretagogue, although potentially not via gastrin (456). Whether these conflicting observations are attributable to species differences (most earlier observations utilized rodent models) remains to be elucidated. The ENS is also thought to mediate parietal and G-cell activation in response to mechanical distension of the stomach (455, 962, 977). The neurohormonal response to gastric stretch is an integral part of the gastric phase of acid secretion. Closer examination, however, reveals that the reports are very conflicting in that it is not clear whether a pure mechanical distension stimulates or inhibits gastrin release (455, 664, 803, 962, 977). A biphasic model characterized by initial inhibition of gastrin secretion under low volumes followed by stimulation under high volumes has been suggested, but awaits further confirmation (977). Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 195 SASCHA KOPIC AND JOHN P. GEIBEL Dietary components, such as amino acids and calcium, can directly promote the secretion of gastrin and can thus sustain acid secretion in the gastric and intestinal phase as digestion progresses (652, 1074). A rise in serum calcium concentrations evokes a similar effect. The correlation between calcium and gastrin is discussed in a separate section (see sect. VD2). It has been unclear for a long time as to how these dietary components activate the G-cell. An involvement of the ENS has been proposed as the most likely explanation in the past. More recent observations, however, strongly indicate that the calcium-sensing receptor (CaSR) represents the molecular link between luminal dietary constituents and G-cell activation (325). The CaSR and its role in the stomach are discussed separately and shall only be summarized at this point (FIGURE 8) (see sect. IVD). First, the same dietary components, i.e., amino acids, amines, and calcium, which have all been shown to trigger gastrin release also function as activators of CaSR (652, 1074). Second, CaSR is expressed on the apical and basolateral side of the G-cell, which allows it to act as nutrient sensor both in the gastric lumen and the circulation (142, 182, 886). Third, direct activation of the CaSR is known to stimulate acid secretion (145, 291, 373). Finally, and most importantly, CaSR (⫺/⫺) animals lack the gastrin secretory response to intraluminal instillation of peptone, calcium, and phenylalanine (325). In light of this evidence, it is highly likely that CaSR is the long elusive luminal nutrient sensor that regulates the secretion of gastrin from the G-cell. The plasma levels of gastrin are closely tied to the intragastric pH. Low intraluminal pH is a potent inhibitor of gastrin release, which serves as a negative-feedback mechanism to impede an overproduction of acid. Conversely, a more alkali intragastric pH induces the secretion of gastrin, which accounts for the commonly observed hypergastrinemia in states of acid suppression, such as during proton pump inhibitor (PPI) therapy. The pH dependency of serum gastrin levels is mainly relayed via somatostatin, as acid directly stimulates somatostatin release (see sect. IIB4). Somatostatin, released by neighboring antral D-cells, in turn acts as the main inhibitor of gastrin secretion (FIGURE 2). The physical proximity to G-cells allows for a fine paracrine regulation of gastrin release. Although it is generally accepted that intragastric pH mostly modulates local somatostatin levels, the G-cell may also directly sense intragastric pH via CaSR. CaSR is acid sensitive, and it has been shown that isolated rat G-cells secrete less gastrin when the extracellular pH is dropped from 7.4 to 5.5 (569). However, more investigations are needed to substantiate this evidence. Furthermore, gastrin release is also inhibited by neuronal regulation by the ENS. The neurotransmitter galanin has been demonstrated to exert a direct inhibitory effect on isolated G-cells (695, 961). C) CELLULAR EFFECTS. Following secretion, gastrin enters the bloodstream and acts on its target cells in an endocrine 196 fashion. Its half-life is determined by its rate of elimination from the plasma which mainly occurs by metabolism in the kidney, gut, and brain (419, 420). The importance of renal elimination is corroborated by the observation that patients with renal failure present with higher plasma gastrin levels (818, 1075). The two primary target cells of gastrin are the histaminesecreting ECL cell and the parietal cell. Gastrin exerts its functions via binding to the cholecystokinin receptor type 2 (CCK2), a seven transmembrane domain G protein-coupled receptor, which is expressed on mature parietal and ECL cells, but also on gastric stem cells (560, 596, 608, 769, 772, 904). On the ECL cell, gastrin binding causes the release of histamine, which in turn stimulates the parietal cell in a paracrine fashion (FIGURE 2) (see sect. IIB3) (412). This activation cascade is commonly referred to as the gastrinhistamine axis. Evidence for a direct, i.e., nonhistaminerelayed, activation of H⫹-K⫹-ATPase in the parietal cell by gastrin exists, but is far less substantiated (459, 1024, 1025). Gastrin may sensitize the parietal cell to subsequent secretagogue stimulation, rather than acting as a bona fide secretagogue itself. Canonically it is widely accepted that gastrin exerts its physiological effects mostly via activation of ECL cells (24, 1131). Knock-out of gastrin leads to a severe impairment of basal and stimulated acid secretion (179, 347). Apart from stimulating acid secretion, gastrin serves as a pivotal proliferative signal for the gastric mucosa in general (60, 410, 534, 631, 816). It is commonly observed that elevated plasma gastrin levels lead to substantial mucosal proliferation (60, 410, 534, 631, 816). This phenomenon has been extensively described in various knockout animals suffering from hypochlorhydria and concomitant hypergastrinemia, but also in patients with ZES (39, 515, 584, 1066). The source of mucosal cell proliferation is progenitor cells located in the isthmus region of the gastric gland (545). Expression of the CCK2 has been confirmed on several gastric progenitor cells (560, 769). Furthermore, gastrin has been shown to stimulate cell migration from the progenitor region along the gastric gland axis (578). Mucosal hyperplasia thus ensues most likely via a direct activation of precursor cells by gastrin. Although hypergastrinemia causes a generalized mucosal hyperplasia, ECL cells seem to be particularly regulated by gastrin, as their relative fraction compared with other mucosal cells increases under prolonged gastrin exposure (60, 410, 631). Conversely, the absence of the CCK2 almost entirely eliminates mature ECL-cells from the gastric mucosa (177, 622). [Somewhat surprisingly this does not occur when gastrin itself is knocked out (179, 347).] It should be noted that the M3 receptor seems to be necessary as a cofactor mediating the trophic effects of gastrin, as its absence is associated with a normal mucosal phenotype despite elevated serum gastrin levels (see above) (9). The mechanism underlying this interdependency between gastrin and the M3 receptor is as of now elusive. The cholinergic and gastrin systems also seem Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH to be intertwined with regard to acid secretion. In the absence of the CCK2 receptor, the parietal cell’s acid secretory response to the secretagogue carbachol (ACh analog) is abolished, while the response to histamine remains intact (543). Again, one can only speculate about the molecular basis of this interaction. In conclusion, gastrin is the most important activator of acid secretion in the stomach. The role of gastrin, and especially its glycine extended forms, has evolved beyond being a mere acid secretagogue to being an important global regulator of cell growth and differentiation. Furthermore, the regulation of gastrin by the levels of plasma calcium provokes the question as to whether gastrin itself in turn has an impact on global calcium homeostasis. A subsequent section makes an attempt at addressing this question (see sect. VD2). 3. Histamine/ECL cell Histamine has been discovered as early as 1910 by Dale, Barger and Laidlow in extracts of ergot fungi (63, 237). In 1920, Popielski for the first time described its effect on the secretion of gastric acid (866). He observed that subcutaneous administration of histamine resulted in increased acid secretion (866). Furthermore, he concluded that this effect was independent of the vagus nerve, as secretion still took place after vagotomy and administration of atropine. This led Popielski to postulate that histamine exerts its effects directly on the level of the gastric gland (866). The hypothesis that histamine acts in a paracrine fashion on parietal cells and that its release is regulated by the levels of gastrin has been put forward for the first time by Emmelin and Kahlson in 1944 (306). At this point, the cellular source of histamine was still obscure. It was only in the late 1960s that histamine had been histochemically localized to the ECL cells of the gastric gland (413, 1084). A) SYNTHESIS AND REGULATION OF RELEASE. Histamine is the effector of the gastrin-histamine axis and directly stimulates the parietal cell to secrete hydrochloric acid (FIGURE 2). Histamine is derived from the amino acid histidine, which is enzymatically converted to histamine by L-histidine decarboxylase (HDC) (957). The effects of genetic HDC deletion are predictably severe: animals lacking HDC have a low basal acid output that does not respond to exogenous administration of gastrin (1066). Histamine is stored in secretory granules of the ECL cell and is released into the surrounding milieu in response to stimulation by gastrin and neuronal signals. Stimulation by gastrin occurs via activation of its GPCR CCK2 (772, 904). Gastrin affects the ECL cell in multiple ways. First, gastrin exposure increases the levels of HDC expression by enhancing its transcription and inhibiting its degradation, to allow for increased synthesis of histamine (268, 331). The molecular mechanism underlying increased HDC transcription is fairly well understood. Following CCK2 activation, increased transcription of HDC is mediated via a PKC- and ERK-dependent pathway (470, 472). The HDC gene promoter is then activated by at least three distinct nuclear factors which bind to gastrin response elements, resulting in gene transcription (889, 890). Apart from augmenting gene transcription, gastrin regulates the degradation of HDC, which further increases intracellular enzyme levels (331, 1214). Second, gastrin enhances the transcription of the vesicular monoamine transporter type 2 (VMAT2; SLC18A2), which is responsible for accumulating histamine in the secretory vesicles (376). Similarly to HDC, this effect depends on PKC and ERK activation and binding of a nuclear factor to a gastrin response element in the VMAT2 promoter region (164, 1154). It should be mentioned that gastrin regulates the transcription of a plethora of other genes which serve a diverse array of roles, ranging from growth to metabolism (346). Amongst many others these include chromogranin A, which is essential for granule packaging and is a precursor of pancreastatin (see sect.VD3) (231). Third, gastrin induces the fusion of secretory granules and the release of histamine into the gland environment. Secretion follows a biphasic elevation of intracellular calcium concentrations after activation of CCK2 (1201). The biphasic increase has been proposed to result from initial IP3-mediated release from intracellular stores, which is followed by subsequent influx of calcium via L-type calcium channels from the extracellular space (1201). The importance of intracellular store mobilization has been contested by a different group, which proposed that solely influx trough L-type, and to a lesser extent N-type, calcium channels triggers the secretory response (673). Lastly, gastrin has a trophic effect on the ECL cell (see sect. IIB2). Apart from gastrin, ECL cells are stimulated by pituitary adenylate cyclase activating polypeptide (PACAP), which is a neuropeptide expressed in the ENS of the gastric mucosa (737, 1054). PACAP has homology to vasoactive intestinal polypeptide (VIP) and binds to a distinct receptor (PAC-1) on the ECL cell (1207, 1208). Binding of PACAP to PAC-1 induces release of histamine (672, 798, 944, 1207). Similar results have been obtained with VIP, which is attributable to partial agonism at PAC-1 (798, 941). Historically, investigations yielded controversial results with regard to the effects of exogenously administrated PACAP on acid secretion. Both an inhibition and stimulation of acid secretion following PACAP injection are reported (760, 862, 944, 1207). This discrepancy is most likely attributable to the fact that PACAP can also act as an agonist of the VIP receptor (VPAC) on the somatostatin-secreting D-cell, leading to a concomitant suppression of acid secretion by somatostatin release (1207). Indeed, if an anti-somatostatin antibody is injected into rats simultaneously with PACAP, acid secretion is elevated threefold from baseline (compared with 1.5-fold in the absence of an anti somatostatin antibody) (1207). Evidence points to the fact that the PACAP- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 197 SASCHA KOPIC AND JOHN P. GEIBEL stimulated release of somatostatin is of particular importance in the mouse, as most studies showing a suppression of acid secretion after PACAP administration were conducted in murine models. PACAP has very similar effects on the ECL cell as gastrin. Similarly to gastrin, PACAP causes histamine release by increasing intracellular calcium concentrations via calcium influx through L-type, but also ligand-gated calcium channels (673). In further analogy to gastrin, PACAP upregulates the expression of HDC and exerts trophic effects on the ECL cell (590, 729, 810). Contradictory results with regard to the effects of acetylcholine on histamine release exist. It has been reported that acetylcholine can either stimulate or has no effect on the secretion of histamine in in vitro experiments on isolated ECL cells (481, 672, 674, 941, 946). In vivo application of muscarinic agonists, followed by measurement of histamine concentrations using microdialysis, also yielded no evidence for cholinergic stimulation (798). Conversely, it is well accepted that adrenergic stimulation leads to an increase in histamine release; however, the physiological relevance of adrenergic activation of ECL cells is not entirely clear (636, 672, 674, 798, 871, 941). The ECL cell is inhibited by a variety of substances, the most prominent of which is somatostatin (204, 590, 798, 941). Somatostatin is produced in D-cells of the oxyntic mucosa and reaches the ECL cell in a paracrine fashion where it binds to the somatostatin receptor (SST2 and potentially SST5) (FIGURE 2) (570, 873). Receptor binding leads to inhibition of histamine exocytosis via blockade of mostly L-type calcium channels (105). This impedes the elevation of intracellular calcium concentrations caused by ECL activators, such as gastrin (see above) (873). In addition, somatostatin also inhibits the proliferation of ECL cells (570). Somatostatin can thus be seen as the global hormonal antagonist to gastrin with regard to ECL cell function and proliferation. The neuronal inhibition of ECL cells is mainly carried out by the neuropeptide galanin (105, 672, 798, 1209). Galanin is localized to neurons of the ENS and demonstrated an inhibitory effect on histamine secretion in in vitro and in vivo models (105, 302, 672, 731, 798, 1209). Similarly to somatostatin, the molecular mechanism underlying its inhibitory effect is an interference with calcium signaling via closure of L-type calcium channels (105). Lastly, prostaglandin E and nitric oxide also act as inhibitors of histamine release (105, 554, 798, 1002). Although neuropeptide YY (PYY) and calcitonin gene-related peptide (CGRP) have also been implicated in playing a role in ECL cell regulation, a detailed discussion is omitted in light of contradictory results which range from stimulation to inhibition of secretion (672, 674, 798, 1210). B) CELLULAR EFFECTS. As mentioned earlier, stimulation of the ECL cell is translated into an elevation in intracellular calcium concentrations, leading to exocytosis of preformed 198 histamine-containing secretory vesicles. The molecular mechanism of vesicle fusion with the apical membrane relies on the formation of the core SNARE complex, consisting of syntaxin, synaptobrevin, and SNAP-25. Synaptotagmin presumably acts as a calcium sensor relaying the intracellular calcium signal to the vesicle fusion protein apparatus. The expression of all SNARE complex proteins has been confirmed in the ECL cell (471, 477, 1215). In accordance with these findings, introduction of the neurotoxins tetanus toxin light chain and botulinum toxin, which cleave constituents of the SNARE complex apparatus and thereby render it nonfunctional, result in inhibition of histamine secretion (477). Very small amounts of histamine are sufficient to induce acid secretion. Histamine acts via the H2 receptor on the parietal cell, which has been discovered by Sir J. W. Black in 1972 (106). For this seminal discovery, he was later awarded the Nobel Prize in Physiology and Medicine. The H2 receptor belongs to the family of seven-transmembrane domain GPCRs. Its activation predominantly leads to increases in the intracellular levels of cAMP, but also of calcium, which serve as stimulatory signals for H⫹-K⫹-ATPase trafficking (67, 189, 738, 840, 1026, 1143). In analogy, pharmacological agents that elevate cAMP, such as IBMX or forskolin, induce acid secretion (1026, 1191). The increase in cAMP is due to activation of adenylate cyclase via Gs. The role of calcium in the process of histamine secretion remains a controversial matter. First, the mechanism leading to histamine-induced increases in intracellular calcium has been subject of discussion. Evidence exists that histamine can, apart from adenylate cyclase, also activate PLC, leading to calcium release from intracellular stores (607, 1142, 1143). Conversely, it has been suggested that the observed increases in intracellular calcium are a byproduct of cAMP-mediated PKA activation, which in turn can regulate the opening of calcium channels (144, 189, 840). Second, it is questionable to what degree the calcium signal is an integral and necessary part of the acid secretory response to histamine (738, 840). Chelation of the transitory calcium increases with BAPTA abolishes only the secretory response of isolated gastric glands to histamine by ⬃40%, while it completely eliminates the response to cholinergic stimulation (738). Also, live fluorescence imaging in isolated glands showed no spatiotemporal correlation between the histamine-induced increases in calcium and the onset of acid secretion, thereby questioning an involvement of calcium in the secretory response (840). H2 receptor knockout animals effectively illustrate the significance of the histamine-gastrin axis in gastric physiology. Lack of the H2 receptor leads to a complete failure of gastrin or histamine to induce acid secretion (584). The secretory response to carbachol, however, remains intact (584). Hypergastrinemia develops as a feedback mechanism with the aim of reestablishing acid secretion, leading to mucosal Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH hypertrophy (584). In light of the central role of the H2 receptor in parietal cell physiology, it has been successfully used as a pharmacological target with the aim of suppressing gastric acid output (see sect. IIC2). 4. Somatostatin/D-cell Somatostatin was isolated for the first time in 1973 from ovine hypothalamus and characterized as an inhibitor of growth hormone release from the pituitary gland (124). A few years later somatostatin was identified in endocrine cells of the stomach, which we now know as D-cells (638). A) SYNTHESIS AND REGULATION OF RELEASE. Somatostatin is a peptide hormone that exists in two primary forms that differ in their respective peptide length. The most abundant form in the gastric mucosa is somatostatin-14 (consisting of 14 amino acids), whereas somatostatin-28 only constitutes a minute fraction of the total gastric somatostatin content (198, 1125). The two forms of somatostatin are cleavage products of a larger 116-amino acid pre-prohormone (preprosomatostatin), which in turn is processed to the 92amino acid-long prosomatostatin (1000). It should be mentioned that other cleavage products, such as antrin or somatostatin-28(1–12) exist and are secreted together with somatostatin (77, 885). Their physiological significance is, however, less well understood. Somatostatin is the global antagonist of the acid secretagogues. It is produced by intestinal and gastric D-cells, the latter of which exist in two populations in the stomach (61): an antral population locally inhibits the release of gastrin from G-cells, whereas a population localized to the acidproducing oxyntic mucosa directly regulates the parietal cell and inhibits histamine release from the ECL cell (FIGURE 2) (19). The morphology of the D-cell is characteristic in that it possesses long cytoplasmic processes, which allow it to communicate with and regulate neighboring cells in a paracrine fashion (620, 632). It is worthwhile to distinguish the two populations of gastric D-cells, as each population possesses unique physiological properties (1202). The antral D-cell is mostly regulated by the local concentrations of gastrin, cholecystokinin, and intraluminal pH. Gastrin induces somatostatin secretion from D-cells, which causes reciprocal inhibition of gastrin release from neighboring G-cells, thereby creating a local negative-feedback loop (976, 1011, 1202). The molecular mechanism underlying this loop is, however, less clear. CCK2 receptor is, if at all, only expressed at very low levels in the antral mucosa (749, 905, 967). It has been proposed that gastrin stimulates somatostatin release in the antrum in a receptor-independent mechanism (1202). This may be accomplished via direct cell-cell contacts between the G- and the D-cell, which have been demonstrated with electron microscopy (620). Conversely, evidence for cholecystokinin and its stimulatory role for somatostatin release via CCK1 is more substantiated (749, 905, 967, 1202). Cholecystokinin is structurally closely related to gastrin (both share an identical 5-amino acid COOH terminus) and also exists in various peptide lengths (767). It is secreted by I-cells of the small intestine following protein and fat-rich chyme entering the duodenum, and thus represents a classical mediator of the intestinal phase of acid secretion (594). As its name implies, cholecystokinin has originally been described as a stimulator of gallbladder contraction; however, its inhibitory influence on gastric acid secretion is now well accepted and extensively described (593, 1203). Cholecystokinin can bind to both the CCK1 and CCK2 receptor with almost equal affinity, whereas the actions of gastrin are almost exclusively mediated by the CCK2 receptor. The dual affinity of cholecystokinin would imply a possible stimulatory effect on acid secretion via activation of CCK2 on ECL cells; however, in vivo the inhibitory effect mediated by activation of CCK1 and CCK2 on D-cells prevails (593, 966, 1202, 1203). One of the most important stimulators of D-cell secretion is the intragastric pH. A seminal observation demonstrating a correlation between gastric acidity and the amount of secreted somatostatin was made in dogs in the 1970s. It has been shown that the amount of somatostatin directly increases in antral venous blood following gastric HCl infusion, while somatostatin levels were unaffected in venous blood from the oxyntic mucosa (982). Similar observations were later made in isolated mouse stomach, however without topographic discrimination (975). Two main hypotheses as to how somatostatin is regulated by intragastric pH exist. The first states that the D-cell can directly act as a pH sensor, and the second postulates that the pH sensing is mediated by neurons, which in turn act on D-cells. To accomplish putative direct pH sensing, several antral D-cells are equipped with a distinct morphological feature. They possess apical projections that are in contact with the glandular lumen, potentially allowing them to constantly monitor the intraluminal milieu (620). These D-cells have been termed open type. Conversely, the D-cells of the oxyntic mucosa are mostly of the closed type, meaning that they are embedded in the mucosa without luminal contact. The molecular identity of the putative apical pH sensor remains elusive. However, the presence of CaSR, which has pH sensing properties, was recently confirmed in preliminary studies on the D-cell and may represent a possible candidate for this mechanism (770). Apart from directly acting on D-cells, the effect of pH on somatostatin secretion may be mediated via afferent spinal neurons. Over 80% of the spinal afferent neurons contain the neuropeptide CGRP (397, 758, 1047, 1116). Perfusion models of antral sleeves have shown that the acid-induced rise in somatostatin is accompanied by a concomitant increase in the concentrations of the neuropeptide CGRP (708). Furthermore, application of a CGRP receptor blocker inhibited the release of somatostatin following acid exposure (708). As D-cells are known to Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 199 SASCHA KOPIC AND JOHN P. GEIBEL express the CGRP receptor, an involvement of CGRP in acid sensing is plausible (558). Again, this provokes the question of how CGRP-containing neurons may sense acidity on a molecular basis. The acid-sensitive channels transient receptor potential vanilloid channel (TRPV1) and the acid-sensing ion channel 3 (ASIC3) had been proposed as molecular acid sensors; however, latest experiments have shown that the increase in CGRP still occurs in the genetic absence of the channels (47, 96, 163). Neuropeptides of the gastric ENS that stimulate the secretion of somatostatin include PACAP and VIP, which both bind to the VPAC receptor expressed on D-cells (199, 657, 1207). The presence of VIP and PACAP containing neurons, which integrate signals from the vagus nerve, has been demonstrated in the gastric mucosa (302, 737). Furthermore, cholinergic signals can act on the antral D-cell via the M3 receptor to promote secretion of somatostatin (140). This is in sharp contrast to D-cells from the oxyntic mucosa that are inhibited by cholinergic signals (197, 200, 1182). As mentioned earlier, the D-cells in the oxyntic mucosa also differ in their morphology. D-cells in the oxyntic mucosa are of the closed type and have thus not been implicated to participate in acid sensing. They exert their acid-suppressive effects by the paracrine regulation of ECL and parietal cells. Further functional divergence between antrum and the oxyntic mucosa has been demonstrated in the regulation of the somatostatin mRNA. For example, suppression of acid secretion with omeprazole in fasted animals markedly decreased somatostatin mRNA levels in the antrum, whereas the levels in the oxyntic mucosa were affected to a much lesser extent, which further corroborates the hypothesis that the antral cells are involved in luminal chemosensation (945). B) CELLULAR EFFECTS. The effects of somatostatin on its target cells are mediated by the SST2 receptor. Knockout of the receptor causes a 10-fold increase in basal acid output, which exemplifies the pivotal role somatostatin plays as a global suppressant of acid secretion (715). Somatostatin acts on all main cell types that are involved in the process of acid secretion, i.e., parietal cells, ECL cells, and G-cells (FIGURE 2). The inhibition of the G- and ECL cells has been discussed in the respective sections. In the parietal cell, somatostatin has a clear direct inhibitory effect on secretagogue-induced acid secretion (827, 1177). This effect is partially attributable to activation of Gi, leading to inhibition of adenylate cyclase and a subsequent decrease in intracellular cAMP levels (827). interest of conciseness, their physiological effects will only be discussed briefly at this point. A) SECRETIN. Secretin is a 27-amino acid peptide hormone that is synthesized in duodenal S-cells and secreted into the circulation in response to a low duodenal pH or passage of digestive products, such as fat (195, 955, 1153). A subpopulation of secretin-producing cells is also present in the gastric mucosa, where it may influence acid secretion in a paracrine manner (191–193). Given its secretory stimulus, it is regarded as a classic effector of the intestinal phase of acid secretion. When it was first discovered in 1902 by Bayliss and Starling (interestingly secretin was the first hormone ever to be discovered), it was noted that secretin induces pancreatic bicarbonate secretion, which leads to a buffering of the gastric acid entering the duodenum (68). In the stomach, secretin acts as an inhibitor of gastric motility and acid secretion (141, 194, 269, 374, 532, 564, 656, 677, 1107). The exact mechanism as to how secretin attenuates the secretion of acid is not exactly known, and several hypotheses have been put forward. For example, it has been shown that secretin induces the secretion of somatostatin from isolated D-cells (141). Increases in somatostatin levels were also observed in isolated perfused stomach models (205, 374). Others have proposed that secretin activates vagal primary afferent neurons, which in turn leads to neuronal modulation of acid secretion (656, 659). In opposition to this theory, it has also been demonstrated that the inhibitory effects of secretin are independent of vagotomy (677). B) OXYNTOMODULIN. Oxyntomodulin is a peptide hormone produced in the mammalian intestine. It is closely related to glucagon and contains its entire amino acid sequence, extended by a COOH-terminal octapeptide (66). In isolated parietal cells, oxyntomodulin acts as an activator of acid secretion (959). The integrated response to oxyntomodulin is, however, opposite. Systemic injection decreases gastric acid secretion in rat, cat, and human test subjects (53, 157, 285, 524, 525, 965). The inhibitory effect is most likely mediated via somatostatin release (53). In conclusion, somatostatin acts as the global brake on acid secretion. By acting on G-cells, ECL cells, and parietal cells, it exerts its inhibitory action on every link in the regulatory chain leading to the secretion of gastric acid. It was recognized in the early 1950s that serotonin was present in the antral mucosa of dog stomachs (323). Serotonin is stored in granules of enterochromaffin cells of the antrum (1099). It is released into the circulation and the gastric lumen in response to vagal stimulation (107, 649). Intraluminal acidification serves as another stimulus for serotonin release (1196). Serotonin has an inhibitory effect on the secretion of gastric acid (107, 153, 521, 650, 720, 903). It is still poorly understood where serotonin interferes with acid secretion. 5. Other substances D) NEUROTENSIN. A variety of other substances have been shown to have either direct or indirect effects on acid secretion. In the 200 C) SEROTONIN. Neurotensin is a 13-amino acid neuropeptide that was originally isolated from calf hypothalamus (161). In the periphery, it is also produced and secreted postprandially by specialized endocrine cells (N-cells) of the Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH small intestine (863). Various investigations have demonstrated that neurotensin suppresses the secretion of gastric acid and delays gastric emptying (25, 108, 486, 985). This has been shown by direct systemic injection, but also by immunoneutralization of endogenous neurotensin in a reverse approach (25, 108, 486, 985). In disagreement with these findings, other investigators could only inhibit acid secretion at unphysiologically high serum concentrations of ⬃750 pmol (747). Of note, physiological postprandial neurotensin levels were measured to be ⬃15 pmol by the same investigators, questioning the role of neurotensin as a physiological endocrine inhibitor of acid secretion (747). Neurotensin is also located to nerve fibers of the enteric nervous system in the stomach, indicating that it may act as a local neuronal rather than an endocrine regulator. It has been proposed that neurotensin may induce the secretion of somatostatin and thereby exert its inhibitory action on acid secretion (53, 414). Most recently, however, the low-affinity neurotenstin type 2 receptor (NTS2) has been identified on the parietal cell, suggesting a direct influence. E) GHRELIN. Ghrelin is a recently discovered 28-amino acid peptide hormone that is synthesized in P/D1 cells of the fundus (242). Since its discovery, multiple functions have been ascribed to it, ranging from being a regulator of appetite to being a modulator of bone remodeling. Its effects on bone are described in a separate section of this review (see sect. VD1). Apart from these functions, ghrelin also has been implicated to affect gastric acid secretion, although it remains a matter of discussion in which direction, as peripherally administered ghrelin has been reported to stimulate, inhibit, or not affect acid secretion (278, 355, 654, 719). The reason for these dichotomic results is largely unclear. The fact that ghrelin circulates in acylated and desacylated forms adds further complexity to the subject (491). Indeed, acylated ghrelin has been shown to stimulate acid secretion following peripheral injection, whereas desacylated ghrelin remained without effect (938). Further investigations are needed to clarify the controversy surrounding ghrelin and its influence on gastric acid secretion. F) NITRIC OXIDE. Nitric oxide (NO) is an important signaling molecule that plays a role in multiple physiological processes, such as vasodilation or the immune response. Indeed, NO has been shown to mediate the hyperemic response of the gastric mucosa that occurs during acid secretion (553). However, NO also directly influences the production of acid. The effect of NO on acid secretion is most likely inhibitory (81, 82, 555, 1002; opposed by Ref. 426). NO is produced by various forms of NO synthases, one of which has been localized at high concentrations in cells in the vicinity of parietal cells, allowing for a putative paracrine regulation (80). NO has been proposed to exert its inhibitory action by either directly inhibiting the parietal cell or by suppressing the release of histamine from ECL cells (81, 82, 555, 1002). Intracellular increases in cGMP concentrations have been observed in both cell types after NO exposure, suggesting that guanylate cyclase is an intracellular target for NO (82, 1002). G) INTERLEUKINS. Interleukins (IL) are cytokines that mainly coordinate immune responses. In particular, IL-1 has been shown to impact gastric acid secretion. IL-1 is a general proinflammatory cytokine that plays an important role in the stomach in the context of Helicobacter pylori infection. H. pylori infection triggers an elevation of IL-1 levels as part of the host’s immune response (65). Peripheral injection of IL-1 can profoundly suppress gastric acid secretion (912, 947, 1059, 1101, 1132). Multiple explanations for this observation have been put forward. It has been suggested the IL-1 acts in the CNS, as intrathecal injection also has an acid-suppressive effect (948, 949). Others have suggested that IL-1 promotes formation of prostaglandins or NO, which in turn inhibit acid secretion (312, 947, 1101). Yet, a direct effect on parietal cells and ECL cells is the most likely explanation, as both cell types express the IL-1 receptor and have been shown to be inhibited in their function in isolated cell models (69, 70, 872, 958). C. The Pharmacological Suppression of Acid Secretion Decreasing gastric acidity is indicated in many pathological contexts, including gastric reflux disease or peptic ulcer disease. This target can be achieved by two main pharmacological approaches: 1) the inhibition of gastric acid secretion or 2) the intraluminal neutralization of already secreted gastric acid (antacids). Gastric acid secretion can be attenuated by either directly blocking its final molecular effector, namely, H⫹-K⫹-ATPase (PPIs and acid pump antagonists), or by interfering with the neurohormonal signaling pathway leading to its secretion (H2 antagonists). The following section attempts to discuss the four most common substance classes employed to increase intragastric pH. 1. Direct pharmacological inhibition of H⫹-K⫹-ATPase The inhibition of H⫹-K⫹-ATPase-mediated proton transport represents the main contemporary pharmacological strategy for reducing gastric acidity. An increase of gastric pH is the main factor ameliorating acid-related disorders and has been show to directly correlate with healing rates of, for example, GERD (74). Two main substance groups exert their acid-reducing effect via inhibiting H⫹-K⫹-ATPase function: PPIs and acid pump antagonists (APAs). Both substances achieve this aim by distinct mechanisms. Omeprazole was the first clinically available PPI (324). The first patent on omeprazole was filed in 1979 by the Swedish company Astra AB (today AstraZeneca). The introduction as a prescription PPI followed in 1989. Today, the omeprazole enantiomer esomeprazole (S-omeprazole) generates the Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 201 SASCHA KOPIC AND JOHN P. GEIBEL second highest revenue of all pharmaceuticals in the United States and is only surpassed by the statin atorvastatin (512). Furthermore, in the United States, PPIs are available as over-the-counter formulations, making them accessible for the broad public. This is partially made possible by the high safety profile of PPIs with a low incidence of unspecific adverse effects. Recently, however, concerns about the long-term effects of chronic acid suppression have emerged with regard to its impact on bone health (see sect. VA). PPIs are delivered as pro-drugs through the bloodstream to the parietal cell. They are weak bases (pKa ⬃4), which can easily pass the cell membrane and accumulate in acidic compartments, such as the secretory canaliculus of the parietal cell. The pro-drug is then converted to the pharmacologically active cyclic sulphenamide by the acidic pH in the secretory canaliculus (670, 1007, 1135). Their specific accumulation in acidic milieus and their pH-catalyzed conversion to active substances confers specificity and thus a high safety profile to PPIs. Once activated, PPIs bind covalently via disulfide bonds to H⫹-K⫹-ATPase, thereby inhibiting its capacity to pump protons (89, 90, 1005, 1006, 1008). The pattern of the cysteine residues, which are involved in PPI binding, differ among the respective members of the PPI family: cysteine-813 reacts with all PPIs. In addition, omeprazole reacts with cysteine-892, lansoprazole with cysteine-321, and pantoprazole and tenatoprazole, respectively, with cysteine-822 (89, 90, 1005, 1006, 1008). Since the binding is covalent and irreversible, the inhibitory effect of PPIs lasts long beyond their plasma half-life, which usually ranges between 0.5 and 2 h depending on the specific PPI (581, 1036). PPIs are generally metabolized by the hepatic cytochrome P-450 system, in particular CYP2C19 and CYP3A4. This is of particular clinical importance, as CYP2C19 polymorphisms are known to exist. These polymorphisms can impact the pharmacokinetics of PPIs by affecting the metabolic rate of CYP2C19, which may have consequences for the optimal therapeutic regimen (582). Esomeprazole and rabeprazole seem to be less dependent on CYP2C19 metabolism (516, 983). Apart from the pattern of cysteine reactivity, half-life and metabolism, PPIs also vary in oral bioavailability (581). Suppression of acid secretion can never be complete, as H⫹-K⫹-ATPase is subjected to a constant turnover (half-life ⬃50 h) and needs to be stimulated for the conversion of the PPI to take place (936). Nevertheless, PPIs are highly effective in reducing gastric acidity. Depending on the PPI and the regimen, overall intragastric pH can be elevated by several pH units, up to a pH of 6 (compared with 1–2 at baseline) (137, 425, 1036). For an excellent summary of PPI efficacy, please refer to Reference 1036. APAs represent the second class of H⫹-K⫹-ATPase inhibitors. Unlike PPIs, they do not undergo irreversible binding, but rather act as potassium competitive antagonists. The 202 duration of inhibition is thus directly dependent on the plasma concentration of the inhibitor. As predicted by homology modeling, mutational analysis, but also recent structural data, APAs bind in the luminal cavity of H⫹-K⫹ATPase in the vicinity of the potassium entry site where they exert their inhibitory action (2, 42, 761, 1104, 1106). Although the inhibition of acid secretion has been shown to be very effective, these substances are generally not in clinical use (577). For example, clinical trials of the APA AZD08650 have shown no additional therapeutic effect compared with the PPI gold-standard, which resulted in abandonment of the drug in a clinical setting (262, 539). 2. H2 antagonists The development of H2 blockers is inseparably intertwined with Sir Black’s discovery of the H2 receptor on the gastric parietal cell at the Smith Kline and French Laboratories (now GlaxoSmithKline) (106). In his original publication, Sir Black also describes burimamide as a competitive H2 antagonist that can effectively inhibit pentagastrin-stimulated gastric acid output in human volunteers (106). Further development of the antagonist led to the synthesis of cimetidine, which was first commercially introduced in 1976 in the United Kingdom, followed by the United States in 1977. Other commonly used members of H2-antagonist family now include ranitidine, famotidine, and nizatidine. H2 antagonists prevent histamine-mediated stimulation of the parietal cell by competitively interfering with its receptor. Although this effectively terminates the gastrin-histamine axis, the parietal cell is still susceptible to cholinergic stimulation via the M3 receptor. This partial inhibition mainly accounts for the lower clinical efficacy of H2 antagonists compared with PPIs, which directly target H⫹-K⫹ATPase as the final target of all parietal cell stimuli (384). For example, a meta-analysis concluded that patients treated for bleeding peptic ulcers are about twice as likely to suffer from persistent or recurrent bleeding if treated with H2 antagonists compared with PPIs (384). Another metaanalysis also demonstrated a higher efficacy of PPIs in treating esophagitis (83% healing rate with PPIs compared with 52% with H2 antagonists)(567). Today, H2 antagonists are largely superseded by PPIs due to their higher clinical efficacy. Furthermore, with the exception of famotidine, H2 antagonists are extensively metabolized in the liver by the CYP-450 system, leading to substantial drug-drug interaction profile (for review, see Ref. 506). 3. Antacids Antacids directly neutralize gastric acid allowing immediate short-term control of heartburn. They exist in various salt formulations, the most common of which are carbonate salts, such as CaCO3, MgCO3, or NaHCO3. The use of calcium carbonate as a dietary calcium supplement is dis- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH cussed in a separate section (see sect. VC). Although each formulation possesses its own spectrum of side effects, a notable condition in the context of this review is the milkalkali syndrome, which is the result of a concomitant overingestion of calcium and alkali, such as CaCO3. Although the syndrome became less prevalent with the introduction of modern ulcer therapies, it still poses a significant risk for patients that may ingest CaCO3 as a calcium supplement for the prevention of osteoporosis or as an antacid on a regular basis. Milk-alkali syndrome presents with the triad of metabolic alkalosis (carbonate), hpercalcemia (calcium), and renal insufficiency. The above average ingestion of calcium leads to increased plasma calcium levels due to excess absorption and impaired renal secretion. PTH levels are low due to negative feedback (6). Hypercalcemia further causes renal vasoconstriction, which decreases the glomerular filtration rate, and renal fluid loss because of activation of the CaSR, which in turn has loop-diuretic-like effects (see sect. IVD3). The activation of the CaSR is further potentiated by the metabolic alkalosis, which increases its sensitivity to calcium. All abnormalities are usually reversible after withdrawal of the offending agent and adequate treatment. III. INTESTINAL CALCIUM ABSORPTION The intestine is responsible for the absorption of dietary calcium into our systemic circulation. Although the kidney also plays a pivotal role in calcium homeostasis by retaining and balancing systemic calcium via regulating its excretion into the urine, renal calcium handling will not be the subject of this review. Current recommendations suggest that an average 40-yrold adult should ingest ⬃1,000 mg of calcium on a daily basis (218). In the United States, this requirement is mostly met (57). Up to 72% of the dietary calcium intake is attributable to dairy products (218). Typically, the intestine absorbs between 25 and 35% of the ingested calcium (1193). This occurs via two distinct pathways: 1) a paracellular pathway and 2) a transcellular pathway. Since a concentration gradient is not a prerequisite for this process, transcellular transport allows us to absorb calcium even when the calcium concentration in the chyme is fairly low. The relative importance of each respective absorption pathway thus alternates with the amount of ingested calcium (46, 824, 1224). The rate of paracellular calcium uptake is canonically thought to remain constant, while transcellular transport can be upregulated under conditions of dietary calcium restriction (46, 824, 1224). This regulation occurs via the active metabolite of vitamin D [1,25(OH)2vitamin D], which serves as a stimulator for transcellular calcium uptake to prevent systemic calcium depletion. A. Transcellular Calcium Absorption 1. Calcium entry Evidence for active transport of calcium across the intestinal epithelium was established very early. With the use of a calcium radioisotope, various groups demonstrated 1,25(OH)2vitamin D-dependent calcium transport against an imposed concentration gradient in the small intestine of the rat (952, 954, 1150). It has also been observed that active transport can be induced by a low-calcium diet, which we now know to stimulate the production of 1,25(OH)2-vitamin D (1133, 1134). The degree of transport was highest in the duodenum and decreased in the more distal segments (952). The duodenum is still considered the primary site where the bulk of transcellular transport occurs. To conduct active transcellular calcium absorption, the enterocyte has to be equipped with an apical calcium entry pathway, a mechanism for cytosolic calcium shuttling, and a basolateral calcium exit pathway (FIGURE 3). As the cytosolic concentration of free calcium is kept at a constant low level with typical concentrations of ⬃100 nM, apical calcium influx follows its electrochemical gradient into the cell. In contrast, the extrusion of calcium on the basolateral membrane against an uphill gradient either directly requires ATP or energy stored in the sodium gradient. A) THE SEARCH FOR THE APICAL CALCIUM ENTRY PATHWAY. Calcium absorption via the paracellular route is tied to a downhill concentration gradient between the luminal and the extracellular compartment and occurs throughout the entire intestine (although solvent drag induced paracellular flux may also play a role at low luminal calcium concentrations; Refs. 266, 1071, 1098). Conversely, transcellular absorption can also take place against an uphill gradient, but requires molecular machinery in the form of distinct calcium transport proteins which are expressed on the apical and basolateral membranes of the enterocyte. This process directly requires energy in the form of hydrolyzable ATP and is alternatively termed “active” transport (versus “passive” paracellular transport). The proximal small intestine, i.e., the duodenum and the jejunum, is the main site for transcellular calcium absorption (825). The molecular identity of the apical calcium entry pathway was unclear for a long time. Early experiments in isolated duodenal brush-border vesicles revealed that calcium uptake was passive, saturable, sensitive to ruthenium red, 1,25(OH)2-vitamin D dependent, and functionally optimal at a pH of 7.5 (740). This black box characterization suggested that a “specific carrier” was responsible for calcium absorption and in retrospect already provided us with accurate key characteristics of the transient receptor potential vanilloid channel type 6 (TRPV6), which was later established as the primary apical calcium uptake channel (740). In subsequent attempts to further unravel the nature of the calcium uptake mechanism, various voltage-gated L-type calcium channel blockers were used (474, 717, 838). Although isolated duodenal cells accumulated less calcium following application Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 203 SASCHA KOPIC AND JOHN P. GEIBEL Enterocyte D D VDR D RXR VDR Transcription Ca2+ PMCA Transcription Ca2+ Ca2+ Na+ Ca2+ Calbindin-D 9k NCX TRPV6 Ca2+ D FIGURE 3. Transcellular and paracellular calcium absorption in the intestine. The transcellular intestinal absorption of calcium relies on apical calcium entry through TRPV6, intracellular calcium transport by calbindin-D9k, and basolateral calcium extrusion via either NCX or PMCA. 1,25(OH)2-vitamin D regulates most of these ion transport proteins on a transcriptional level. 1,25(OH)2-vitamin D passes the plasma membrane of the enterocyte and binds to its receptor (VDR), which then heterodimerizes with RXR to initiate transcription. Evidence also suggests that 1,25(OH)2-vitamin D regulates the permeability of tight junctions, which gate the paracellular absorption of calcium. D, 1,25(OH)2-vitamin D. of the inhibitors and 1,25(OH)2-vitamin D stimulation (717), in vivo calcium entry proved to be fairly insensitive to their effects (with the exception of verapamil, which demonstrated some degree of inhibition if applied at very high concentration in the millimolar range) (474, 838). The conflicting reports may be attributable to the different experimental models that were used, as isolated single cells do not allow discrimination between apical and basolateral transport mechanisms. Furthermore, it has been argued that Ltype calcium channels may play a role in the stimulatory pathway of vitamin D, rather than in calcium uptake per se (717). In conclusion, an involvement of L-type calcium channels seemed rather inconclusive and the identity of the calcium entry channel remained elusive. B) TRPV6. The cloning of the calcium transport protein subtype 1 (CaT1) in 1999 finally marked a turning point in the search for the elusive intestinal calcium entry channels (838). The work was pioneered by Hoenderop and colleagues who had identified the main calcium entry protein in the kidney (epithelial calcium channel type 1, ECaC) via an expression cloning strategy a few months earlier (474). 204 CaT1 was identified by a similar approach. A rat duodenal cDNA library was functionally screened using a calcium uptake assay in a Xenopus oocyte expression system (838). This screening process yielded the 727-amino acid protein CaT1, which showed a 75% sequence homology to rabbit ECaC. Homology analysis also demonstrated a relationship to the vanilloid reptor type 1 (VR1), a nonspecific cation channel that is activated by capsaicin, the pungent ingredient in chili peppers, and mostly mediates pain signaling through afferent sensory neurons (838). The nomenclature changed over time as new channel proteins were identified, and today we consider CaT1, ECaC, and VR1 to be members of the same transient potential receptor vanilloid (TRPV) ion channel family. Literature now refers to ECaC as TRPV5, to CaT1 as TRPV6, and to VR1 as TRPV1. The structure of TRPV6 was predicted to have six transmembrane domains and four ankyrin repeat domains, which serve as cytoskeletal linking sites (838). Channel conductance was not dependent on other ions and was inhibitable by a low extracellular pH (838). Calcium uptake was reduced by as much as ⬃70% at a pH of 5.5, which con- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH firmed the early black box data observations made in intestinal brush-border vesicles (838). This behavior seemed counterintuitive to the investigators, as TRPV6 expression was highest in the duodenum, which is exposed to an acid load from the stomach (838). Duodenal pH has been reported to be as low as ⬃6.1– 6.6 but is even lower acutely after gastric emptying (⬃5.4), which would entail significant inhibition of TRPV6-mediated calcium uptake (284, 290, 838). Conductance was also modestly sensitive (10 – 15% inhibition) to L-type calcium channel blockers at high concentrations, which partially clarified the preceding ambiguous observations made by other groups with these inhibitors (318, 337, 717, 838). Subsequently, the human analog of rat TRPV6 was cloned (it had 97% sequence homology and was rather confusingly named ECaC2), and its expression was confirmed in human duodenum (64). The generation of a TRPV6 antibody allowed for first localization studies, which confirmed an apical localization of TRPV6 and thus reaffirmed its role as the primary apical calcium entry pathway in the intestine (1220). Tissue expression of TRPV6 varies among species (461). In humans, TRPV6 was identified in the duodenum, jejunum, stomach, esophagus, kidney, placenta, mammary gland, pancreas, prostate, testis, and salivary gland, but was also found to be upregulated in a series of malignancies, including prostate, breast, colon, and ovarian cancer (461, 792, 836, 839, 1168, 1220). The role of TRPV6 in these tissues mostly remains to be elucidated. TRPV6 displays some unique biophysical properties. As mentioned earlier, TRPV6 consists of six transmembrane segments, like many voltage-gated cation channels. Unlike these channels, TRPV6 lacks the voltage sensor domain in the fourth ␣-helix and is in a constitutively open state at resting membrane potential. Analysis of the quarterny structure shows that it assembles in tetramers (476). The ankyrin domains were implicated to be responsible for tetramer formation; however, more recent structural data obtained by crystallography question this hypothesis (311, 848). It is thought that heterotetramers can also be formed with subunits of closely related TRPV5 (476). In contrast to most other members of the TRP channel family, TRPV6 is a very selective channel for calcium (475, 1058, 1197). The relative permeability of TRPV6 for calcium over sodium (PCa/PNa) is ⬎100, whereas TRPV1, for example, has a selectivity of PCa/PNa ⬃10 (although this has recently been shown to be variable) (206, 475, 1197). This high selectivity is crucial for the maintenance of a constant membrane potential in the enterocyte during calcium absorption. TRPV6 is strongly inward rectifying, which has been attributed to magnesium ions plugging the channel pore for outward ion movement during states of depolarization (1126). Magnesium also exerts a voltage-independent inhibitory effect on TRPV6 current; however, the mechanism underlying this observation is unclear (475, 1126). Furthermore, TRPV6 gating is sensitive to intracellular calcium. Increases in calcium were shown to inhibit the channel, resembling a negative-feedback mechanism (475). Although global calcium concentrations in the cell largely remain constant, the channel microenvironment is exposed to fluctuations in local calcium. This feedback loop may be important for finely tuning the amount of calcium influx and preventing calcium overload of the enterocyte. The putative mechanism of this regulation will be discussed later in this section. In 2001 it was demonstrated that ATP modulates TRPV6 activity by preventing channel rundown (475). Recent work by Al-Ansary et al. (15) suggests that ATP directly binds to the channel, thereby inhibiting inactivation by locking the channel in the open conformation. Binding of ATP may be antagonized by channel phosphorylation through PKC (15). In conclusion, TRPV6 is precisely regulated by its own microenvironment. Calcium, magnesium, and protons have an inhibitory effect on the channel, whereas ATP prevents channel inactivation. It is important to remember that apart from being a nutrient, calcium is a crucial intracellular messaging molecule. Therefore, the enterocyte has to tightly control its intracellular concentration and adapt uptake to energy status and basolateral extrusion. To ensure this delicate intracellular homeostasis, TRPV6 is associated with and regulated by a variety of auxiliary proteins. The first protein that has been identified to interact with TRPV6 was the S100A10-annexin2 complex (1112). The S100A10-annexin2 complex has been implicated to play a role in protein trafficking and membrane anchoring. Its association with TRPV6 is essential for channel trafficking to occur, as a disruption of the interaction leads to cytosolic scattering of the channel (1112). Formation of the S100A10-annexin2 complex and its association with TRPV6 involves activation of PKA and calcineurinA (CnA) (117). Another protein that is required for TRPV6 membrane insertion is rab11a (1111). In analogy to S100A10-annexin2, perturbation of rab11a binding results in decreased surface expression of TRPV6 and channel retention in the cytosol (1111). Furthermore, the protein kinases SGK1 and WNK3 are known to promote membrane insertion of TRPV6; however, their exact site of action is still unclear (114, 1031). Once trafficked to the membrane, physical channel stability is maintained by a variety of auxiliary proteins. The COOH-terminal tail of TRPV6 contains a PDZ protein binding motif. PDZ proteins serve as membrane anchors and protein scaffolds and mediate the assembly of multiprotein complexes, thereby regulating channel activity. The PDZ protein sodium hydrogen exchanger regulating factor (NHERF4) (aka PDZK2) has recently been identified to interact with TRPV6 (574, 1113). It has been speculated that NHERF4 serves as a scaffold for TRPV6 at the apical pole, which is underlined by the observation that knockdown of NHERF4 by RNAi leads to decreased TRPV6 current in HEK293 cells (574, 1113). Apart Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 205 SASCHA KOPIC AND JOHN P. GEIBEL from trafficking to the apical membrane, channel number can be regulated by internalization and degradation. One possibility of decreasing functional channels at the cell surface is protein ubiquitination. The process of ubiquitination involves enzymatic tagging of target proteins, thus directing them to the proteasome or lysosome for rapid degradation. The degradation tag is transferred to the target protein by E3 ubiquitin protein ligases, such as Nedd4 –2. It is already well understood how Nedd4 –2-mediated ubiquitination can decrease the number, but also directly modulate the activity, of the epithelial sodium channel (ENaC) in the kidney (825). A recent study demonstrated that a similar mechanism applies to TRPV6 (1212). Coexpression of Nedd4 –2 and TRPV6 in oocytes resulted in decreased calcium flux and channel numbers (1212). Nedd4 –2-mediated TRPV6 ubiquitination could thus serve as a mechanism of rapidly regulating channel retrieval from the plasma membrane (1212). Several other proteins have been identified to associate with TRPV6 that do not affect channel numbers, but rather directly regulate channel activity. The nisnap1 gene was identified in the late 1990s; however, its function remained elusive (992). Recently, TRPV6 was shown to interact with nisnap1 (971). It is expressed in the intestine, but not the kidney, which is unusual as TRPV6 and TRPV5 are generally regulated by similar auxiliary proteins. Electrophysiological measurements showed that nisnap1 inhibits TRPV6 without affecting its surface expression (971). Very similar functional properties were attributed to RGS2, a protein that is mainly known to alter the GTPase activity of G proteins. In analogy to nisnap1, RGS2 can inhibit TRPV6 at the plasma membrane without affecting its trafficking dynamics (970). As previously discussed, the activity of TRPV6 is finely regulated by intracellular calcium concentrations. Elevations in intracellular calcium levels exert an autoinhibitory effect on channel opening (475). Shortly after the identification of TRPV6, calmodulin (CaM) was shown to bind to the channel in a calcium-dependent manner, and it was speculated that it mediates the calcium feedback response (461, 791). Subsequent investigations demonstrated that CaM may indeed represent the molecular calcium sensor (263, 619). Elegant FRET studies reported that CaM dynamically associates with TRPV6 in the presence of calcium and that this association is terminated when intracellular calcium is depleted (263). The association between TRPV6 and CaM also correlates with decreased current flux through the channel (263). Although the ankyrin repeat domains of the channel were thought to possibly serve as binding sites for CaM, more recent structural insights provide rebutting evidence for this hypothesis (848). Instead, the COOH-terminal tail of TRPV6 has been suggested as the site where CaM binding occurs (263, 619, 791). Hence, CaM can tune ion flux through TRPV6 and 206 most likely mediates the channel’s sensitivity to intracellular calcium. Phosphorylation by kinases and dephosphorylation by phosphatases represent a common cellular strategy for rapidly modulating channel gating properties. The non-receptor tyrosine kinase Src has emerged as a candidate for the direct phosphorylation of TRPV6, thereby increasing channel conductance (1042). Src was previously shown to modulate TRPV4 activity (1178). The effects of Src are antagonized by the phosphatase PTP1B. Both enzymes act on the tyrosine residues Y161/162, which are located in the NH2terminal tail of the channel (1041). An interesting interaction without direct relevance for the intestine has been reported between renal TRPV5 and klotho (170). Klotho is a -glucuronidase with a transmembrane anchor that can be cleaved, resulting in shedding of the enzymatically active domain of the protein into the urine (170, 612). Klotho can then increase TRPV5-mediated calcium uptake by hydrolyzing N-linked oligosaccharides on extracellular channel domains, thereby preventing channel retrieval from the plasma membrane (170). Although a recent report indicates that klotho can also affect TRPV6 activity in vitro, it is not expressed in the intestine and thus may only interact with renal TRPV6 (612, 683). Although this finding has no implications for intestinal calcium uptake, various bacteria and neutrophils produce -glucuronidase, which may affect TRPV6 activity in the intestine (359, 1004). Recently, Stumpf et al. (1046) described an association between cyclophilin B (CyB) and TRPV6 in the placenta. Coexpression of both proteins in oocytes increased calcium uptake (1046). CyB was also detected by Western blot analysis in the small intestine and colon; however, colocalization and functional studies in intestinal tissue remain to be performed (1046). C) THE TRPV6 KNOCKOUT CONUNDRUM. As will be discussed in more detail later, 1,25(OH)2-vitamin D is one of the key hormonal regulators of systemic calcium homeostasis (see sect. IVA). Increased levels of 1,25(OH)2-vitamin D lead to enhanced intestinal calcium absorption. Shortly after cloning of TRPV6, it was recognized that the channel is positively regulated at the mRNA level by 1,25(OH)2-vitamin D (614, 1030, 1109, 1110). The TRPV6 promoter has multiple binding sites for the 1,25(OH)2-vitamin D receptor (VDR) and is thus directly sensitive to increases in 1,25(OH)2-vitamin D levels (736). Consequently, VDR-deficient animals display a marked decrease in TRPV6 mRNA levels, whereas administration of 1,25(OH)2-vitamin D in wild-type animals results in an increase in mRNA transcription (1030, 1109, 1110). In conjunction with the rapidly expanding characterization of the channel itself, these observations supported the dogma that TRPV6 is the essential player Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH in transcellular calcium uptake and that this pathway is strongly regulated by 1,25(OH)2-vitamin D. With the introduction of novel genetic techniques, a TRPV6 (⫺/⫺) mouse was created in 2007 by Bianco et al. (94). The animals presented with decreased intestinal calcium uptake, as measured by serum concentrations of a calcium radioisotope following gavage, decreased femoral bone density (9.3%) and increased 1,25(OH)2-vitamin D levels, as a result of feedback regulation (94). These findings were in accordance with the postulated role of TRPV6 as the primary 1,25(OH)2-vitamin D-sensitive calcium uptake mechanism in the intestine. However, the generation of another TRPV6 (⫺/⫺) animal line by Benn et al. (76) a year later spawned a controversy in the field. In these animals, baseline calcium uptake was identical between wild-type and (⫺/⫺) groups. Surprisingly, calcium uptake could be increased in the (⫺/⫺) group by a low calcium diet and, more importantly, by 1,25(OH)2-vitamin D (an observation that was also confirmed by another group; Ref. 615) (76). These findings profoundly questioned the role of TRPV6 in calcium absorption and suggested that a different molecular target of 1,25(OH)2-vitamin D mediates the increase in calcium uptake after exposure. All these observations were in sharp contrast to the initial report by Bianco et al. (94). However, Benn et al. (76) also reported that compared with wild-type animals calcium uptake was reduced in TRPV6deficient animals that were fed a low-calcium diet. This suggests that the animals may not be able to adequately increase absorption, when confronted with a low dietary availability of calcium, which in turn suggests a role of TRPV6 during states of dietary calcium insufficiency. These controversial findings allow several conclusions and speculations. 1) As with any model of targeted gene disruption, a compensatory mechanism may be in place that masks and distorts the physiological importance of TRPV6. The animals may upregulate other, yet unidentified, calcium transport mechanisms to compensate for the loss of TRPV6 function. 2) Rather than being a constitutively active pathway, transcellular calcium absorption through TRPV6 only occurs in states of low dietary calcium intake. This hypothesis has been put forward very early and has been reaffirmed by a recent study that observed an increase in bone turnover in TRPV6 (⫺/⫺) animals on a low-calcium diet (46, 666, 824, 1224). Mobilization of bone calcium may be necessary in these animals to maintain systemic calcium concentrations, which are double challenged by the lack of TRPV6 and the insufficient calcium intake (666). Furthermore, it has been extensively described that a low-calcium diet induces gene expression of TRPV6 [presumably through an increase in 1,25(OH)2-vitamin D levels], suggesting a role of the channel during states of insufficient dietary calcium supply (76, 132, 639). 3) 1,25(OH)2-vitamin D may have many more targets in the intestinal mucosa than previously anticipated and may also regulate calcium uptake through the paracellular pathway (76, 615). In conclusion, the generation of TRPV6 (⫺/⫺) animals has sustainably challenged our model of transcellular calcium absorption by questioning the relative importance of TRPV6 and by introducing new potential targets of 1,25(OH)2vitamin D regulation. D) GENETIC POLYMORPHISMS OF TRPV6. Single nucleotide polymorphisms (SNPs) are variations in the genomic sequence that occur in one single base. If the frequency of a SNP or a specific set of SNPs (haplotype) increases in a population over time compared with other SNPs, it can be concluded that this set of SNPs is associated with an evolutionary advantage, meaning that this gene locus was under selection. Recent reports from various groups indicate that TRPV6 was subjected to strong selection (12, 502, 1023, 1050). Traces for selection can be found in any non-African population (12, 502, 1023). The selective event in Europeans has presumably occurred ⬃7,000 years ago and coincides with the development of agriculture (502). This correlation also holds true for other populations (502). It has been speculated that a change in diet or resistance to emerging disease led to selection and fixation of the now conserved haplotype (502). Interestingly, selection took place in parallel in many populations, pointing towards a strong selective stress that developed independently in each region (502). The electrophysiological properties of ancestral and derived TRPV6 were investigated by two groups (502, 1050). Hughes et al. (502) observed no statistically significant divergence in channel behavior. The derived channel only displayed a tendency towards decreased sensitivity to the autoinhibition by calcium, albeit not significant (P ⫽ ⫺0.094) (502). The authors speculated that differences in protein-protein interactions may have led to selection (502). Conversely, Sudo et al. (1050) reported increased calcium influx through the derived channel, which may constitute an evolutionary advantage by facilitating dietary calcium uptake. Regardless of the controversial functional data, these insights indirectly underline the importance of TRPV6 and provide a counterweight to the conclusions drawn from the TRPV6 (⫺/⫺) animal model. It is unquestionable that TRPV6 was under parallel independent selection in many regions across the planet. This provokes the question of how strong selection can occur for a derived channel whose physiological difference to the ancestral form seems to be very subtle, yet complete disruption of the channel in the mouse model only causes a very mild phenotype. This inconsistency adequately exemplifies the caveat that underlies the conclusions drawn from (⫺/⫺) animals and reminds us of the fact that we are far from understanding the exact physiology of transcellular calcium uptake. E) CAV1.3. TRPV6 may not be the only channel mediating apical calcium absorption in the intestine. A recent investi- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 207 SASCHA KOPIC AND JOHN P. GEIBEL gation provides another explanation for the initially observed partial sensitivity of transcellular calcium uptake to L-type calcium channel blockers. The voltage-gated calcium channel Cav1.3, a member of the L-type calcium channel family, was recently identified in the apical membranes of the distal jejunum and proximal ileum (751). Previous observations were emulated, as the investigators again demonstrated a decrease in calcium absorption following application of L-type calcium channel inhibitors in the corresponding segments (751). The authors argued that uptake through Cav1.3 may have previously been misinterpreted as paracellular calcium uptake (751). However, it should be noted that the calcium uptake assay used in this report did not discriminate between transcellular and paracellular calcium movement. Subsequently, it has been observed that L-type inhibitor-sensitive calcium flux is linked to stimulation of glucose uptake through the glucose transporter type 2 (GLUT2) (692, 750). Cav1.3 may serve as an alternative calcium entry pathway that is active in states of luminal calcium abundance and that coregulates glucose absorption (692, 750). Further investigations will be needed to determine the contribution of Cav1.3 to dietary calcium absorption. 2. Calbindin-D 9k The following section will address the question of what happens to dietary calcium after it enters the enterocyte. It should be considered that transcellular calcium uptake and cytosolic calcium homeostasis are two contradicting requirements for the enterocyte. A prominent transcellular flux of free calcium will inevitably interfere with housekeeping functions of the cell, such as intracellular signaling. Furthermore, it has been calculated from in vivo data that if calcium were to diffuse freely (simple diffusion) through the cytosol, uptake rates would only be 1/70th of the actually measured values (129). Simple diffusion would constitute a bottleneck in the process of transcellular calcium absorption if cytosolic calcium concentrations should be kept low. A partial solution to this problem was found as early as 1966, when Wasserman et al. (1151) identified a 1,25(OH)2-vitamin D-inducible calcium binding protein in the chick intestine. The authors observed that calcium radioisotopes traveled faster across a cellophane membrane if suspended in intestinal homogenates from rachitic, i.e., 1,25(OH)2-vitamin D deficient, chicks than if suspended in the homogenates from rachitic animals treated with 1,25(OH)2-vitamin D (1151). This indicated that calcium was bound to a protein in the enterocyte and that expression of this protein was controlled by 1,25(OH)2-vitamin D (1151, 1152). Subsequently, the calcium binding protein was further characterized (1073, 1149, 1152). Expression levels were shown to be highest in the duodenum and to gradually decrease in more distal segments, which correlated with the degree of calcium uptake that has been attributed to each intestinal segment respectively in prior functional investigations (1073). The mammalian isoform, 208 which had a lower molecular mass of ⬃9 kDa (hence the name calbindin-D 9k), compared with the 28 kDa of the avian isoform, was later identified (138, 280, 283, 358). Concerning the functional role of calbindin-D 9k, it had already been speculated very early after its discovery that it may serve as a calcium shuttling protein (951). Indeed, initial calculations and later very basic experimental data confirmed that calbindin-D 9k may mediate facilitated diffusion of calcium between the two poles of the enterocyte, in analogy to the transport of oxygen by myoglobin in the muscle (321, 602). In a very fundamental investigation, calcium flux was measured between chambers that were separated by dialysis membranes. A 51% increase in transchamber calcium flux occurred in the presence of calbindin-D 9k (321). However, it is hard to relate this number to physiological values, given the simplification underlying the experimental model. It has subsequently been calculated that calbindin-D 9k may facilitate diffusion of calcium by a factor of up to ⬃60 (129). Rather than envisioning calbindin-D 9k as a protein that individually shuttles calcium ions across the cell, one should imagine calbindin-D 9k as a calcium gradient amplifier (129, 602). As the intracellular concentration of free calcium is in the nanomolar range, the intracellular gradient of free calcium between the apical and basolateral pole can also only be in the nanomolar range. Diffusional flux, however, directly correlates with the concentration gradient and can in consequence only be very small. Calbindin-D 9k is expressed in the enterocyte in the micromolar range and dynamically binds and releases calcium (714). Since the association between calbindin-D 9k and calcium is dynamic, the local concentration of calbindin-D 9k-bound calcium will directly correlate with the concentration of free calcium. It is thus the concentration gradient of calbindin-D 9k-bound calcium throughout the cell that determines the flux rate of calcium. As this gradient can be in the micromolar range, calbindin-D 9k serves as a calcium gradient amplifier (129, 602). It should be noted that the real experimental data on calbindin-D 9k are fairly scarce and that the bulk of scientific effort has gone into mathematical modeling of facilitated diffusion and calcium binding kinetics (129, 321, 322, 602, 1021). Functionally, several correlations between calbindin-D 9k and calcium uptake were identified. The calbindin-D 9k content of each intestinal segment correlates linearly with its ability for calcium uptake (129, 824, 1021). Although this relation holds true on the experimental and the mathematical modeling level, it does not prove causality (129, 824, 1021). Furthermore, 1,25(OH)2-vitamin D and a lowcalcium diet induce calbindin-D 9k on both the mRNA and protein level, suggesting that it is involved in the process of regulated calcium absorption (289, 604). In accordance with these findings, VDR-deficient animals have a decreased calbindin-D 9k mRNA content that cannot be rescued by exogenous 1,25(OH)2-vitamin D administration (663, 1194). Although a 1,25(OH)2-vitamin D responsive Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH element (VDRE) had been identified in the 5=-flanking region of the calbindin-D 9k gene, later mutational analysis demonstrated that this site was not essential for transcriptional regulation of calbindin-D 9k by 1,25(OH)2-vitamin D (217, 240). Hence, it is not clear how 1,25(OH)2-vitamin D exactly regulates calbindin-D 9k on a molecular level. Interestingly, intestinal calbindin-D 9k mRNA levels can be rescued in VDR (⫺/⫺) animals by a high-calcium/phosphorus/lactose diet, while extraintestinal calbindin-D 9k mRNA levels are unaffected (663). This suggests that, apart from endocrine regulation through 1,25(OH)2-vitamin D, intestinal calbindin-D 9k is also regulated by local factors (663). A direct short-term stimulatory effect of oral calcium intake on calbindin-D 9k levels had been observed before in a wild-type background (647). The mechanisms underlying the effect of diet on calbindin-D 9k levels are, however, still obscure. Despite the correlation between calbindin-D 9k levels and 1,25(OH)2-vitamin D and the mathematical and experimental models, which support its role as a calcium shuttling protein, no clear evidence for the involvement of calbindin-D 9k in transcellular calcium absorption existed. This had sparked controversy among investigators. In fact, a very early study concluded that although 1,25(OH)2-vitamin D induced both calbindin-D 9k and calcium absorption, a discrepancy existed in the time course of both effects (424). Later, synthetic 1,25(OH)2-vitamin D derivatives, designed for a maximized effect on cell differentiation and suppressed calciotropic activity, failed to increase serum calcium levels while causing a sevenfold increase in calbindin-D 9k mRNA [native 1,25(OH)2-vitamin D increases both serum calcium and calbindin-D 9k mRNA](604). In 2006, a calbindin-D 9k (⫺/⫺) mouse was created by Kutuzova et al. (613). The animals presented with no apparent phenotype abnormalities and had normal serum calcium concentrations (613). Subsequent analysis of these animals showed that they responded equally well to 1,25(OH)2vitamin D with regard to calcium absorption as wild-type animals (13). Of note, calcium absorption was assessed by measuring serum calcium following gavage of a calcium radioisotope and did not discriminate between transcellular and paracellular absorption (13). A different group replicated the findings made in the calbindin-D 9k (⫺/⫺) mouse and also did not find any appreciable variation in phenotype or serum calcium concentrations (639). However, the investigators observed that during preweaning, when both calcium demand and absorption are at their peak, gene expression of TRPV6 and the basolateral calcium extruder, plasma membrane calcium ATPase isoform 1b (PMCA1b), were highly induced in the calbindin-D 9k (⫺/⫺) animals (128, 639). This has been interpreted as a compensatory upregulation of calcium transport proteins to ensure sufficient uptake in a state of high metabolic demand for calcium (639). Subsequently, a TRPV6 and calbindin-D 9k double (⫺/⫺) animal was created. The mature animals show no disturbances in calcium homeostasis, although, similarly to TRPV 6 (⫺/⫺) animals, they cannot increase calcium uptake in response to a dietary calcium challenge to the same extent as wild-type animals (76). In light of the insights gained through knockout animals, the role of calbindin-D 9k remains somewhat unclear. Again, it is difficult to dissect the physiological function of calbindin-D 9k in these animal models, given the assumption that the organism will pursue compensation for the loss of a gene product. In conclusion, it is undisputed that calbindin-D 9k is regulated by 1,25(OH)2-vitamin D, that it can bind calcium and that theoretical modeling and very fundamental experimental models verify that it can facilitate diffusion of calcium across the enterocyte. The (⫺/⫺) models suggest that animals can compensate for the loss of calbindin-D 9k and maintain normal calcium homeostasis (13, 76, 613, 639). Furthermore, TRPV6, calbindin-D 9k and double (⫺/⫺) animals can still increase their calcium uptake in response to 1,25(OH)2-vitamin D or a low-calcium diet, respectively, albeit not to the same extent as wild-type animals (13, 76, 615). One may conclude that neither of these proteins is necessary for transcellular uptake; however, it seems more likely that 1,25(OH)2-vitamin D has more targets than previously postulated and that compensatory mechanisms are in place. 3. Basolateral calcium extrusion Once calcium reaches the basolateral membrane, it is extruded into the extracellular space, which completes the process of intestinal absorption. As extracellular calcium concentrations are higher than cytosolic concentrations, this process requires energy. Two proteins are responsible for this task. 1) The PMCA extrudes calcium at the direct expense of ATP, whereas 2) the sodium-calcium exchanger (NCX) utilizes the energy stored in the sodium gradient to transport calcium out of the cell (FIGURE 3). It is this basolateral exit process that requires energy during transcellular calcium uptake and that allows us to absorb calcium against an uphill gradient when dietary concentrations are low. A) THE PLASMA MEMBRANE CALCIUM ATPASE. The plasma membrane calcium ATPase (PMCA) is a virtually ubiquitous protein that is responsible for intracellular calcium homeostasis by pumping calcium into the extracellular milieu, thereby keeping intracellular concentrations low. The pump belongs to the family of P-type primary ion transport ATPases, which among others also includes gastric H⫹-K⫹ATPase (see sect. IIA1). Four isoforms of the protein exist; however, variety is increased by splicing (562, 1043). The PMCA1b splice variant is most predominant in the small intestine and the duodenum in particular, which suggest an involvement of this isoform in the process of transcellular calcium absorption (341, 492). Given the ubiquitous expression of PMCA, a detailed review of its structure and Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 209 SASCHA KOPIC AND JOHN P. GEIBEL function will be omitted. For a current and detailed review, please refer to Reference 1043. PMCA was first characterized in the 1960s in the membrane of erythrocytes (956). Very early experiments in basolateral membranes isolated from enterocytes identified a calciumdependent enzyme with phosphatase activity, which served as first evidence for PMCA in the intestine (100, 742). Subsequent investigations in basolateral vesicles from rat intestine concluded that two distinct calcium transport mechanisms existed in their membranes: an ATP-dependent (PMCA) and a sodium-dependent (NCX) mechanism (382, 457, 573). Furthermore, it was shown that inhibition of calmodulin halved the amount of ATP-dependent calcium transport (573). Today, we know that PMCA is highly regulated by CaM, which can increase both the affinity of the pump to calcium and its turnover speed by a factor of up to 10 (526, 627, 628, 756). Interestingly, it has also been suggested that calbindin-D 9k can directly stimulate calcium transport via PMCA (1136, 1137). In addition to intracellular proteins, PMCA is also quantitatively regulated by endocrine factors. 1,25(OH)2vitamin D can increase both the PMCA mRNA and protein content in enterocytes (35, 36, 62, 146, 382, 489, 614, 823, 1109; contested by Ref. 1138). The data on the effects of a low-calcium diet on PMCA transcription are more controversial, as one study suggests induction whereas two other studies observed reduced mRNA levels (146, 583, 639). Of note, these observations were made in different species (chick vs. mouse). The sensitivity of the tissue to 1,25(OH)2-vitamin D is also reported to decrease with age, which may represent another confounding factor underlying these observations (35, 36). In conclusion, the transcriptional regulation of PMCA1b by 1,25(OH)2-vitamin D and its decreasing expression along the length of the small intestine correlate well with the canonical model of transcellular calcium absorption. It is challenging to investigate the functional contribution of PMCA1b in the process of calcium absorption, given its role as a housekeeping protein. PMCA1 is crucial during development, which results in embryonic death if knocked out (814, 1198). Conversely, heterozygote (⫹/⫺) animals present with no apparent phenotype, albeit parameters linked to calcium homeostasis were not assessed (814). B) THE NCX. Long before the molecular identities of PMCA and NCX were known, it had been observed that calcium uptake in the intestine was dependent on extracellular sodium (713). The authors concluded that “a sodium, calciumexchange diffusion carrier may exist at the basal membrane of the cell,” which proved to be a remarkably accurate prediction (713). As mentioned before, later investigations delineated between an ATP and a sodium-dependent transport of calcium on the basolateral enterocyte membrane (382, 457, 573). 210 For a recent review on the structure and function of NCX, please refer to Reference 687. In brief, NCX exists in three isoforms (NCX1–3), which are expressed in a broad variety of cell types (687). The function of NCX has mainly been investigated in excitatory tissues, given its role as a highcapacity calcium extrusion mechanism following excitation. To transport calcium against its strong electrochemical gradient, NCX has to utilize three sodium ions to accomplish extrusion of one calcium ion (85, 460). NCX1 is the predominant isoform in the small intestine, where it has been detected on the mRNA and the protein levels (275, 688). However, NCX had been postulated to play a more important role during calcium absorption in the kidney than in the enterocyte, hence not much data on intestinal NCX are available to us (473). Furthermore, genetic disruption of NCX1 is embryonically lethal, which imposes some limitations on our experimental methodology (600). A more recent study provides some functional evidence for NCX in the intestine. By measuring intracellular calcium concentrations with a fluorescent indicator dye, Dong et al. (275) demonstrated that calcium uptake in a sodium-free environment (to run NCX in its reversed configuration) was significantly decreased when NCX was pharmacologically inhibited. Despite our knowledge that NCX exists in enterocytes and that it can extrude calcium under experimental conditions, it is difficult to assess its relative contribution to transcellular calcium absorption. In addition, NCX is not subjected to regulation by 1,25(OH)2vitamin D, which further contributes to the ambiguity concerning its importance in the process of calcium absorption. This has been observed very early, when exogenous administration of 1,25(OH)2-vitamin D to vitamin D-deficient animals did not increase sodium-dependent calcium transport, while doubling transport through PMCA (382). Furthermore, it was recently shown that a calcium-depleted diet decreases duodenal NCX1 mRNA levels (583). Of note, two members of the potassium-dependent sodium calcium exchanger (NCKX) family, namely, NCKX 3 and 4, were also identified in the small intestine (658, 687, 688). However, further functional investigations will be needed to clarify their role. B. Paracellular Calcium Absorption If we plot the amount of duodenal calcium absorption as a function of the luminal calcium concentration, we can observe that the absorption curve is comprised of two distinct kinetic components: a saturable/exponential component and a nonsaturable/linear component (824, 825, 1134). The saturable component represents transcellular calcium uptake through the enterocyte, as both the number of calcium transport proteins and their turnover rate is limited. The nonsaturable component reflects calcium uptake through the paracellular pathway. The saturable compo- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH nent is less pronounced in the jejunum and disappears completely in the ileum, indicating that transcellular calcium absorption is restricted to the proximal segments of the intestine, as discussed previously (825) [this has been contested more recently, when transcellular flux was also noted in the ileum (46)]. Compared with the transcellular pathway, the paracellular route has not received much scientific attention. It has been put forward that the rate of paracellular calcium absorption is constant across the length of the intestine and is neither sensitive to 1,25(OH)2-vitamin D nor a low-calcium diet (824, 825, 1224). However, provided that enough dietary calcium is available to saturate the transcellular pathway, observations indicate that net calcium absorption is highest in the ileum, which has been attributed to the sojourn time, rather than alterations in paracellular permeability (290, 710). In the rat, the chyme spends some 74% of its transit time in the ileum, which allows for a long exchange period between lumen and plasma (290). The diffusion rate itself is fairly low and only amounts to 2% of the rate if calcium were to diffuse freely between intestinal lumen and plasma (290). This effect is a consequence of the diffusion barrier that tight junctions, which act as functional gating molecules of the paracellular pathway, impose on calcium flux. Epithelial tight junctions are adhesion points between two neighboring cells that seal their intercellular space against a lumen, thereby restricting ion and water movement between the two compartments. As water cannot be directly transported, its movement is tied to ion fluxes, which in turn are determined by their respective concentration gradients across the epithelium. Each epithelial cell expresses an annulus of tight junction proteins at the apical end of the lateral membrane. Tight junctions are protein complexes that consist of a variety of intra- and transcellular proteins. A detailed review of their structure would exceed the scope of this article (for a recent review, please refer to Ref. 999). Briefly, the composition of the involved proteins determines the pore size, ion selectivity, and thereby the relative leakiness of each tight junction and the whole epithelium in general. Furthermore, tight junctions create a lateral diffusion barrier for membrane proteins and help to maintain the functional polarity of the epithelial cell. It is important to recognize that tight junctions are not static complexes, but can be rather dynamically regulated (999). This allows the epithelium to change its ion and water permeability in response to various stimuli (999). As discussed previously, the rate of the nonsaturable component of calcium absorption is documented to be fairly constant and insensitive to 1,25(OH)2-vitamin D, which indicated that 1,25(OH)2-vitamin D may have no effect on paracellular transport (824, 825, 1224). However, in the late 1990s, Chirayath et al. (201) postulated that 1,25(OH)2-vitamin D can increase paracellular calcium flux in confluent Caco-2 cell cultures, which form an epithelia-like monolayer with tight junctions. This conclusion is based on the observations that 1,25(OH)2vitamin D decreased the transepithelial electric resistance, which is often used as a measure of tight junction permeability, and induced bidirectional, i.e., also “serosal” to “mucosal,” calcium flux in the cultured monolayers (201). A more recent investigation further substantiated this hypothesis. It has been shown that 1,25(OH)2-vitamin D regulates the mRNA levels of some tight junction proteins in rat duodenum, which may alter their gating characteristics (354, 614). For example, the mRNA levels of claudin-3, a protein that directly determines tight junction permeability, were decreased 2.2-fold following 1,25(OH)2-vitamin D administration (614). Conversely, claudin-2 and -12 mRNA and protein levels were increased (354). Functional observations in Caco-2 monolayers further demonstrate that overexpression of claudin-2 and -12 increases electrical conductivity and facilitates paracellular calcium flux (354). All of these events are tied to a 1,25(OH)2-vitamin D-mediated promotion of transcriptional events. However, it is known that 1,25(OH)2-vitamin D can also exert short-term nongenomic effects (1122) (see sect. IVA6B). A recent investigation presents evidence for augmented solvent-drag paracellular calcium flux after acute administration of 1,25(OH)2-vitamin D (1098). Solvent-drag mediated flux may occur at low calcium concentrations in the absence of a calcium gradient. In this model, calcium is dragged through the paracellular space by water flux that is fueled by the hyperosmolar milieu in the paracellular space (266, 626). 1,25(OH)2-vitamin D was shown to induce this calcium flux in the presence of initially equimolar calcium concentrations between the mucosal and serosal compartments (1098). Over the last years, evidence for the regulation of the paracellular pathway by 1,25(OH)2-vitamin D has slowly accumulated. It is apparent that the canonical dogma which postulates that 1,25(OH)2-vitamin D only targets the transcellular pathway has to be revisited. Still, more experimental investigations will be needed to ultimately clarify the effects of 1,25(OH)2-vitamin D on paracellular calcium transport. Should tight junctions indeed be subjected to regulation by 1,25(OH)2-vitamin D, this mechanism may partially explain the sensitivity of calcium uptake in TRPV6 and calbindin-D 9k (⫺/⫺) animals to 1,25(OH)2-vitamin D. C. Alternative Models of Transcellular Calcium Absorption Two alternative models of transcellular calcium absorption have been put forward. One suggests that calcium is transported through the enterocyte by vesicles (vesicular transport model) rather than facilitated diffusion, and the other postulates that 1,25(OH)2-vitamin D can induce intestinal calcium absorption in a rapid, nongenomic fashion via a putative 1,25(OH)2-vitamin D surface receptor (transcaltachia). Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 211 SASCHA KOPIC AND JOHN P. GEIBEL The assumption that calcium is transported through the cell in vesicles is corroborated by a handful of observations. It has been demonstrated that 1,25(OH)2-vitamin D treatment increased the number of supranuclear lysosomes in rachitic chicks, compared with nontreated animals (247). It was later shown that the calcium content of lysosomes can more than double following treatment with 1,25(OH)2-vitamin D, suggesting that they may play a role in the process of transcellular calcium movement (780). Compared with the cumulative evidence that is in accordance with our canonical model of calcium absorption, the role of vesicular transport of calcium is still fairly obscure. The model of transcaltachia mainly relies on the observation that 1,25(OH)2-vitamin D can exert effects that are too acute to be attributable to transcriptional events. For example, intestinal calcium absorption was increased in chicks within 14 min of 1,25(OH)2-vitamin D exposure, an onset which is too rapid as to be of a genomic nature (785). It should be noted that rapid effects of 1,25(OH)2-vitamin D have also been suggested to influence the paracellular pathway (see above). Undoubtedly a careful evaluation of the route of calcium flux, i.e., transcellular versus paracellular, is necessary. At least, isolated intestinal cells respond with increased uptake of radiolabeled calcium to acute 1,25(OH)2vitamin D exposure (568). It has been postulated that apart from the VDR, a membrane-bound 1,25(OH)2-vitamin D exposure receptor, the 1,25(OH)2-vitamin D-MARRS (membrane-associated, rapid response, steroid binding) protein, mediates the acute effects of 1,25(OH)2-vitamin D (568, 779, 785). IV. REGULATION OF CALCIUM HOMEOSTASIS Eucalcemia is maintained by the concerted effort of vitamin D, PTH, and, to a lesser extent, calcitonin. All three hormones can influence serum calcium concentrations by acting on the intestine, the kidney, or bone. 1,25(OH)2-vitamin D, the active vitamin D metabolite, primarily modulates the intestinal absorption of calcium and will therefore be discussed in most detail (FIGURE 4). Apart from hormonal regulators that influence the absorption, excretion, and deposition of calcium, our body needs a mechanism that allows it to sense the current levels of plasma calcium. This task is fulfilled by the CaSR. It oversees the precise regulation of the calcitropic hormones. A. Vitamin D As discussed previously, vitamin D is one of the key regulators of calcium homeostasis. Our body has two sources for vitamin D, namely, a dietary source (vitamin D2⫹3) and an endogenous source that relies on ultraviolet (UV) light catalyzed synthesis in the skin (vitamin D3) (FIGURE 5). 212 Nomenclature in this case is fairly misleading, as vitamins are per definition substances that cannot be generated by our body and have to be ingested from an external source. The fact that we can synthesize vitamin D3 in our skin classifies the substance as a prohormone rather than a vitamin. As we will see, our current nomenclature is a byproduct of the historic events leading to the discovery of vitamin D. 1. Historical perspective From a historical perspective, the identification of vitamin D is closely intertwined with attempts to understand the pathophysiology of rickets. Rickets is characterized by childhood skeletal deformities resulting from inadequate osteoid mineralization and calcification of cartilage due to decreased serum calcium levels during development. The adult form equivalent of rickets is termed osteomalacia. With the onset of industrialization, rickets became a prevalent problem in the 18th, 19th, and the beginning of the 20th century. In fact, the disease was so widespread at the beginning of the 20th century that an investigation conducted by the German pathologist Schmorl on 386 children, who had died before the age of 4 years, concluded that 90% of them had had rickets (968). Even in present times nutritional rickets still remains a major public health concern in developing countries (303, 757, 809). The seminal observations that led to the identification of vitamin D were provided by Mellanby in 1919 (733). He observed that dog pups who were fed a severely restricted diet consisting of porridge or bread were consistently developing rickets (733). Development of rickets could be averted if their diet was supplemented with cod liver oil, which we now know to contain a high concentration of vitamin D (733). Mellanby (733) concluded that “rickets is a deficiency disease which develops in consequence of the absence of some accessory food factor or factors.” Vitamin A had been discovered shortly before, and it was subsequently speculated that it may represent the factor promoting bone formation (733). This hypothesis was later rebutted by American biochemist McCollum who concluded that “a substance which is distinct from fat-soluble [vitamin] A” must be responsible for preventing rickets (727). Furthermore, he stated that his experiments “demonstrate the existence of a fourth Vitamin [vitamin D] whose specific property [. . .] is to regulate the metabolism of bones” (727). In parallel to the unraveling of the dietary component of rickets, scientists were independently discovering the importance of sunlight for disease prevention. The Polish pediatrician Raczynski was most likely the first to demonstrate evidence for this hypothesis experimentally (881). He kept one dog pup in the shade while a littermate was kept in the sunlight. Both dogs were breastfed by their mother. After 6 wk, the bones of the dog that was kept in the shade contained 36% less calcium (881). These observations were followed up by the German pediatrician Huldschinsky, who healed rachitic children after exposing them intermittently for 2 months to the UV rays generated by a mercury vapor quartz lamp (504). Hess and Unger (447) rep- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH Hypocalcemia Stimulates PTH secretion Parathyroid glands Increases bone resorption Bone PTH Increases renal calcium absorption Stimulates 1,2 1,25(OH)2-D synthesis Kidney Intestine eases renal Increases calcium absorption Increases intestinal calcium absorption 1,25(OH)2-D FIGURE 4. Endocrine regulation of serum calcium levels. Calcium homeostasis is mainly regulated by PTH and 1,25(OH)2-vitamin D. Both hormones act at their respective target organs to increase serum calcium levels. licated these findings by using sunlight treatment. Hess (446) later summed up the impact of these revolutionary observations, which now seem intuitive to us: “We have known that a growing plant cannot thrive in the dark, but have failed to realize that the same laws apply to growing animals.” It was later recognized that it was sufficient to irradiate the food administered to animals rather than the whole animal to prevent development of or heal rickets. Thus initially “inert” dietary substances with no antirachitic properties could be activated by UV light (385, 448 – 450, 505, 1037). It was concluded that irradiation caused the conversion of a biological precursor to an active form and that the same mechanism was physiologically taking place in the skin. Initially, it was speculated that cholesterol may serve as this pro-vitamin. It was Windaus and Hess (in collaboration with Rosenheim) who were the first to uncover its exact molecular identity. They stated: “We conclude from our experiments with complete certainty that ergosterol [. . .] represents the anti-rachitic provitamin (1166). In 1928, Windaus received the Nobel Prize in Chemistry for “the services rendered through his research into the constitution of the sterols and their connection with the vitamins.” The irradiation product of ergosterol was later purified and named vitamin D2 (ergocalciferol) (43, 896, 1164, 1167). Although these findings solved the question as to how UV irradiation generates Vitamin D2 from ergosterol, which has antirachitic properties if ingested, the molecular mechanisms underlying the antirachitic effects of cutaneous sunlight exposure still remained obscure. Since ergosterol is an exclusive Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 213 SASCHA KOPIC AND JOHN P. GEIBEL Keratinocyte Dietary vitamin D 7-dehydrocholesterol UV-B (290-315nm) Intestinal uptake Previtamin D Isomerization Vitamin D Vitamin D Vitamin D DBP DBP Hepatocyte 1,25(OH)2-vitamin D Vitamin D Mitochondria: CYP27A1 ER: CYP2R1 25(OH)-vitamin D 25(OH)-vitamin D DBP Proximal tubule Glomerular filtration 25(OH)-vitamin D PTH 25(OH)-vitamin D DBP Mitochondria: CYP27B1 Megalin Calcium 1,25(OH)2-vitamin D Target organs FIGURE 5. Vitamin D metabolism. Vitamin D can either be synthesized in the skin or absorbed from our diet. It is then transported to the liver where it undergoes 25-hydroxylation by one of two hepatic enzymes (CYP27A1 or CYP2R1). During transport through the circulation, vitamin D is bound to a carrier protein (DBP). The 25(OH)-vitamin-D-DBP complex passes the glomerular filter and is scavenged from the primary urine by the apical megalin receptor of the proximal tubule. Here, 25(OH)-vitamin D is converted to the active vitamin D metabolite 1,25(OH)2-vitamin D. DBP, vitamin D binding protein. component of yeast and fungal membranes, a different precursor substance had to exist in animal skin. Again, it was Windaus and colleagues who identified 7-dehydrocholesterol as the provitamin in porcine skin, which is converted to vitamin D3 (cholecalciferol) under irradiation (1165). After these discoveries, industrially produced vitamin D has rapidly been used in medical applications and as a food fortification. To- 214 day, the main portion of dietary vitamin D ingestion in the United States stems from fortified dairy products (150). 2. Intestinal vitamin D absorption To exert its antirachitic effects, dietary vitamin D has to be absorbed into our circulation. Early everted gut sack exper- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH iments demonstrated that this process has linear, nonsaturable, and passive kinetics, suggesting that no specific carrier mechanism for vitamin D is in place (484). These observations were later replicated in in vivo models (483). Absorption is highest in the proximal and mid small intestine (484). Since vitamin D is fat soluble, its absorption mechanism is similar to that of dietary lipids. In an aqueous media, vitamin D aggregates in micelle-like structures (735). Its absorption is aided by the secretion of bile acids, which is underscored by the observation that patients suffering from cholestasis can present with vitamin D deficiency and develop bone disease, such as osteomalacia or osteoporosis (245, 445, 563, 721, 894, 953, 1018, 1080). Apart from bile salts, formation of mixed micelles containing monoglycerides and free fatty acids represents another factor that aids in vitamin D absorption (884, 1082; contested by Ref. 483). These substances increase micelle size, which promotes the solubilization of vitamin D, thereby increasing uptake (884). Clinically, pancreatic insufficiency, causing an impairment of triglyceride breakdown through insufficient lipase secretion, leads to decreased vitamin D absorption (1080, 1127). This is a particular problem in cystic fibrosis patients, who often develop pancreatic and concomitant vitamin D insufficiencies (33, 230, 625, 678, 924). Following uptake into the enterocyte, vitamin D is packed into chylomicrons and secreted into the lymph (288, 953). It has been demonstrated that after intestinal administration of radioactive labeled vitamin D3, up to 90% of the recovered radioactive tracer was associated with the chylomicron fraction of the collected intestinal lymph (288). Furthermore, patients suffering from the autosomal recessive chylomicron retention disease (Anderson disease; OMIM 246700), which causes impairment of chylomicron processing and secretion in the enterocyte, can present with insufficient levels of fat-soluble vitamins, such as vitamins D and E (535). The chylomicron remnants, which include vitamin D, are then scavenged by the liver after the lymph has entered the circulation through the thoracic duct (287, 407). However, it has been demonstrated in vitro and in vivo in hepatectomized and normal rats that vitamin D can directly transfer from chylomicrons to a vitamin D binding protein (DBP; see sect. IVA5) in the blood plasma (286, 288). It has therefore been suggested that at least a fraction of hepatic vitamin D uptake is mediated through DBP rather than chylomicrons (286; contested by Ref. 407). In the liver, vitamin D is then hydroxylated at its 25 position to 25(OH)-vitamin D. The metabolism of vitamin D will be the subject of a later section. The intestinal absorption of 25(OH)-vitamin D has been investigated by many groups, with the aim of optimizing vitamin D administration in a therapeutic context by bypassing the first metabolic step in the liver (219, 245, 287, 445, 645, 721, 1018, 1019, 1127). In general, enteric up- take of 25(OH)-vitamin D is more effective than that of vitamin D, which is partially attributable to a comparably lower dependency on bile acid secretion (219, 245, 287, 645, 1018, 1019). The observation that patients with cholestasis still absorb 25(OH)-vitamin D effectively, whereas vitamin D absorption is impaired, corroborates this hypothesis (1018). There is some controversy with regard to the transport of 25(OH)-vitamin D following its absorption (287, 701, 1019). It has been argued that it may be transported predominantly in the protein fraction of the lymph (287), i.e., not in chylomicrons, or that it is directly absorbed into the portal blood (701, 1019). 3. Cutaneous vitamin D synthesis Cutaneous synthesis is our second source of vitamin D. Cutaneous production depends on exposure to UVB (290 – 315 nm) light (FIGURE 5). The UVB photons convert 7-dehydrocholesterol, which is located in the plasma membrane of keratinocytes, to previtamin D3 (1117–1120). This dependency on sunlight causes a seasonal variation in vitamin D3 production, with synthesis being low during the winter months when the radiation angle of the sun flattens (187, 478, 538, 1035, 1156). In consequence, changes in latitude result in similar variability in production. Further factors that decrease production include pigmentation of the skin and application of sunscreen (209, 723), whereas an increase in altitude promotes production (478). Following conversion from 7-dehydrocholesterol, previtamin D3 isomerizes to vitamin D3 (479). The isomerization process is temperature dependent and fairly slow (479, 1117). It has been calculated that the half-life for the formation of vitamin D3 is ⬃2.5 h (1085). Interestingly, in vitro experiments conducted in isotropic medium demonstrated that the isomerization rate was 10 times slower than in in vivo experiments (1085). This was later attributed to the fact that amphiphatic interactions with phospholipids of the cell membrane stabilize the previtamin D3 conformer which then isomerizes to vitamin D3 (1086). The cellular microenvironment of the reaction thus greatly optimizes the isomerization to vitamin D3. After its synthesis, vitamin D3 is bound to DBP and carried though the bloodstream to its target organs. Observations made in patients indicate that the vitamin D3 plasma levels peak ⬃2 days after sunlight exposure, which is due to the slow isomerization rate in the skin (7). 4. Vitamin D metabolism and its regulation Irrespective of the source (endogenous or exogenous), vitamin D is metabolized in the liver to 25(OH)-vitamin D (FIGURE 5). Evidence for the existence of biologically active vitamin D metabolites emerged in the 1960s (686, 799). 25(OH)-vitamin D was identified by means of injecting rats with radiolabeled vitamin D and subsequent silicic acid column chromatography of lipid extracts from serum and var- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 215 SASCHA KOPIC AND JOHN P. GEIBEL ious tissues (686). Chromatography revealed the presence of a vitamin D metabolite in serum, liver, bone, and feces in rats and in human serum (256, 686). Furthermore, it was shown that the vitamin D metabolite was biologically active by reverting rickets in diseased animals and by increasing intestinal calcium transport and bone metabolism (686, 752). The metabolite was characterized as 25(OH)-vitamin D and subsequently successfully synthesized (111, 112). It was soon recognized that hepatectomized rats were not effectively converting vitamin D to 25(OH)-vitamin D, suggesting that the liver was the main organ responsible for 25-hydroxylation (864, 865). The ability of the liver to metabolize vitamin D was also confirmed in perfused livers and tissue homogenates (490). Historically, a long controversy existed with regard to the subcellular localization of the enzyme that hydroxylates vitamin D (25-hydroxylase) in the liver, as enzyme activity was observed in both mitochondrial and microsomal fractions of liver homogenates (93, 102, 103, 258, 696, 1053). Extensive investigations indicated that both the microsomal and mitochondrial 25hydroxylase are members of the cytochrome P-450 family (102–104, 696, 835). Both fractions demonstrated distinct enzymatic kinetics. The mitochondrial enzyme was characterized as a low-affinity high-capacity enzyme, whereas the microsomal fraction displayed high-affinity low-capacity characteristics (103, 104, 356, 696). The mitochondrial 25-hydroxylase (CYP27A1) was first purified to homogeneity from rabbit liver mitochondria (235). It was demonstrated that this cytochrome P-450 was not specific to vitamin D and could also hydroxylate other substrates, most notably cholesterol (27-hydroxylation), which represents an important step in the formation of bile acids (235). In retrospect, CYP27A1 had been purified 4 years earlier; however, only the 27-hydroxylation of cholesterol had been investigated and its effects on vitamin D had remained obscure (1162). Subsequently, the enzyme’s cDNA was cloned from rabbit, rat, and human, and its dual role in vitamin D and steroid conversion was confirmed (26, 149, 1048, 1103). The full gene structure was identified a few years later (646). Although no crystal structure of the enzyme is available to us, a homology model based on other CYP family members has been proposed (874). In the liver, CYP27A1 is expressed on the mRNA level in hepatocytes, endothelial, stellate, and Kupffer cells (1078). Furthermore, CYP27A1 expression was confirmed in a variety of other tissues, including duodenum, adrenal glands, kidney, lung, vascular endothelium, brain, retina, skin, muscle, and osteoblasts; however, their potential contribution to vitamin D metabolism remains unclear (26, 51, 135, 184, 370, 383, 508, 640, 898, 979). Interestingly, an extrahepatic conversion of vitamin D had been suggested previously to occur in the kidney and the intestine (1097). Marked differences in CYP27A1 activity can be observed between males and females. For example, CYP27A1 enzyme activity and mRNA expression were demonstrated to be increased in female rats 216 (933, 1078). Higher expression in females was also confirmed in biopsy samples from human subjects (370). A regulation via sex hormones may underlie this phenomenon, as injection of estradiol was shown to induce CYP27A1 activity (933). Interestingly, seasonal variations in expression were also observed, which may represent a confounding factor for decreased 25(OH)-vitamin D levels during the winter months (370). It should be noted that CYP27A1 can also hydroxylate vitamin D3 at other positions (402, 950). These include positions 27 and 26; however, the ratio for 25-:27-:26hydroxylation has been estimated to be only 100:15:3, which demonstrates that 25-hydroxylation of vitamin D3 is the most essential reaction catalyzed by the enzyme (950). More importantly, CYP27A1 can also use its own product 25(OH)-vitamin D as a substrate to further act as a 1␣hydroxylase and produce the hormonally active form of vitamin D, namely, 1,25(OH)2-vitamin D (50, 51, 950). As will be discussed later, this reaction is normally catalyzed in the kidney by another CYP family member (CYP27B1). At the moment, it is not clear what the physiological significance of 1␣-hydroxylation by CYP27A1 is. Mutations in CYP27A1 cause the autosomal recessive disorder cerebrotendinous xanthomatosis (CTX; OMIM 213700). The disease was first described in 1937 and is characterized by cholestanol deposits that are most prominent in tendons, especially the Achilles tendon, the brain, and the lung. Patients present with progressive neurologic defects, atherosclerosis, and cataracts and commonly suffer from diarrhea. The inadequate bile acid synthesis was first noted in 1974 by Setoguchi et al. (993). Shortly after the cDNA of CYP27A1 was cloned, it was demonstrated that mutations of this enzyme were responsible for CTX (148). In agreement with the dual role of CYP27A1, CTX patients also suffer from osteoporosis, low 25(OH)-vitamin D levels, and impaired intestinal calcium absorption (83, 320). Three CYP27A1 mutations that are known to cause CTX and still lead to protein expression were recreated in vitro, and enzymatic activity was assayed. Depending on the expression system, these mutants showed lower or higher 25hydroxylation activity than the wt enzyme, which led the authors to questions the enzyme’s role in vitamin D metabolism (403). It should be considered that 1) many more (⬃38) mutations underlying CTX exist, 2) by far not all patients exhibit disturbances in bone or vitamin D homeostasis, and 3) the investigated mutant enzymes may only cause disturbances in cholesterol, rather than vitamin D metabolism (83, 320, 403). In the light of these limitations, it should be questioned whether CTX represents an apt model system to evaluate CYP27A1 in the context of vitamin D metabolism. With the introduction of novel genetic tools, a cyp27a1 (⫺/⫺) mouse was created in 1998 (918). However, the phe- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH notype of CTX could not be reproduced, albeit fecal bile acid content was markedly decreased (918). Rather surprisingly, serum 25(OH)-vitamin D levels were increased in (⫺/⫺) animals, which either pointed to compensatory upregulation of other, maybe microsomal, enzymes or a noninvolvement of cyp27a1 in vitamin D metabolism in the mouse (918). The (⫺/⫺) mouse model was later characterized in more detail, but no new conclusions with regard to vitamin D were drawn (902). Another caveat that needs to be considered when assessing the physiological importance of CYP27A1 for vitamin D metabolism is that the enzyme cannot 25-hydroxylate dietary vitamin D2 (402). This observation underlines the necessity for another 25-hydroxylase which physiologically metabolizes vitamin D2. The microsomal 25-hydroxylase was a clear candidate for this process; however, it has only recently received more extensive scientific attention. The molecular identity of the microsomal 25-hydroxylase was unclear, until it was unmasked by Cheng et al. (184) in 2003 as CYP2R1. CYP2R1 is expressed on the mRNA level in a plethora of tissues, but most prominently in the liver and testes (184). As illustrated by a recent investigation, the testes may play a role in calcium homeostasis. Patients with testiculopathy (and concomitant lower CYP2R1 expression) were shown to have decreased 25(OH)-vitamin D levels and osteoporosis (333). The protein’s crystal structure has recently been resolved in complex with vitamin D3 (1045). Unlike CYP27A1, CYP2R1 has been shown to 25hydroxylate both vitamin D2 and vitamin D3, which may be a solution to the enigma of vitamin D2 metabolism (184). The physiological relevance of CYP2R1 was underscored by the characterization of a patient who presented with low 25(OH)-vitamin D levels and was shown to have a transition mutation in the CYP2R1 gene (183). Furthermore, a remarkable large-scale study has recently tried to establish a correlation between 25(OH)-vitamin D status and the genotype of 33,996 individuals. It was found that lower 25(OH)-vitamin D levels correlated with variants in the CYP2R1 gene (1146) (a smaller study came to similar conclusions, Ref. 139). This represents an outstanding finding, as the influence of CYP27A1 mutations on 25(OH)-vitamin D production is far less conclusive. Given the disproportionate quantity of scientific data, it is at this moment difficult to evaluate the relative contribution of each 25-hydroxylase to vitamin D metabolism, but more and more evidence for the importance of CYP2R1 is accumulating. The regulation of 25-hydroxylation has been the subject of controversy in the past, which is partially due to the fact that multiple enzymes may metabolize vitamin D, and that it was challenging to experimentally discriminate between these enzyme entities. Thus evidence which supports (91, 92, 741, 1078) and questions (258, 1097) the existence of 25-hydroxylase regulation can be found. A detailed analysis of these observations is beyond the scope of this review, but the most recent investigation should be considered in more detail, given the advances in our experimental repertoire: it has been demonstrated in the rat that 1,25(OH)2-vitamin D can downregulate hepatic CYP27A1 transcription with a concomitant reduction in enzyme activity (1078). These observations strongly corroborate the hypothesis that the 25-hydroxylation step is subjected to negative-feedback regulation. The exact mechanism of this regulatory mechanism remains elusive, especially since the CYP27A1 gene is not under control of a VDRE (369, 988, 1078). Following its synthesis, 25(OH)-vitamin D binds to DBP and is transported to the kidney, where it undergoes further conversion to 1,25(OH)2-vitamin D, the hormonally active form of vitamin D (FIGURE 5). Historically, 1,25(OH)2vitamin D was first identified in the nuclei of intestinal cells as an uncharacterized vitamin D metabolite (429, 634). The biological activity of the metabolite was determined to be much higher than that of vitamin D, and it was eventually isolated and identified as 1,25(OH)2-vitamin D (480, 586, 633, 800). 1,25(OH)2-vitamin D is up to 10 times more potent than vitamin D. The importance of the kidney for the synthesis of 1,25(OH)2-vitamin D was soon discovered, as nephrectomized rats were neither able to convert 25(OH)vitamin D, nor absorb calcium effectively (339, 458). Briefly thereafter, patients with chronic renal insufficiency were shown to lack the capability to metabolize 25(OH)vitamin D, which further established the role of the kidney as the major conversion site (724). The impairment of 1,25(OH)2-vitamin D synthesis in the course of chronic renal deficiency is a cofactor in the development of renal osteodystrophy, a bone mineralization deficiency due to deranged mineral balance. Of note, the kidney is not the exclusive site of CYP27B1 expression. 1␣-Hydroxylase has been detected in a variety of other tissues, including the placenta, decidua, skin, brain, vascular endothelium, pancreas, colon, but also in monocytes and dendritic cells (451– 453, 603, 1204 –1206). The role of extrarenal 1,25(OH)2vitamin D synthesis is not entirely clear. Beyond modulating calcium homeostasis, vitamin D has been shown to have immunomodulatory and antiproliferative effects (see sect. IVA6). Given these observations, it has been speculated that extrarenal local 1,25(OH)2-vitamin D production and paracrine secretion may represent important factors for the maintenance of the “barrier function” in these tissues (451, 453). In the kidney, 1␣-hydroxylation of 25(OH)-vitamin D takes place in the inner mitochondrial membrane of the epithelial cells of the proximal tubule (393). The cellular uptake mechanism of 25(OH)-vitamin D by the tubule cells will be subject of a later section. The 1␣-hydroxylase responsible for the conversion is also a member of the cytochrome P-450 family (CYP27B1) and was first cloned in 1997 by St-Arnaud et al. from rat cDNA (1034). Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 217 SASCHA KOPIC AND JOHN P. GEIBEL The human homolog was cloned shortly thereafter (349, 748). Interestingly, mapping of the CYP27B1 gene revealed that the locus was identical to the gene locus of the autosomal recessive disorder pseudo vitamin D deficiency rickets, type 1A (PDDR1A, OMIM 264700) (1034). PDDR is characterized by low serum calcium, secondary hyperparathyroidism, and low 1,25(OH)2-vitamin D levels (869) (of note, the original article wrongly suggests an autosomal dominant inheritance pattern). Patients can exhibit rickets or osteomalacia due to increased mobilization of calcium. The gene locus of PDDR1A had been mapped by linkage analysis; however, before the cloning of CYP27B1, it was not clear whether a defect in the enzyme itself or disturbances in its regulation were responsible for the disease (617, 618, 1223). The clear-cut phenotype of PPDR1A effectively illustrates the pivotal role of CYP27B1 and the lack of redundancy at this essential step of vitamin D metabolism. The characteristic features of PDDR1A can essentially be emulated if cyp27b1 is knocked out in a mouse model (238, 822). The serum calcium levels and the secondary hyperparathyroidism can be normalized if these animals are fed a high-calcium, phosphorus, lactose diet, albeit bone growth remains impaired (239). The activity of CYP27B1 is subjected to tight hormonal regulation. The key regulator of 1,25(OH)2-vitamin D synthesis is PTH, which is secreted by the parathyroid glands in response to low serum calcium concentrations in an effort to increase calcium uptake and release bone calcium into the circulation. The regulation of calcium homeostasis by the parathyroid glands and the CaSR will be covered in subsequent sections (see sect. IV, B and D). Apart from this stimulatory input, CYP27B1 is under negative control by its own product. 1,25(OH)2-vitamin D represses CYP27B1 on a transcriptional level (116, 125, 443, 591, 764). Although studies in (⫺/⫺) animals suggest that the VDR is essential for autoinhibition to take place, the promoter region of CYP27B1 does not include a canonical VDRE (125, 591, 764). It is thus most likely that the transcriptional regulation through 1,25(OH)2-vitamin D is indirect (125, 591). Alternatively, so-called E-box-type elements were recently proposed to act as negative VDREs (575). Moreover, CYP27B1 can be directly regulated by the local calcium concentrations. High extracellular calcium inhibits 1,25(OH)2-vitamin D synthesis, whereas low calcium concentrations induce its production (109). It has been proposed that changes in calcium modulate VDR expression and thereby the sensitivity of cell to the local negative feedback by 1,25(OH)2-vitamin D (702). Some evidence suggests that the CaSR mediates the regulatory effects of calcium on CYP27B1 activity (FIGURE 8) (702). Other factors that regulate CYP27B1 activity include fibroblast growth factor 23 (FGF23), calcitonin, prolactin, sex steroids (at least in avian species), and phosphate (716, 913, 1067, 1068, 1217). 218 Apart from regulating the synthesis of the vitamin D metabolites, our body also tightly controls their degradation. The first step of vitamin D catabolism is 24-hydroxylation, which is carried out by the mitochondrial enzyme CYP24A1. The primary site for vitamin D catabolism is the kidney, but CYP24A1 is also strongly expressed in other extrarenal tissues, such as the intestine, osteoblasts, keratinocytes, prostate, placenta, brain, and heart (11, 181, 796). CYP24A1 can hydroxylate both 25(OH)-vitamin D and 1,25(OH)2-vitamin D, thereby creating 24,25(OH)2vitamin D and 1,24,25(OH)3-vitamin D, respectively (14). 24-Hydroxylation is followed by a series of oxidation/reduction reactions which finally yield the excretable product calcitroic acid (703, 893). At least in the proximal tubule of the kidney, the regulation of CYP24A1 is reciprocal to that of CYP27B1. 1,25(OH)2-vitamin D upregulates CYP24A1, thereby stimulating its own breakdown, whereas PTH inhibits CYP24A1 (37, 180, 812, 1069, 1222). While 1,25(OH)2-vitamin D increases the transcription of CYP24A1 (the gene has two upstream VDREs), PTH exerts its inhibitory effects by decreasing CYP24A1 mRNA stability (1222). In osteoblastic and distal convoluted tubule cell lines, PTH has synergistic effects with 1,25(OH)2-vitamin D in inducing CYP24A1 (37, 1189). In analogy to CYP27B1, CYP24A1 is further regulated by FGF23, calcitonin, and phosphate (361, 513, 1175). 5. Vitamin D transport and cellular uptake The hypothesis that vitamin D may be bound to a carrier substance in serum was first expressed in the late 1950s, when it was demonstrated that the alpha fraction of human serum had high anti-rachitic properties (1079). It was later shown that DBP is identical to the group specific component (Gc) protein, which had been characterized independently by Hirschfeld and colleagues around the same time (236, 464, 465). DBP (⬃58 kDa; 458 amino acids) is synthesized in the liver and is closely related to albumin and ␣-fetoprotein, which are all derived from the same ancestral gene (221, 875, 1158, 1187, 1188). The crystal structure of DBP has been resolved at a resolution of 2.3 Å in complex with 25(OH)-vitamin D (1121). DBP can bind vitamin D and all of its metabolites (408). There is, however, a difference in the relative affinity for the vitamin D steroids, with the affinity for 25(OH)-vitamin D being highest (Kd ⬃10⫺8 M), followed by 1,25(OH)2-vitamin D and vitamin D (Kd ⬃10⫺7 M) (408). In humans, vitamin D2 and D3 metabolites are bound with equal affinity to DBP (406). Given its long plasma half-life, 25(OH)-vitamin D is also measured as the primary clinical parameter to assess the vitamin D status of patients. Although the plasma concentration of DBP is ⬃4 – 8 M, only ⬍5% of the binding sites are occupied by vitamin D sterols (408, 409). DBP has a very fast turnover rate. It has been estimated that up to 28% of DBP are replaced every day (557). This turnover entails a high demand for synthesis output by the liver. In consequence, patients with liver disease demonstrate lower DBP and total vitamin D levels than healthy subjects (97, 409). This rela- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH tionship is reversed during pregnancy, when the levels of DBP and vitamin D sterols are increased (97, 409, 609, 685, 892). Of note, DBP is not the exclusive carrier substance for vitamin D sterols. Albumin and lipoproteins were shown to transport a fraction (⬃15%) of vitamin D (1015). Given its multitude of functions, DBP has been regarded as an essential protein. Indeed, an analysis of over 80,000 human serum samples showed that DBP was present in all of them, which led to the hypothesis that deleterious mutations of DBP were lethal (213). Rather surprisingly, DBP (⫺/⫺) mice thrive well with growth curves identical to their littermates, although their 25(OH)-vitamin D and 1,25(OH)2-vitamin D levels (total) are severely decreased (937). If challenged with a low vitamin D diet, the DBP (⫺/⫺) animals develop secondary hyperparathyroidism, leading to defects in bone mineralization (937). It is unclear, however, to what extent albumin and lipoproteins compensate for the loss of the primary vitamin D carrier substance. Not much is known about the cellular uptake of vitamin D. As all other steroids, free vitamin D can passively diffuse through the plasma membrane because of its lipohilic nature. However, due to the high concentration of DBP and its affinity towards vitamin D ligands, only a fraction of vitamin D circulates in the free form. For example, 0.003% of 25(OH)-vitamin D is transported as an unbound sterol in serum, which raises the question whether passive diffusion represents a sufficient uptake pathway (98). At least 25(OH)-vitamin D has been proposed to be delivered to the proximal kidney tubule for further conversion to 1,25(OH)2-vitamin D by a different and remarkable mechanism. Rather than diffusing passively, it has been proposed that the 25(OH)-vitamin D-DBP complex passes the glomerular filter and is endocytosed by the epithelial cell of the proximal tubule (FIGURE 5) (805). The endocytotic process is mediated by megalin (aka gp330), which is a multifunctional clearance receptor on the luminal membrane. Mice lacking functional megalin were shown to lose DBP and vitamin D in the urine and develop vitamin D deficiency (805). A kidney specific megalin (⫺/⫺) animal was recently created, and the observations made in the global (⫺/⫺) animal, which had a very low perinatal survival rate of 2%, could essentially be replicated (643). Furthermore, defects in cubulin, a membrane protein that colocalizes with megalin, cause a similar phenotype (806). The experimental data are further supported by clinical observations made in patients suffering from Fanconi syndrome. Fanconi syndrome is a global reabsorption deficiency of the proximal tubule, which can develop as a result of heavy metal poisoning and/or drugs or may have inherited causes. These patients were shown to lose DBP in their urine, which may reflect the inability of the tubule cell to endocytose DBP (805, 1076, 1100). A similar endocytotic uptake mechanism has been proposed for mammary cells, which can also convert 25(OH)-vitamin D to 1.25(OH)2-vitamin D(926). 6. Cellular effects of vitamin D The cellular effects of 1,25(OH)2-vitamin D can be categorized into two major pathways, which are defined by their respective speed of onset: 1) slow genomic responses and 2) rapid nongenomic responses. Both pathways require binding of 1,25(OH)2-vitamin D to its intracellular receptor, the VDR (503, 787). The VDR (NR1I1, nuclear receptor subfamily 1, group I, member 1) belongs to the superfamily of nuclear receptors, which amongst others also includes the estrogen, testosterone, or glucocorticoid receptors. VDR was first identified in the late 1960s in the chromatin fraction of chick intestinal mucosa, where 1,25(OH)2-vitamin D increases the rate of intestinal calcium uptake (430). The 427-amino acid protein (molecular mass 48.3 kDa) was cloned in 1988 by Baker et al. (59). The crystal structure of the VDR is available to us with 1,25(OH)2-vitamin D bound to the receptor’s ligand binding domain (914). A) GENOMIC EFFECTS. Following ligand binding, nuclear receptors typically act as transcription factors and induce or repress the transcription of certain target genes. In the case of VDR, 1,25(OH)2-vitamin D binds to the receptor, which subsequently heterodimerizes with the retinoid X receptor (RXR) (FIGURE 3) (1027, 1083). The VDR-RXR complex then interacts with a VDRE in the 5= promoter region of the regulated gene resulting in transactivation. Alternatively, it has been proposed that VDR can bind to the VDRE before ligand binding occurs (920). I) Intestine. The intestine is one of the primary target sites of 1,25(OH)2-vitamin D. 1,25(OH)2-vitamin D upregulates the expression of intestinal TRPV6, calbindin-D 9k, and PMCA, which are canonically regarded to mediate the process of transcellular calcium absorption (FIGURE 3) (see sect. IIIA). Furthermore, 1,25(OH)2-vitamin D may modulate calcium uptake through the paracellular route (see sect. IIIB). By increasing the amount of absorbed calcium, 1,25(OH)2-vitamin D directly elevates serum calcium levels. This represents one of the final links in the regulatory chain of calcium homeostasis which starts with sensing of low calcium levels by the parathyroid gland and ends with increased synthesis of 1,25(OH)2-vitamin D by the kidney. The significance of 1,25(OH)2-vitamin D for intestinal calcium absorption is illustrated by 1,25(OH)2-vitamin D-deficient patients, which absorb up to 80% less calcium from their meal compared with healthy individuals (996). Although similar results were obtained in animal models, probably one of the more illustrative observations has been made in VDR-deficient animals (825, 1110). Deletion of VDR correlates with a massively impaired capacity to absorb intestinal calcium resulting in low plasma calcium levels and hyperparathyroidism (1110). This phenotype is re- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 219 SASCHA KOPIC AND JOHN P. GEIBEL versible by intestine-specific expression of VDR on a VDR (⫺/⫺) background (712, 1181). Thus animals that lack the VDR, except in the intestine, present with normal serum calcium and PTH levels (1181). In conclusion, it is unquestionable that 1,25(OH)2-vitamin D and its receptor are the key regulators of intestinal calcium absorption. II) Bone. Some evidence exists that 1,25(OH)2-vitamin D may have direct influences on the formation of bone (FIGURE 4). The VDR is expressed in osteoblasts, osteoclasts, and chondrocytes (115, 533, 623, 730). Most of the direct effects of 1,25(OH)2-vitamin D are thought to be mediated by osteoblasts. 1,25(OH)2-vitamin D has been shown to promote osteoblast differentiation from mesenchymal stem cells and to regulate the synthesis of various osteoblast proteins, such as osteocalcin, alkaline phosphatase, collagen I, osteopontin, and RANKL (45, 78, 79, 665, 709, 804, 870, 925). In general, however, the effects of 1,25(OH)2-vitamin D on osteoblast maturation and protein synthesis are very pleiotropic and depended on the duration of the exposure and the differentiation stage at which the osteoblast is exposed (817). Effects of 1,25(OH)2-vitamin D on the mineralization of bone have also been reported. For example, an increase in the mineralization of extracellular matrix was observed following concomitant 1,25(OH)2-vitamin D and vitamin K exposure (599, 745). Yet, the overall direct influence of 1,25(OH)2-vitamin D on bone metabolism is somewhat obscure. This is illustrated by VDR-deficient animals. Naturally, these animals develop rickets and osteomalacia due to impaired intestinal calcium absorption. If the animals are, however, maintained normocalcemic, the skeletal phenotype is completely rescued (21, 661). Although, these observations question the importance of 1,25(OH)2-vitamin D as a direct regulator of bone metabolism, a subsequent investigation by Panda et al. (821) came to a different conclusion. The authors reported that osteoblast numbers, mineral apposition rates, and overall bone volume were reduced in normocalcemic (rescue diet) cyp27b1/VDR (double ⫺/⫺) animals, suggesting that the 1,25(OH)2-vitamin D system is necessary for intact bone formation (821). The reason for these discrepant findings is generally unclear; however, differences in the length of exposure to the rescue diet have been put forward as a possible cause (821). For a more detailed review of the effects of vitamin D on bone, please refer to References 1033, 1114. III) Kidney. The kidney is not only the major site of 1,25(OH)2-vitamin D synthesis (see above), but also represents a vitamin D target organ. The kidney acts as key regulator of calcium homeostasis by changing the amount of calcium that is reabsorbed from the primary urine. The majority of the calcium that is filtered through the glomerulus is reabsorbed in the proximal tubule through the paracellular space, with the amount of absorbed calcium gradually decreasing along the nephron. Fine regulation of calcium absorption occurs in the distal tubule and collecting 220 duct. The mechanism by which the distal tubule conducts transcellular calcium absorption is highly analogous to the proximal intestine. The epithelial cells of the distal tubule express TRPV5 (the “sister” channel of TRPV6) as the apical calcium entry channel, calbindin-D 28k and NCX1 and PMCA1b as basolateral calcium extruders (203, 474, 610, 837, 922). In analogy to the intestine, 1,25(OH)2-vitamin D upregulates the majority of these proteins in an effort to increase renal calcium reabsorption (203, 474, 610, 837, 922). B) NONGENOMIC EFFECTS. In contrast to the genomic effects of 1,25(OH)2-vitamin D, which have been known for decades, the rapid cellular responses have only recently received more scientific attention. While the transcriptional events of 1,25(OH)2-vitamin D take place on a time scale of a few hours to days, the rapid nongenomic responses occur within minutes of exposure. One of the first evidences, which in retrospect can be attributed to a nongenomic response, was the observation made in 1941 by Selye that intraperitoneal injection of steroids had an anesthetic effect (990). Interestingly, the rapid responses to 1,25(OH)2-vitamin D also require the presence of the VDR, as these responses cannot be elicited in VDR deficient animals (503, 787). It should be noted that other proteins such as 1,25(OH)2-vitamin-DMARRS have also been suggested as candidates for a membrane associated 1,25(OH)2-vitamin D receptor (782). Attempts at identifying the subcellular localization of the VDR in the rapid response context have yielded that VDR is also present in plasma membrane invaginations, the socalled caveolae (503, 802). These VDR-containing membrane microdomains have been identified in multiple tissues, including the intestine, kidney, and lung, and are identified by coexpression of caveolin-1, which is used as a marker protein for caveolae (503, 802). Functionally, not many rapid response effects of 1,25(OH)2-vitamin D have been characterized. For example, it has been demonstrated that 1,25(OH)2-vitamin D can influence ion channel gating in osteoblasts, modulate the contraction of cardiomyocytes, lead to insulin secretion in pancreatic -cells via elevating intracellular calcium, and cause photoprotection in keratinocytes (273, 540, 1088, 1200, 1216). In the intestine, the phenomenon of transcaltachia (see sect. IIIC) has been attributed to rapid actions of 1,25(OH)2-vitamin D (801). In addition to 1,25(OH)2-vitamin D, the VDR also binds the secondary bile acid lithocholic acid (704). Secondary bile acids are bile acids that have been metabolized by the intestinal gut flora. Lithocholic acid is toxic and has been implicated to play a role in intestinal carcinogenesis (601). It has been suggested that the VDR may serve as a secondary bile acid sensor and induce lithocholic acid breakdown through CYP3A activation (537). The noncanonical VDR stimulation by lithocholic acid may thus serve as an autoprotective mechanism (704). Apart from inducing CYP3A, lithocholic acid has been demonstrated to increase expres- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH sion of TRPV6 in intestinal cell lines, which corroborates its role as a physiological VDR agonist (517). We are far from understanding the full spectrum of the effects of 1,25(OH)2-vitamin D, and unfortunately, the scope of this review does not allow a detailed analysis of these processes. In general, our knowledge concerning the physiological role of 1,25(OH)2-vitamin D is massively expanding beyond the horizon of calcium homeostasis. Evidence is accumulating that 1,25(OH)2-vitamin D can influence the renin-angiotensin-aldosterone system and may thereby act as a regulator of blood pressure (660, 662, 1219). Furthermore, low vitamin D status is associated with an increased incidence of colorectal, ovarian, and breast cancer(363, 364, 642, 984). Vitamin D also acts as a potent modulator of the immune response and cell proliferation. B. PTH Experiments dating back to the beginning of the 1900s have demonstrated that surgical removal of the parathyroid gland results in tetany. It was recognized very early that administration of calcium could ameliorate or prevent the manifestation of tetany, and it was subsequently concluded that the parathyroid glands play an important role in calcium metabolism (690, 826, 939). In 1925, extracts from the parathyroids were for the first time shown to control tetany in dogs (216). This finding marked the discovery of PTH, which is one of the three prime hormones regulating calcium homeostasis. 1. Production and secretion PTH is a 84-amino acid peptide hormone produced in the parathyroid glands. Its amino acid sequence was first established in 1970 in bovine (126, 788). Cloning of the human cDNA followed a decade later (440). The PTH gene encodes a 115-amino acid precursor hormone (preproPTH), which is enzymatically cleaved in a two step process to its mature 84-amino acid secreted form (565). The NH2-terminal prepro-signaling sequence is necessary for correct hormone processing and trafficking (340, 546). This knowledge is mainly founded on truncation studies and observations made in patients with mutations in the PTH gene, which can result in familial isolated hypothyroidism, a disorder characterized by hypocalcemia and low PTH levels (38, 340, 828, 1055). For example, a well-characterized T-to-C point mutation in the prepro signaling sequence that causes FIH leads to accumulation of the precursor hormone in the ER (243, 546). The impaired processing triggers ER stress, ultimately apoptosis and PTH insufficiency (243). Following its cleavage to mature PTH, the hormone is stored in secretory vesicles and released into the circulation in response to low plasma calcium. The regulation of PTH secretion is extremely tight, given the body’s need to maintain the calcium concentration within a narrow window (1.1–1.3 mM). Small alterations in calcium homeostasis can have deleterious effects, for example, on the excitability of neurons and muscles. The low plasma half-life of PTH of ⬍5 min allows for a precise regulation of this balance (95). To achieve controlled and rapid on-demand secretion of PTH, the parathyroid is equipped with an ultrasensitive extracellular CaSR, which constantly monitors the plasma calcium levels and triggers intracellular signaling events and PTH release upon imminent drops in calcium levels (FIGURE 6) (see sect. IVD). PTH is further regulated on a transcriptional level by 1,25(OH)2-vitamin D, creating a negative-feedback loop (154, 930, 931). 2. PTH1R PTH exerts its physiological effects via activation of a membrane-bound GPCR, the parathyroid hormone receptor type 1 (PTH1R) (PTH2R is mostly expressed in the CNS and tissues that are not involved in calcium handling and will thus not be reviewed). Of note, PTH1R is not exclusively located at the plasma membrane but can also localize to the cell nucleus. The physiological significance of nuclear PTH1R is currently unclear but may represent a novel signaling paradigm for the actions of PTH (830, 856, 857, 1155). Full-length PTH is not required to activate PTH1R. The NH2-terminal domain of PTH mediates most of the physiological effects of the hormone and is responsible for binding in the ␣--␣ binding fold of PTH1R (861). This is why clinically PTH(1–34) is used as a PTH analog with identical biological activity (753, 867, 919, 1094). Conversely, NH2terminal truncation of PTH(1–34) to PTH(2–34) changes the characteristics to a partial receptor agonist, whereas further truncation to PTH(3–34) results in loss of biological activity (1094). PTH1R was first cloned from opossum in 1991, which was followed by identification of the highly homologous human cDNA shortly thereafter (536, 964). In the nonactivated state the receptor is expressed as a homodimer at the cell surface, which dissociates upon PTH binding (860). PTH1R is a member of the class B (class 2, secretin family) GPCRs. As many other GPCRs, it undergoes N-linked glycosylation at four asparagine residues (1218). Mutational analysis revealed that site-specific mutation of all four sites decreases cell surface expression, whereas impairment of fewer glycosylation sites does not seem to have significant effects on trafficking or ligand binding (1218). Furthermore, the extracellular domain of the receptor includes a characteristic disulfide bond pattern involving six cysteine residues (392). These residues are thought to be essential for stabilizing the hydrophobic ␣--␣ binding pocket for PTH, which is conserved among all members of class B Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 221 SASCHA KOPIC AND JOHN P. GEIBEL Ca Ca Ca Calcium Ca PLC Gq PIP2 DAG Kinase phosphorylation Inhibition of cell growth IP3 Calcium 1,25(OH)2-D D PLA2 ER D VDR D RXR VDR AA Increases VDR expression Nucleus Inhibits PTH secretion Reduces PTH synthesis Parathyroid gland PTH FIGURE 6. CaSR signaling in the parathyroid gland. Increased serum calcium levels lead to an inhibition of PTH secretion. Serum calcium levels are measured by the CaSR receptor. Activation of CaSR causes generation of arachidonic acid (AA) metabolites, which inhibit the release of PTH and increase the expression of VDR, thereby increasing the cell’s sensitivity to the negative feedback exerted by 1,25(OH)2-vitamin D. 1,25(OH)2-vitamin D suppresses the synthesis of PTH. Furthermore, CaSR activation inhibits parathyroid gland growth. GPCRs and has recently been crystallized in the presence of PTH (861). Binding of PTH causes activation of at least two distinct G proteins. G␣q/11 mediates intracellular calcium release via phospholipase C (PLC) activation and increases in inositol trisphosphate (IP3), whereas G␣s activates adenylyl cyclase leading to rises in cAMP (5, 188, 808, 859). PTH1R can be regulated on a variety of levels, ranging from trafficking and internalization to direct protein interactions at the cell surface. Desensitization of PTH1R is mediated by GRK2 binding/phosphorylation and -arrestin binding, which uncouples the receptor from its associated G proteins and triggers its internalization (267, 326, 330, 1123). For 222 example, knockout of -arrestin causes increased and sustained levels of the second messenger cAMP in primary osteoblast cultures upon PTH stimulation (327). PTH1R also associates with the scaffolding protein NHERF1, which stabilizes the receptor at the cell membrane and prevents its endocytosis and desensitization (1022, 1139). This effect of NHERF1 is partially attributable to a prevention of an interaction between -arrestin and PTH1R (1140). Colocalization of NHERF1, -arrestin, and PTH1R has been demonstrated and suggests that NHERF1 is constitutively bound, whereas -arrestin association is more dependent on receptor activation (580). Interestingly, it has recently become apparent that -arrestin not only plays a role in receptor desensitization, but also mediates activation of downstream signaling cascades, such as MAPKs, in a G Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH protein-independent manner (380). Current scientific effort thus focuses on the development of so-called “biased” PTH1R agonists, which can selectively trigger G protein- or -arrestin-dependent signaling events, allowing for a more selective therapeutic repertoire (381, 1160). Furthermore, NHERF may also modulate the cell’s response to PTH binding. In the presence of NHERF2, the cell’s calcium response via PLC is augmented, whereas the cAMP response is dampened, presumably via recruitment of G␣i (698). Both NHERF and -arrestin provide effective examples of how receptor-associated proteins can modulate canonical signaling events or even, as is the case with -arrestin, initiate signaling events in their own right. PTH1R can also be regulated by its external environment. The extracellular receptor domain can be cleaved by metalloproteases, resulting in receptor degradation (579). PTH binding prevents this proteolytic cleavage, thus stabilizing PTH1R at the cell surface. The physiological importance of this mechanism is not yet fully understood. However, MMPs are involved in bone remodeling and may in consequence locally regulate the sensitivity of osteoblasts to PTH (579). 3. Cellular effects PTH exerts its effects in two primary target tissues: bone and the kidney. In the kidney, PTH causes phostphaturia, increases calcium absorption, and induces the synthesis of 1,25(OH)2-vitamin D. In-detail analysis of renal phosphate handling is beyond the scope of this review and has been summarized previously (334, 765). In brief, phosphaturia mainly results from downregulation of the Na-Pi transporter type IIa (NaPi-IIa) at the apical membrane of the proximal tubule, thereby reducing the amount of reabsorbed phosphate from the primary urine. Rather than directly modulating the transporter’s activity, PTH exposure mainly affects the number of active cotransporters on the plasma membrane. Activation of basolateral PTH1R causes retrieval of NaPiIIa and targets it for lysosomal degradation, resulting in diminished Pi reuptake (566, 682, 847). Apart from NaPiIIa, at least two other apical phosphate transporters are present in the proximal tubule: NaPi-IIc and PiT-2 (986, 1124). Currently, there is some evidence that PTH can also regulate NaPi-IIc and PiT-2, but additional studies remain to be conducted (855, 987). Although a contribution of these transporters to renal phosphate reabsorption is highly likely, knockout studies suggest that NaPi-IIa is responsible for ⬃80% of total phosphate transport, thus representing the major uptake mechanism (72, 468). It has been recognized for over 30 years that PTH can stimulate the synthesis of the active vitamin D metabolite 1,25(OH)2-vitamin D in the kidney (116, 338) (see sect. IVA4). 1,25(OH)2-vitamin D in consequence enhances the intestinal and renal uptake of calcium in an effort to counteract hypocalcemia, which initially led to secretion of PTH. The PTH-stimulated increase in 1,25(OH)2-vitamin D levels is achieved on a transcriptional level. PTH upregulates the transcription of CYP27B1, the mitochondrial enzyme which is responsible for the conversion from 25(OH)-vitamin D to 1,25(OH)2-vitamin D. Transcriptional upregulation occurs via PTH binding to PTH1R, leading to increases in the second messenger cAMP and activation of PKA (125, 442, 763, 764, 921). Apart from inducing the synthesis of 1,25(OH)2-vitamin D, PTH can directly upregulate the renal reabsorption of calcium. The regulation of calcium absorption by PTH occurs in distal segments of the nephron, mostly in the distal convoluted tubule and the connecting tubule (99, 379, 1003, 1172). In close analogy to the duodenum, these segments express calcium transport proteins, which are responsible for mediating the process of active transcellular calcium absorption in the kidney, namely, TRPV5, calbindin-D 28k, and NCX1. PTH can regulate all of these protein levels on a transcriptional level (1108). Interestingly, this seems to be accomplished independently of 1,25(OH)2-vitamin D, which also positively regulates most of these transporters (see sect. IVA6) (1108). It is therefore difficult to dissect the relative contribution of each of the two hormones in the physiological regulation of the calcium transport proteins. In addition to transcriptional activation, PTH was shown to cause direct phosphorylation of TRPV5, thereby increasing its opening probability (249). The channel is phosphorylated at threonine-709 in a PKA-dependent fashion (249). Elevation of intracellular cAMP levels and concomitant PKA activation are classical downstream effects of PTH1R activation in the kidney (248, 1172). In bone, PTH exerts a dichotomous effect depending on the pattern of exposure. It is well documented that pulsatile PTH exposure has anabolic effects on bone mass, whereas continuous release increases plasma calcium by bone catabolism (FIGURE 7) (27, 348, 401, 469, 1065). The observation that intermittent PTH administration increases bone mass has led to the use of PTH as a treatment strategy for osteoporosis (621, 671, 897). The enhanced bone formation mainly results from an increase in osteoblast numbers. This phenomenon has been partially attributed to a PTHmediated induction of osteoblast differentiation and an inhibition of their apoptosis (75, 274, 518, 530, 531, 684, 969, 1032). Multiple mechanisms underlying the anti-apoptotic effects of intermittent PTH on osteoblasts have been suggested. Among others, these include runt-related transcription factor 2 (Runx2)-mediated transcription of survival genes and increased DNA repair (75, 969). It has further been shown that fibroblast growth factor 2 (FGF2) is partially needed as an endogenous cofactor for the anabolic effects of PTH to take place (934). Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 223 SASCHA KOPIC AND JOHN P. GEIBEL Intermittent Chronic PTH PTH1R OPG RANKL Osteoblast proliferation RANK Osteoclast differentiation H+ Osteoblast Osteoclast V-ATPase FIGURE 7. The effects of PTH on bone. PTH has a dual effect on bone. Intermittent PTH exposure causes osteoblast proliferation, leading to an increase in bone mass. Continuous PTH exposure results in RANKL upregulation and concomitant OPG suppression (OPG serves as a decoy receptor for RANKL and prevents its interaction with osteoclast RANK). The stimulated RANKL-RANK interaction leads to osteoclast proliferation and increased bone turnover. Continuous PTH exposure, on the other hand, mainly affects osteoclast numbers and activation, thereby increasing bone turnover. Since osteoclasts are canonically thought to not express PTH1R (although this view has recently been challenged, Ref. 260), the catabolic effects of PTH are relayed through osteoblast signaling. The PTH-induced crosstalk between osteoblasts and osteoclast is mainly mediated by receptor activator of nuclear factor B (RANK), osteoprotegrin (OPG), and RANK ligand (RANKL). Both RANKL and OPG are expressed by osteoblasts and exert opposing actions on osteoclasts. RANKL promotes osteoclastogenesis by binding to RANK on osteoclasts. Conversely, OPG serves as a soluble decoy receptor for RANKL and thus inhibits its interaction with RANK, thereby suppressing osteoclastogenesis. In accordance with this model, RANKL-deficient animals develop osteopetrosis because of insufficient osteoclasts activation (592). Sustained PTH exposure affects RANK-RANKL signaling by downregulating antiresorptive OPG, while simultaneously stimulating production of RANKL by osteoblasts (FIGURE 7) (350, 497, 679, 689). The enhanced RANK-RANKL signaling induces formation of osteoclasts, which in turn leads to enhanced bone resorption and elevates serum calcium levels. In the intestine, several observations suggest that PTH may have a direct, i.e., non-1,25(OH)2-vitamin-D mediated, effect on intestinal calcium absorption. Both isolated enterocytes and intestinal loops demonstrated an increase in calcium uptake following acute PTH exposure (781, 783, 784). However, more investigations are needed to clearly establish a direct regulatory role of PTH in the context of intestinal calcium uptake. 224 4. PTH fragments It should be noted that full-length PTH(1– 84) is not the only circulating form of the hormone in the body. Various PTH fragments can be found in the circulation, which partially originate directly from the parathyroid gland and partially represent products of peripheral cleavage. The parathyroid itself releases COOH- and NH2-terminal hormone fragments, which are generated by cysteine proteases (cathepsin B and H) in distinct secretory vesicles of the gland (418, 427, 693). Interestingly, the fraction of secreted hormone fragments changes with extracellular calcium conditions. It has been reported that more fragments are released under conditions of hypercalcemia, when secretion of fulllength PTH is suppressed (417, 418, 605, 726). Peripheral proteolysis represents the second source of PTH fragments. This process occurs predominantly in liver and the kidney (127, 234, 989). The group of fragments that have received the most amount of scientific attention is the large NH2terminally truncated non-PTH(1– 84) fragments. PTH(7– 84) is the quantitatively major member of this group, which is secreted by the parathyroids (233). The group of nonPTH(1– 84) fragments can represent up to 20% of circulating PTH, but can increase in patients with renal failure dramatically to up to 50% because of impaired renal clearance (130, 131, 444, 648). This is of particular interest, as it has become recently apparent that the non-PTH(1– 84) fragments exert biological activity. In general, non-PTH(1– 84) fragments antagonize the effects of PTH in its primary target tissues, bone and the kidney but also the parathyroid gland directly. It has been shown that PTH(7– 84) can inhibit PTH release from the parathyroid, presumably in an autocrine fashion, despite low serum calcium concentra- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH tions (493). In bone, PTH(7– 84) blocks the effects of PTH on calcium mobilization in thyroparathyroidectomized rats (786). More detailed investigations revealed that PTH(7– 84) reduces calcium release from bone in vivo and inhibits the formation of osteoclast-like cells in murine primary marrow cultures (272). In the kidney, PTH(7– 84) can inhibit the formation of 1,25(OH)2-vitamin D, presumably via a posttranscriptional mechanism (768, 1102). Since activation of PTH1R requires an intact NH2-terminal domain of PTH, it has been speculated that non-PTH(1– 84) fragments exert their function through a pathway distinct of PTH1R. The existence of a COOH-terminal PTH receptor has thus been postulated (270 –272, 786). The molecular identity of this receptor is, however, not yet resolved. Another mechanism through which non-PTH(1– 84) fragments may exert their biological activity is downregulation of PTH1R. It has been demonstrated that PTH(7– 84) causes internalization of PTH1R, thus offsetting the effects of PTH by decreasing the number of available receptors at the cell surface (1022). In conclusion, non-PTH(1– 84) fragments act as PTH antagonists and are secreted by the parathyroid in response to hypercalcemia. 2. The calcitonin receptor Calcitonin exerts its physiological functions via activation of the calcitonin receptor. The calcitonin receptor is a seventransmembrane domain GPCR. In particular, it is a member of the family B subfamily of GPCRs (669). It shares significant homology with other receptors of the family, which include the PTH, GHRH, PACAP, VIP, secretin, glucagon, and glucagon-like peptide receptors. The calcitonin receptor is expressed in the two most extensively described calcitonin target tissues, i.e., osteoclasts and the kidney, but also in other adult tissues, such as the prostate, CNS, skeletal muscle, and placenta (17, 329, 428, 790, 878, 1174). Multiple isoforms of the calcitonin receptor occur in the body, which results from different RNA splicing directed by tissue-specific promoters (17, 30, 389, 606). Activation of the calcitonin receptor mostly translates into a rise of intracellular cAMP levels via Gs-dependent activation of adenylate cyclase (167, 332, 435, 669). Although the cAMP/PKA pathway appears to be dominant, activation of both PLC and PLD have also been reported (167, 332, 776). 3. Cellular effects A) OSTEOCLASTS. 1. Production and secretion Shortly after the discovery of calcitonin and its hypocalcemic effects, investigators set out to identify its physiological site of action. First evidence for an effect of calcitonin on bone metabolism came from experiments on rat embryonic bone in tissue culture. It was observed that calcitonin caused a decreased basal release of calcium from these preparations (343). These observations served as the first evidence of how calcitonin lowers serum calcium levels. Furthermore, calcitonin blocked the resorptive actions of PTH on bone, albeit only temporarily (342, 343). After 4 – 6 days of combined treatment (calcitonin ⫹ PTH), calcium release rose again (342). This desensitization to the effects of calcitonin has been coined as the calcitonin “escape” phenomenon (342). Today we know that the transient effect of calcitonin on osteoclasts is attributable to a downregulation of surface calcitonin receptors and their synthesis (882, 1061, 1129, 1130). Calcitonin is encoded by the CALCA gene and is initially synthesized as a 141-amino acid precursor (preprocalcitonin), which is later processed to the mature 32-amino acid hormone (637). The same gene also encodes the neuropeptide CGRP. Production of either peptide is dependent on tissue-specific RNA splicing. Although encoded by a different gene, amylin also belongs to the calcitonin peptide family. All three peptides, i.e., calcitonin, CGRP, and amylin, share some overlapping functions with regard to osteoclast suppression (16, 229, 1199). Calcitonin is released from the C-cells in response to rising concentrations of plasma calcium. The CaSR is responsible for the molecular process of calcium sensing on the parafollicular cells (351, 367, 728). As alluded to before, calcitonin directly inhibits the action of osteoclasts, causing the balance between bone absorption and formation to shift towards anabolism. Calcitonin exerts its inhibitory effects on osteoclasts via activation of its receptor, which is expressed in abundance on their surface (428, 790, 878). Exposure to calcitonin triggers distinct morphological changes in the osteoclast. Osteoclasts are highly motile cells that resorb bone via formation of so-called resorptive pits, which are membrane invaginations that are luminally acidified by active proton secretion. Calcitonin has been shown to inhibit the formation of these resorptive bays in vitro (487, 1057). Furthermore, osteoclast motility is markedly decreased, causing the cell to C. Calcitonin Calcitonin is a peptide hormone that has been discovered by Copp et al. in 1962 as a factor that reduces serum calcium concentrations (160, 227). Calcitonin production was initially falsely ascribed to the parathyroid glands, and it was only later that the thyroid gland had been established as the source of calcitonin (463). The primary sites of calcitonin production are the parafollicular cells (C-cells) of the thyroid gland. Calcitonin exerts its hypocalcemic effects primarily by inhibition of osteoclast activity. It should be noted that the importance of calcitonin in day-to-day calcium homeostasis in humans is rather negligible (see sect. IVC4). For this reason, it will only be reviewed concisely. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 225 SASCHA KOPIC AND JOHN P. GEIBEL enter a state of functional quiescence (169). Apart from directly altering osteoclast function, calcitonin also affects osteoclast differentiation. Calcitonin inhibits the formation of multinucleated mature osteoclasts by arresting their differentiation in more immature stages (1060). Renal calcium handling is the second organ function that is influenced by calcitonin. However, it is not entirely clear whether calcitonin causes calciuria or enhances calcium reabsorption from the urine. The conflicting results may be to some extent attributable to species differences. In humans, calcitonin likely increases the excretion calcium through the urine and thereby acts in concert with its inhibitory action on osteoclasts to lower serum calcium levels (31, 32, 143, 215, 819, 1016). Conversely, most studies demonstrating an increase in the renal reabsorption of calcium and magnesium were conducted in rats, rabbits, or mice (84, 158, 159, 265, 304, 877, 883, 1225). A single investigation established a calcium-conserving effect of calcitonin in human (160). The primary site of action for calcitonin in the rat is the thick ascending limb (TAL) of the loop of Henle, where calcitonin has been demonstrated to bind its receptor, increase local adenylate cyclase activity, and promote calcium reabsorption (166, 168). Apart from enhancing the reabsorption of calcium, calcitonin also promotes the vectorial transport of NaCl in the rat TAL, thereby amplifying the corticomedullary concentration gradient, which is a prerequisite for the subsequent concentration of urine in the collecting duct (265, 304). In the rabbit, the calcium-conserving effects of calcitonin seem to be mediated by the distal tubule (1225). duced in HEK-293 cells following calcitonin exposure, suggesting a regulatory role of calcitonin in vitamin D catabolism (361). In addition to its putative direct effect on calcium reabsorption, calcitonin may thus affect normal calcium metabolism indirectly by modulating the levels of circulating 1,25(OH)2-vitamin D. B) KIDNEY. It has also been speculated that calcitonin may act directly on the collecting duct in a similar fashion to antidiuretic hormone (ADH or vasopressin), i.e., to concentrate the urine by increasing the reabsorption of water from the primary urine (251). Indeed, calcitonin was shown to increase the apical expression of aquaporin 2 (AQP2) in principal cells of the collecting duct (119). Apical insertion of AQP2 and subsequent transepithelial water movement is the primary mechanism by which ADH causes concentration of the urine to lower plasma osmolarity. In conclusion, the direct impact of calcitonin on renal calcium handling is quite vague and may be of minor importance. However, calcitonin also has another, indirect effect on calcium homeostasis. Calcitonin was shown to be an important regulator of the expression of CYP27B1, the renal enzyme responsible for the conversion of 25(OH)-vitamin D to 1,25(OH)2-vitamin D (1009, 1217). In normocalcemic rats, CYP27B1 mRNA levels were inducible by calcitonin administration, leading to an increase in the production of 1,25(OH)2-vitamin D (1009, 1217). Furthermore, CYP24A1 was in- 226 4. The relevance of calcitonin for calcium homeostasis The relevance of calcitonin for day-to-day calcium balance is highly debatable. This is corroborated by fundamental observations during conditions of decreased or increased calcitonin levels, neither of which result in an appreciable phenotype in terms of calcium balance. For example, patients with medullary carcinomas of the thyroid (MTC), a tumor of the thyroid C-cells resulting in the hypersecretion of calcitonin, were shown to have normal bone mineral densities (1176). Animal studies further substantiate this conundrum. A reduction in serum calcitonin levels by thyroidectomy in rats did not impact serum calcium levels (223, 1064). In light of this evidence, the question arises as to what the physiological role of calcitonin is. It has been suggested that calcitonin is an evolutionary remnant (462). This is substantiated by the fact that calcitonin from other species is more potent than human calcitonin. Teleost calcitonin has the highest biological activity in humans, which may be a result of their higher dependence on the hormone. For example, salmon calcitonin has an approximately sixfold higher affinity to calcitonin receptor than human calcitonin (28, 328). Furthermore, it is less effectively eliminated by the kidney, resulting in a longer plasma half-life (405). The differences in the biological activity between calcitonin forms led to the introduction of salmon calcitonin as a treatment for skeletal disorders, such as osteoporosis or Paget’s disease (1077). Given its ambiguous role in regular calcium homeostasis, it has been postulated that calcitonin may be of importance during states of high calcium demand, such as during lactation (1170). It has been demonstrated that calcitonin and CGRP (⫺/⫺) mice show greater loss of skeletal mass during lactation than wt animals (1170). Since calcitonin and CGRP are encoded by the same gene, animals were controlled by CGRP substitution, which was without effect (1170). Another mechanism by which calcitonin may be osteoprotective during lactation or pregnancy is by inducing the renal synthesis of 1,25(OH)2-vitamin D (1217). D. The CaSR The CaSR is a G protein-coupled membrane-bound receptor that is the primary sensor for calcium and is the first link in the regulatory chain of calcium homeostasis. By regulating the release of PTH from the parathyroid to modulate Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH current serum calcium levels, it presides over the subsequent hierarchical cascade of vitamin D synthesis and calcium handling in the vitamin D target organs, such as the intestine, kidney, and bone. More recent investigations have demonstrated that the CaSR is not exclusively expressed in the parathyroid gland, but is also present locally in the vitamin D target organs. This diverse expression suggests that CaSR can modulate organ function locally and outside of the strict PTH-vitamin D-organ axis. Thus a short and local feedback loop is created, which allows the organs to respond rapidly to the local calcium environment. A detailed description of the receptor’s role in each tissue is beyond the scope of this article, but an attempt will be made to identify the key features of CaSR in these local sites in a subsequent section (FIGURE 8). For excellent in-depth reviews, please refer to Refs. 372, 711, 906. The importance of CaSR as a regulator of calcium balance is underlined by clinical pathologies that are caused by its mutation. Loss-of-function mutations cause familial hypocalciuric hypercalcaemia (FHH; OMIM 145980) and neonatal severe hyperparathyroidism (NSHPT; OMIM 239200), whereas activating mutations cause autosomal dominant hypocalcemia (OMIM 601198). These mutations mostly change the threshold of receptor activation in either direction. Currently, ⬃300 different mutations are reported, two-thirds of which represent inactivating mutations (241). Given its broad expression pattern, the deranged sensing of blood calcium levels in these disorders not only affects the secretion of PTH from the parathyroid glands, but also causes local dysfunction in other organs, such as the kidney where calcium absorption is perturbed. 1. Structure and signaling The CaSR was first cloned by Brown et al. (134) in 1993 from bovine parathyroid using a Xenopus oocyte expression cloning system. Cloning of the human CaSR followed 2 years later (366). The CaSR is a 1,028-amino acid protein that belongs to the superfamily of classic 7-transmembrane domain G protein-coupled receptors (134, 366). It is mainly expressed as a homodimer on the cell surface (55). The dimerization process has been shown to take place in the ER and is mediated by the formation of disulfide bonds between cysteine residues (C129, C131) and noncovalent interactions of leucine residues (L112, L156) in the extracellular domain of the receptor (54, 529, 858, 888, 1213). Following assembly in the ER, the CaSR undergoes N-linked glycosylation in the Golgi apparatus, some of which is pivotal for cell surface expression (887). The trafficking between ER and Golgi apparatus is regulated by the small GTP-binding protein Rab1 (1221). Knockdown and mutations of Rab1 in HEK cells results in decreased numbers of CaSR at the cell surface (1221). Conversely, the internalization of CaSR is thought to be mediated by ubiquitination by the E3 ligase dorfin (498). On the cell surface, the CaSR resides in caveolin-1-rich plasma membrane domains, which also contain associated signaling proteins (571). These signaling complexes are formed with the help of scaffolding proteins. The COOHterminal tail of CaSR binds to filamin A, an actin binding protein (48, 467). Silencing of filamin A with siRNAs results in the attenuation of MAPK signaling by the receptor (496) (see below). In lack of a crystal structure of CaSR, the exact binding sites of calcium on the extracellular domain remain subject of speculation. So far, applicable structural data has only been obtained from the metabotropic glutamate receptor type I (mGluR1), which belongs to the same family of type C GPCRs. In this model, glutamate binds to key residues which are located in a cavity, embedded in between two lobular domains (LB1 and LB2) of the extracellular tail (611). This structural hallmark has been aptly coined the receptor’s Venus fly trap module. This motif is conserved among other GPCRs of the same family. Multiple attempts to identify the calcium binding sites have been undertaken using computational homology modeling (499, 500, 1014). These models have postulated between one and five calcium binding sites (499, 500, 1014). With the employment of mutational analysis, it has been possible to validate functionality of some of these putative binding sites (500). Monitoring of the intracellular calcium response to increasing extracellular calcium levels in CaSR transfected HEK cells had indicated previously that the Hill coefficient for this response was ⬃3.1, suggesting that multiple calcium binding sites may exist (834). It should be noted that the CaSR can also be stimulated by other polyvalent cations (Mg2⫹, Pb2⫹, Cd2⫹, Fe2⫹, Ba2⫹, Ni2⫹, Co2⫹, or Gd3⫹) and larger polycationic molecules, such as spermine, spermidine, putricine, protamine, and neomycin (134, 416, 880). Furthermore, several substances can allosterically modify the receptor and potentiate its sensitivity to its direct agonists. These include pharmacological small molecule substances (calcimimetics) that are in clinical use for the treatment of conditions, such as secondary hyperparathyroidism, and L-type amino acids, which enable the CaSR to act as a nutrient sensor (220). Truncation studies have demonstrated that the Venus fly trap motif is necessary for allosteric modification by L-type amino acids (759). The affinity of calcium to the CaSR can also be modulated by changes in extracellular pH (279, 879). An acidic extracellular milieu has been shown to decrease the sensitivity of CaSR to its agonists, whereas an increased extracellular pH has converse effects (879). The intracellular domain of CaSR contains five PKC phosphorylation sites (366). Mutational analysis demonstrated that PKC-mediated phosphorylation of the CaSR at Thr888 blunts its response to extracellular calcium, as evidenced by inhibited calcium release from intracellular Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 227 SASCHA KOPIC AND JOHN P. GEIBEL Calcium-sensing receptor Kidney Proximal tubule Collecting duct Distal convoluted tubule CaSR CaSR CaSR HPO42– CaSR CaSR NaPi-IIa Na+ H+ Ca2+ V-ATPase TRPV5 25(OH)-vitamin D CaSR CaSR Mitochondria: CYP27B1 H2O 1,25(OH)2-vitamin D AQP2 Intestine Thick ascending limb of the loop of Henle Enterocyte ROMK K+ 1 CaSR CaSR NKCC2 2 CaSR Na+ CFTR 2Cl– Cl– K+ 3 1 2 Ca2+ H2O Stomach Bone G-cell PPIs APAs CaSR CaSR H+ H,K-ATPase CaSR CaSR K+ ? Gastrin CaSR ECL-cell + parietal cell activation Differentiation and function ? RANKL RANK ? Osteoblast Circulation H+ V-ATPase Parietal cell FIGURE 8. CaSR in the gastrointestinal tract, kidney, and bone. Kidney: the effects of CaSR activation on ion transport in various nephron segments are shown. In the proximal tubule, CaSR stimulates phosphate absorption and 1,25(OH)2-vitamin D synthesis. In the thick ascending limb of the loop of Henle, CaSR inhibits apical potassium channels (ROMK), thereby inhibiting NKCC2 (potassium recycling). The resulting changes in the lumen-positive potential inhibit paracellular calcium uptake. In the distal convoluted tubule, CaSR presumably stimulates apical calcium entry through TRPV5. In the collecting duct, CaSR stimulates proton extrusion through the V-type ATPase and inhibits urine concentration through AQP2. Stomach: in the parietal cell, CaSR induces acid secretion by activating H⫹-K⫹-ATPase. In the G-cell, CaSR activation results in gastrin secretion. Of note, CaSR serves as a luminal nutrient and calcium sensor on the G-cell. Bone: CaSR on osteoblasts presumably regulates their differentiation and RANKL expression. Intestine: in the intestine, CaSR activation reduces water secretion by inhibiting chloride secretion through CFTR. 228 Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org Osteoclast GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH stores (56, 246). The phosphorylation occurs in response to receptor activation and thus represents an autoinhibitory feedback mechanism (246). -Arrestins are most likely involved in the process of PKC-associated desensitization (681, 853). Conversely, dephosphorylation of the Thr-888 residue is carried out by a calyculin-sensitive phosphatase, thereby restoring the receptor’s initial sensitivity (246). Another mechanism of desensitization is mediated by a G protein receptor kinase (GRK), most likely by interfering with G␣q regulated pathways (see below) (681, 853). Once stimulated, the CaSR activates a variety of intracellular signaling cascades. Being a GPCR, most of these processes are mediated by G proteins. Specifically, G␣q/11, G␣i, and G␣12/13 have been shown to be coupled to the CaSR (40, 175, 495, 849). The expression of all subunits was confirmed in bovine parathyroid (1115). G␣i mediates the suppression of cAMP levels by inhibiting adenylyl cyclase and activates the ERK/MAPK pathway (175, 250, 375, 572). Activation of G␣q/11 results in increased intracellular calcium concentrations via activation of PLC and IP3 triggered calcium release (133, 1010). As demonstrated in HEK cells, this cascade can also activate further downstream phospholipase A2 leading to production of arachidonic acid metabolites (415). G␣12/13 is thought to regulate phospholipase D and phosphatidylinositol 4-kinase (PI 4-K); however, this interaction has only been demonstrated in heterologous cell culture system (494, 495). 2. CaSR in the parathyroid CaSR regulates parathyroid function at three levels: 1) the release of PTH from secretory granules, 2) de novo synthesis of PTH, and 3) parathyroid cell growth. Activation of CaSR by increasing plasma calcium results in an inhibition of PTH release, thereby lowering calcium levels. It is thought that this response is mediated by the generation of arachidonic acid metabolites via G␣q and PLA2 activation (FIGURE 6) (121, 152). Cultured porcine parathyroid cells demonstrated an increase in arachidonic acid production after CaSR stimulation while PTH release was inhibited (121). Furthermore, exogenous administration of arachidonic acid suppressed PTH release from the parathyroid cells (121). Similar effects were demonstrated for the arachidonic acid metabolites 12- and 15-hydroxyeicosatetranoic acid, suggesting that they represent the downstream effectors of arachidonic acid production (120). Apart from directly controlling PTH release, CaSR also modulates PTH synthesis. PTH gene transcription is mainly regulated by 1,25(OH)2-vitamin D. Binding of 1,25(OH)2vitamin D to the VDR causes a decrease in pre-pro-PTH mRNA levels creating a negative-feedback loop (154, 930, 931). However, it was recognized before the identification of the CaSR that serum calcium can modulate the actions of 1,25(OH)2-vitamin D on PTH gene transcription (930). It was shown that increases in calcium can potentiate the inhibitory effects of 1,25(OH)2-vitamin D (930). This effect is most likely mediated by CaSR, whose activation can decrease PTH transcription by augmenting the inhibitory effects of 1,25(OH)2-vitamin D. Molecularly this is achieved by upregulating the expression of the VDR (151, 162, 362, 653, 916). The current working model states that activation of CaSR causes an increase of arachidonic acid metabolites and activation of the MAPK pathway, which in turn results in increased VDR mRNA levels (FIGURE 6) (151). This allows the parathyroid to adjust its 1,25(OH)2-vitamin D sensitivity to the current plasma calcium levels. The molecular mechanisms underlying the trophic effects of CaSR activation are less clear. Earlier observations had already suggested that hypocalcemia is associated with parathyroid cell proliferation (778). Currently, the CaSR specific calcimimetics provide the most useful insight into the regulation of parathyroid growth by CaSR. Calcimimetics administered in the context of both animal models and clinical studies of hyperparathyroidism demonstrate that activation of CaSR leads to a reduction in gland size (510, 589, 746, 1128). Conversely, inactivating mutations of CaSR result in parathyroid enlargement. Parathyroid-selective genetic disruption of G␣q was furthermore shown to cause moderate hyperparathyroidism with increased plasma PTH levels and gland hyperplasia, suggesting a role of G␣q in the regulation of parathyroid cell growth (849). Similar findings were reported in G␣q/11 double KO animals (1159). 3. CaSR in the kidney The CaSR acts as an important regulator of ion and water homeostasis in the kidney. It should be noted that it can exert its effects on calcium transport independently of other hormonal regulators, such as PTH and 1,25(OH)2-vitamin D. The CaSR is expressed along most of the nephron, albeit in varying subcellular localizations (FIGURE 8) (907, 908). In the proximal tubule, CaSR is localized apically at the base of the brush border, where it has been implicated to play a role in phosphate transport (52, 907, 909). The primary regulator of phosphate transport in the proximal tubule of the kidney is PTH. In brief, increased PTH levels inhibit phosphate reabsorption from the lumen. Activation of the apical CaSR can partially reverse the effects of PTH and restore phosphate absorption (52). Conversely, PTH and high phosphate levels reduce CaSR expression (909). Furthermore, it is likely that the CaSR mediates the inhibitory effects of calcium on 1,25(OH)2-vitamin D synthesis in the proximal tubule (109, 702). In the thick ascending limb of the loop of Henle, CaSR is located on the basolateral membrane (907). In this nephron segment, the receptor acts as a major modulator of monovalent and polyvalent ion absorption. Activation of CaSR leads to an inhibition of the apical renal outer medullary Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 229 SASCHA KOPIC AND JOHN P. GEIBEL potassium (ROMK; Kir1.1) channel, mainly through arachidonic acid metabolites created by PLA2 (1147, 1148). Apical ROMK releases potassium ions into the lumen, which in turn are needed to fuel apical ion uptake through the Na⫹-K⫹-2Cl⫺ (NKCC2) cotransporter. By decreasing apical potassium efflux, CaSR inhibits sodium and chloride uptake through NKCC2 (906). This correlation is reflected in much earlier observations, which report that calcium infusions can decrease tubular sodium clearance (300, 718, 1052). In addition, impairment of NKCC2 has also implications for calcium absorption. Reduced NKCC2 activity decreases the lumen-positive potential and negatively affects countercurrent multiplication, and in consequence the nephron’s ability to concentrate urine (434). Both mechanisms will lead to impaired calcium absorption (434). Calcium absorption in the medullary portion of the thick ascending limb is thought to occur predominantly as passive uptake through the paracellular route (995). Similar observations have been made when blocking NKCC2 pharmacologically with the loop diuretic furosemide (299). It has thus been proposed that activation of basolateral CaSR has “loop diuretic-like” effects, reducing NaCl but also calcium absorption in the kidney (434). In contrast to the medullary section, the cortical portion of the thick ascending limb has been proposed to have predominantly active calcium uptake properties, which are under the hormonal regulation of PTH and calcitonin (344, 509). PTH increases calcium absorption in this segment, and it has been shown that, similarly to phosphate absorption in the proximal tubule, activation of CaSR can suppress the effects of PTH (264, 754). The absorption of NaCl does not seem to be affected by CaSR (264, 754). The distal convoluted tubule and the connecting tubule are responsible for the fine-tuning of calcium reabsorption in the kidney. To achieve this goal, they are equipped with molecular machinery, similar to that in the duodenum (TRPV5, calbindin-D 28k, NCX1, and PMCA1b) to absorb calcium against its electrochemical gradient through the transcellular pathway (680). In analogy to the proximal small intestine, these transporters are predominantly regulated through 1,25(OH)2-vitamin D, but also PTH. CaSR colocalizes with TRPV5 in this segment (1090). Its activation causes increase calcium influx through TRPV5 and may thereby locally and rapidly adapt active absorption to the urine calcium concentration (FIGURE 8) (1090). Apart from regulating calcium and phosphate absorption in the kidney, CaSR modulates proton and water movement in the collecting duct. In the intercalated cells of the collecting duct, apical V-ATPase acidifies the urine in an effort to maintain systemic acid-base homeostasis. It has been shown that luminal calcium and neomycin can induce V-ATPase activity via activation of CaSR, thereby causing proton secretion into the urine (FIGURE 8) (901). Since the formation 230 of calcium kidney stones is dependent on luminal pH, it has been speculated that this may represent an autoprotective mechanism that prevents nephrolithiasis (901). Furthermore, stimulation of apical CaSR in the principal cells of the collecting duct leads to decreased ADH (vasopressin)-stimulated water reabsorption through AQP2 (FIGURE 8) (942, 943). Taken together, activation of CaSR has diuretic effects via inhibiting NKCC2 in the thick ascending limb of the loop of Henle and by inhibiting AQP2-mediated water reabsorption the collecting duct. 4. CaSR in the gastrointestinal tract The CaSR is distributed along most of the gastrointestinal tract, ranging from the stomach to the large intestine (186, 360, 744, 932, 998). We are now only slowly beginning to unravel its function in this diverse array of tissues. In the stomach, CaSR localizes to the basolateral membrane of the acid-secreting parietal cells and to all membranes of the gastrin-secreting G-cells (FIGURE 8) (142, 182, 886). Primary cultures of G-cells were shown to release gastrin after stimulation of the CaSR with calcium (142, 886). The release is mediated via calcium influx into the cytosol through nonselective cation channels opening after CaSR stimulation (142). These findings provide the molecular basis for the observation that rises in serum calcium can increase serum gastrin levels (see sect. IIB2). The apical expression of CaSR in G-cells theoretically enables it to act as a luminal nutrient sensor modulating gastric acid secretion and other parameters. In recent studies there is direct evidence showing that gastrin levels increase in mice after calcium and L-type amino acid ingestion (325). This effect was abolished in CaSR (⫺/⫺) animals (325). In healthy human test subjects, pharmacological stimulation of CaSR leads to a concomitant increase in gastrin levels and gastric acid output (165). CaSR on G-cells was thus postulated to play an important role in the gastric phase of acid secretion by maintaining acid output by maintaining gastrin secretion (325). Apart from being expressed on G-cells, CaSR is also localized on the basolateral membrane of the acid-secreting parietal cell, where it exerts effects that are independent of gastrin and other secretagogues. Activation of parietal cell CaSR has been reported to increase H⫹-K⫹-ATPase-mediated proton secretion, thereby acidifying the gastric lumen (FIGURE 8) (145, 291, 373). This stimulatory effect was demonstrated for direct activators, such as calcium or Gd3⫹, but also allosteric modifiers, such as L-type amino acids (145, 291, 373). In parallel to other tissues, the intracellular activation signal for H⫹-K⫹-ATPase is mediated by rises in intracellular calcium, PLC, MAPK, and PKC (899). In conclusion, both rises in luminal and plasma calcium concentrations can induce gastric acid secretion either indirectly through gastrin release or directly through parietal cell activation. The physiological significance of this observation remains the subject of speculation, but may be linked Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH to facilitating calcium uptake by increasing acid output. As described in a subsequent section, it has been speculated that gastric acid increases the bioavailability of ingested calcium (see sect. V). Alternatively, CaSR may primarily function as a nutrient sensor in the stomach (amino acid sensing), which maintains constant acid output in the gastric (apical G-cell sensing) and postprandial (basolateral parietal cell) phase of digestion, when circulating levels of amino acids are high (325). In the intestine, functional investigations on CaSR have mainly been conducted in colonic epithelia, where CaSR localizes to both the basolateral and apical membranes of the colonic crypt (173, 186, 360). Expression patterns vary slightly in the small intestine, with general basolateral expression and additional weak apical expression in the villus (173, 360). Furthermore, CaSR is expressed in both the Meissner’s and Auberach’s plexuses. Early experiments on single perfused colonic crypts demonstrated that intracellular calcium concentrations could be increased when exposing the crypts to classic CaSR agonists and that forskolinstimulated fluid secretion could be inhibited (186). This and subsequent investigations indicate that CaSR plays an important role as a modulator of colonic fluid secretion (185, 186). Subsequently, attempts have been made to take advantage of the “constipatory” effects of CaSR activation in pathophysiological settings. Activations of CaSR in the course of diarrheagenic enterotoxin exposure was shown to decrease fluid secretion via increased breakdown of cyclic nucleotides (371). Although the potential clinical applications of ameliorating the symptoms of secretory diarrhea are promising, more efforts will have to be made to fully unravel the physiological role of CaSR in intestinal ion and fluid transport. So far it is not clear whether intestinal CaSR can modulate calcium absorption, as is the case in the kidney. 5. CaSR in bone It is well established that CaSR is expressed in osteoblasts, osteoclasts, and their respective precursors (172, 174, 542, 1183–1185, 1192). The functional role of CaSR in these cells is, however, less clear. Undoubtedly, both cell lines are exposed to local fluctuations in calcium concentrations making an adaptive response to the calcium environment plausible. Indeed, changes in extracellular calcium concentration have been shown to regulate various cell functions, mostly in in vitro models. Extracellular calcium can stimulate the proliferation, migration, and differentiation of osteoblasts (174, 297, 1183, 1184, 1186). Similarly, calcium was proposed as a differentiation signal for osteoclasts (542, 544, 734). Significant doubt about the in vivo importance of CaSR in bone has emerged with the generation of the CaSR (⫺/⫺) mice. Although CaSR knockout results in rickets, these animals suffer from severe hyperparathyroidism, which did not allow a discrimination between the effects of high PTH and CaSR on bone turnover (365). Con- comitant genetic ablation of the parathyroid gland or PTH secretion, however, revealed that the skeletal phenotype of CaSR single mutation (⫺/⫺) could mostly be rescued, suggesting that the skeletal abnormalities were due to high circulating PTH levels rather than CaSR inactivation (598, 1096). Furthermore, CaSR does not seem to be the exclusive calcium-sensing mechanism in osteoblasts, as changes in extracellular calcium can still elicit functional responses in CaSR (⫺/⫺) osteoblasts (852). This observation has been attributed to another GPCR with calcium-sensing capabilities, namely, GPRC6A (850, 851). Although GPRC6A has a higher activation threshold for calcium, it also responds to the CaSR allosteric activator R568 (851). GPRC6A activation may thus represent a confounding factor in most in vitro studies on osteoblasts and their modulation by CaSR. Also, GPRC6A knockout leads to osteopenia, further underlining the possibility of an alternate calcium-sensing pathway in bone (850). Osteoblasts extracted from these GPRC6A-deficient animals show decreased sensitivity to extracellular calcium and in vitro mineralization defects (854). Although these observations have profoundly questioned the physiological significance of CaSR in bone, closer examination still favors a role of CaSR in bone turnover. With the recent advances in genetic methods, an osteoblast-specific CaSR (⫺/⫺) model has been created (171). These animals have severely stunted growth and skeletal development, clearly suggesting an involvement of CaSR in normal osteoblast function (171). The previous conflicting evidence gained from global CaSR (⫺/⫺) models with survival rescue by elimination of PTH synthesis have been attributed to the possible expression of alternate CaSR splice variants, which may compensate for the deletion of full-length CaSR in these animals (171, 915). In an attempt to further elucidate the function of CaSR in osteoblasts, the reverse approach has been executed by specifically upregulating CaSR in osteoblasts with use of a constitutively active receptor mutant (296). Upregulation of CaSR results in bone loss, as evidenced by a decrease in bone volume and density, specifically of trabecular bone (296). These findings are accompanied by an increased number of osteoclasts, whereas osteoblast parameters were essentially unchanged (296). Activation of CaSR has been speculated to promote RANKL production by osteoblasts, which serves as an osteoclastogenic signal (296). Osteoblasts may thus recruit osteoclasts and induce their maturation via CaSR signaling and increased RANKL expression, which would explain the observed increase in bone turnover and osteoclast numbers in the setting of constitutive CaSR activation (241). V. THE STOMACH AND CALCIUM Preceding parts of this review have independently summarized the physiology of acid secretion, intestinal calcium absorption, and their respective regulation. The following Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 231 SASCHA KOPIC AND JOHN P. GEIBEL section will attempt to illustrate the functional intersections between these seemingly unrelated fields. In particular, the question of whether acid is needed to absorb calcium effectively from the gut or whether the stomach contributes to the regulation of calcium homeostasis by secretion of an endocrine substance will be investigated. A. Proton Pump Inhibitors and the Risk of Fracture In May 2010, the Food and Drug Administration (FDA) released the following safety announcement: “Healthcare professionals and users of proton pump inhibitors should be aware of the possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors, and weigh the known benefits against the potential risks when deciding to use them” (319). Proton pump inhibitors (PPIs, see sect. IIC1) are in widespread use for the treatment of acid-related disorders, such as gastroesophageal reflux disease (GERD) or gastric ulcer disease. They exert their curative effects by inhibiting the acid output of the stomach. Over the recent years, mostly epidemiological evidence has accumulated which links the intake of PPIs to an increased risk of sustaining fractures, especially in the elderly population. Yang et al. (1190) published one of the earliest and largest studies investigating this potential correlation in 2006. Examining a population of over 13,000 hip fracture cases and over 135,000 controls over the age of 50, the authors concluded that long-term (over 1 year) PPI use was associated with an increase in hip fractures (AOR ⫽ 1.44) (1190). Although the likelihood of sustaining a fracture following PPI intake may seem fairly low, the implications for public health are substantial. This has multiple reasons: PPIs represent the third most commonly prescribed medication in the United States and are also available as over-the-counter formulations. Furthermore, there is an ongoing debate whether PPIs are overprescribed, putting certain populations at unnecessary risk of side effects. In combination with the high incidence of osteoporotic fractures, the mean incidence of hip fractures alone between 1986 and 2005 was 957 per 100,000 women over the age of 65 per year, a small increase in risk suddenly has implications for a very large population (123). The roots of this controversy may potentially be traced back to the 1940s and 1950s. Before the advent of PPIs, total and partial gastrectomies or vagotomies were performed to control acid-related disorders. It was soon apparent that patients who underwent these radical surgical procedures developed osteoporosis/-malacia (58, 305, 732, 876). A study that assessed the prevalence of osteomalacia in gastrectomized patients concluded that up to 12% of patients (19% of females) had histologically overt osteomalacia, although general disturbances in calcium metabolism were estimated to occur in up to 28% of patients (208, 368). Other inves- 232 tigations came to lower prevalence results of ⬃5–10% (1091). The osteomalacia was also shown to translate into an increased incidence of fractures in these patients (795). Naturally, gastrectomy represents a radical intervention, and the reasons for this correlation may be multifactorial, but reduced acid output may be of significance. Back in the field of PPIs, the seminal epidemiologic investigation by Yang et al. was subsequently followed up by a number of studies, which also focused on other types of fractures, other populations, and other drugs reducing gastric acid output, such as H2 receptor antagonists (202, 228, 252, 394, 400, 559, 868, 923). Although their conclusions were somewhat controversial, a recent meta-analysis supports the initial hypothesis that a correlation between PPI intake and fracture risk (hip, spine, and any-site fractures) exists (1195). The meta-analysis considered 11 studies and identified an overall odds ratio of 1.30 for all fracture types combined (1195). There was no association between H2 blocker intake and an increase in fracture risk, although some single studies supported a link (228, 1195). Another meta-analysis came to a comparable conclusion with regard to an increased fracture risk under PPI exposure (616). B. Gastric Acid and Intestinal Calcium Uptake A variety of reasons could theoretically account for the observation that PPIs increase the likelihood of fractures. The most prominent hypothesis assumes that the reduced acidity in the stomach impairs the intestinal absorption of dietary calcium. This assumption is based on both patient observations and experimental animal data. Alas, the number of animal studies, which in contrast to investigations in humans per default allow more radical experimental designs and genetic manipulation, is very small. 1. Effects of gastrectomy, vagotomy, and PPIs on mineral metabolism in humans Before discussing the reports that try to correlate PPI use with calcium uptake, it is worthwhile to examine older literature on patients that had undergone partial or total gastrectomy. As discussed previously, these procedures are known to be linked to bone disease. In contrast to PPIs, which eliminate the singular factor of acid secretion, gastrectomies also influence gastric emptying, the emulsification of food stuffs, and food habits. It is thus more difficult to draw conclusions on the influence of acid secretion on calcium absorption from gastrectomized patients than from individuals on PPI therapy. A further caveat lies in the type of gastrectomy, as different surgical procedures are and were in use. Some surgeries bypass the duodenum (Billroth II, Roux-en-Y, total gastrectomy), whereas some leave the duodenal passage intact (Billroth I). Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH A common finding among gastrectomized patients is their low 25(OH)-vitamin D levels, while levels of 1,25(OH)2vitamin D seem to be increased (101, 244, 378, 511, 794, 972, 1081). The pathophysiological reason for this is not entirely clear. It has been argued that bone disease and low 25(OH)-vitamin D are a result of impaired vitamin D absorption following surgery; however, the consensus seems to be that uptake rates of vitamin D is not impaired in these patients (245, 378; contested by Ref. 1081). The vitamin D insufficiency may also be a byproduct of improper nutrition (378). As fat and milk intolerance can develop, especially in surgeries which exclude the duodenum (Billroth II), a change of dietary habits with insufficient intake of the fatsoluble vitamin D may be an underlying cause. Indeed, long-term longitudinal studies suggest that maintaining body weight reduces the risk of developing bone disease after gastrectomy, which emphasizes the role of adequate nutrition (667, 668). On the other hand, Billroth I and II patients show the same loss in bone density, although Billroth II surgery (bypassing of the duodenum) is associated with a much higher degree of fat malabsorption (110, 651). A different investigation even concluded that Billroth I patients have a higher loss in bone density than Billroth II patients (794). A more recent report suggests that the problem underlying bone disease after gastrectomy may be impaired calcium absorption, rather than dietary vitamin D deficiency (244). It has been shown that high 1,25(OH)2vitamin D levels accelerate the breakdown of 25(OH)-vitamin D (210 –212, 244). The observed low 25(OH)-vitamin D levels in gastrectomized patients may thus be a byproduct of increased catabolism, and not insufficient intake, whereas the high 1,25(OH)2-vitamin D levels may represent compensatory upregulation due to insufficient calcium absorption or intake (244). Calcium absorption in gastrectomized patients (Billroth I ⫹ II) has been reported to be in the low-normal range, while 1,25(OH)2-vitamin D levels are increased (794). In line with these findings, secondary hyperparathyroidism, an indicator of compensatory upregulation due to low serum calcium levels, is a known finding after gastrectomy (101, 244, 1171). Other investigations on intestinal calcium absorption in gastrectomized patients came to very contradictory results, ranging from increased to impaired absorption (8, 34, 255, 398, 585, 794). Many of these reports failed to assess 1,25(OH)2-vitamin D and PTH levels, which means that adaptive mechanisms may mask the insufficient baseline uptake of calcium in the intestine (8, 34, 255, 398, 585, 794). In conclusion, the exact pathogenesis of postgastrectomy osteopenia remains somewhat unclear. The disorder may be attributable to vitamin D insufficiency, impaired calcium absorption, inappropriate diet, or a combination of all factors. The intrusiveness of gastric surgery makes it difficult to dissect the influence of gastric acid on these parameters. This is why vagotomized patients are a somewhat more apt patient population to study the effects of gastric acid on calcium uptake, albeit the number of studies on this cohort is very limited. Vagotomy abolishes the parasympathetic input to the stomach and thereby decreases the amount of secreted acid. Although the gross anatomy of the stomach remains intact, other parameters, such as gastrin levels, are also deranged given the important role of the vagus nerve in the regulation of gastric acid secretion (see sect. IIB). While bone disease is generally not reported in these patients, low 25(OH)-vitamin D levels are common (514, 793). Similarly to gastrectomy, the 1,25(OH)2-vitamin D levels are concomitantly elevated, suggesting adaptive upregulation potentially to compensate for decreased calcium absorption (793). As discussed previously, the decreased 25(OH)-vitamin D could be indicative of augmented catabolism of the vitamin (244). Serum calcium is commonly decreased or in the lower normal range, while intestinal calcium absorption is increased, presumably in response to elevated 1,25(OH)2vitamin D (110, 974). Secondary hyperparathyroidism does not manifest (793, 974). In general, the disturbance in mineral metabolism is more pronounced in gastrectomized patients than in vagotomized patients, as evidenced by the higher incidence of bone disease and secondary hyperparathyroidism. It is challenging to draw clear conclusions on the influence of gastric acid on calcium absorption in either patient group. Yet, it is apparent that compensatory mechanisms are in place in these patients, as evidenced by the increased 1,25(OH)2vitamin D and PTH levels. Less efficient calcium uptake due to decreased acid output may be one explanation for this, but without further analysis this conclusion remains speculative. With the advent of PPIs and H2 blockers, the number of surgical interventions to control acid-related disorders decreased massively. Given their high specificity, PPIs selectively eliminate gastric acid output. Several investigations that try to tie PPI intake to a disturbance in mineral metabolism exist. Graziani et al. (396) observed in eight healthy volunteers that postprandial calcium concentrations did not increase in subjects on a PPI regime (omeprazole 20 mg 3⫻ daily), whereas control subjects demonstrated a clear spike in serum calcium levels. Urine calcium excretion was also reduced compared with the control group (396). A similar effect of PPIs was later observed by two independent groups in patients undergoing hemodialysis (395, 423). It should be noted that neither of these studies directly assessed intestinal calcium absorption, but rather measured serum calcium as an indirectly related parameter. More recently, intestinal calcium absorption was measured by O’Connell et al. (807) using a radiolabeled calcium isotope. The investigators reported that 7 days of PPI (omeprazole 20 mg 1⫻ daily) intake significantly reduced calcium absorption in elderly women under fasting conditions compared with the placebo group. Although these studies support a role of PPIs in reducing calcium uptake, conflicting Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 233 SASCHA KOPIC AND JOHN P. GEIBEL evidence exists. Several investigations that assessed calcium absorption using radioactive tracers and a whole gut lavage technique found no evidence for a decrease in absorption under short-term PPI treatment (421, 991, 1173). It is not clear why these discrepancies in the outcomes of the trials exist. There is, however, variability in the experimental technique to measure calcium uptake, the cohorts investigated (young vs. postemenopausal women vs. dialysis patients) and the form of calcium administration (calcium salts vs. whole meals), which may partially account for the divergent results. Indeed, different calcium salts are absorbed with different effectiveness in acid suppressed individuals, which will be subject of later discussion (see sect. VC). Furthermore, different populations may have different capacities for endocrine compensation. In summary, it is experimentally difficult to unmask the potential correlation between a reduction in gastric acidity and calcium absorption, given our body’s high capacity for compensation. In addition, slight alterations in mineral homeostasis may take years to manifest themselves clinically, for example, in osteopenia or fractures. Without following up on test subjects on a long-term basis, snapshot measurements which may still lie within clinically normal range can be misleading. 2. Effects of gastrectomy, vagotomy, and PPIs on mineral metabolism in the animal model To further elucidate the problem of osteopenia following gastrectomy or PPI use, several animal studies tried to replicate and expand the observations made in human test subjects. For example, Axelson et al. (49) measured serum calcium concentrations in rats who had undergone parathyroidectomy and various surgical procedures to reduce gastric acid output (vagotomy, antrectomy, gastrectomy). While parathyroidectomy alone predictably reduced serum calcium levels, the gastric operations (with intact parathyroid glands) had little to no effect on calcium concentrations (49). Interestingly, intestinal calcium absorption was even increased in the latter group. The authors attributed this observation to a compensatory upregulation of PTH secretion and concomitant 1,25(OH)2-vitamin D production. To eliminate this factor, gastrectomy or fundectomy was conducted after parathyroidectomy, thereby depriving the animals of their compensatory machinery. This intervention resulted in massive hypocalcemia and death after a few days, which led the authors to conclude that acid secretion is important for the maintenance of calcium homeostasis (49). Another investigation in rats that had undergone antrectomy (Billroth I) observed a significantly decreased absorption of calcium (345). Fundectomy did not affect calcium absorption (927). However, both studies employed the balance method to calculate calcium absorption, which is considered less accurate than using radiotracers. In pigs, 234 total gastrectomy causes massively reduced calcium uptake and secondary hyperparathyroidism (700). In this study, the duodenum was surgically bypassed by esophagojejunostomy, thereby eliminating the site of maximal active calcium absorption and limiting the conclusion that can be drawn (700). In addition, several reports of vagotomy in a rat model are available to us (49, 307, 308, 928). It has been demonstrated that vagotomy alone has no effect on the rate of intestinal absorption (307, 928). Secondary hyperparathyroidism was observed by one group, while PTH levels were reported to be unaffected by the other group (307, 928). 1,25(OH)2-vitamin D was not measured, which would have provided further evidence of compensation due to decreased calcium bioavailability. However, if vagotomy and parathyroidectomy are performed together, intestinal calcium absorption is significantly impaired compared with vagotomy or parathyroidectomy alone (307). This is in accordance with the low serum calcium concentrations found in gastrectomized and parathyroidectomized rats (49). PPIs were also used to relate acid secretion to bone disease in rats. Bone weight did not change in rats that were treated for 4 wk with omeprazole (841). Alas, calcium absorption was not measured in these animals, and a decrease in bone weight represents a very terminal and long-term outcome. A more recent investigation by Schinke et al. (963) demonstrates that mice which have been genetically manipulated to be achlorhydric (CCK2 ⫺/⫺) have decreased serum calcium levels as well as develop osteoporosis and secondary hyperparathyroidism in an effort to maintain calcium balance (963). This study is especially noteworthy, as acid secretion is knocked out selectively in this mouse model while the stomach remains intact (stomach morphology). Furthermore, a genetic mutation that has been associated with osteopetrosis (a disease characterized by increased bone density) due to osteoclast malfunction was also shown to cause decreased gastric acid secretion. These patients present with lower serum calcium values. Rather than being a product of impaired bone resorption (osteoclast defect), the hypocalcemia may thus be related to impaired intestinal calcium absorption (gastric acid secretory defect) (963). C. Calcium Salts Many investigators have employed calcium salts to determine the efficacy of intestinal calcium absorption. Furthermore, calcium salts are in wide clinical use as a dietary supplement. As will be discussed in this section, calcium salts differ in their bioavailability, which not only represents a potential source of error in experimental designs, but more importantly, has extensive clinical implications. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH Calcium salts represent the most common supplementation form of calcium for individuals who do not meet their adequate daily intake. The indications for supplementation can be diverse, but mostly include conditions such as osteoporosis/-penia or preventative intake after menopause, during glucocorticoid intake or if lactose intolerant. In the year 2000, the National Health Interview Survey concluded that 11% of Americans ingest calcium supplements on a daily basis (739). Females account for 80% of this population, mostly to ensure supply after menopause (739). Calcium salts exist in multiple formulations. The most commonly used salts are calcium carbonate, calcium citrate, calcium lactate, and calcium gluconate. Calcium carbonate is the most widely used formulation, because it contains the highest percentage of elemental calcium per weight (40%), which equates to small tablet size and easier ingestion (997). In comparison, calcium citrate contains 21% elemental calcium, calcium lactate 14%, and calcium gluconate 9% (997). However, the calcium salts do not only differ in their calcium fraction, but are massively divergent with regard to their solubility in water. Calcium carbonate is the least water-soluble salt at a neutral pH. For example, calcium citrate dissolves 17 times more readily in water than calcium carbonate (997). Very fundamental in vitro solubility experiments have shown that after 1 h in 500 ml of water only 1% of the initial 500 mg of calcium carbonate are dissolved at 37°C (997). The solubility of calcium carbonate can be greatly improved by an acidic environment (390, 997). Adjusting the pH to 5.5 in the same experiment dissolves 86% of the calcium carbonate; further lowering it to 2.5, a value that can be expected in the stomach, increases the dissolved fraction to 100%. Given these differences in solubility, a plethora of studies have investigated the bioavailability of the various calcium salts, mostly focusing on the difference between calcium carbonate and calcium citrate. Again, the conclusions are heterogenic. Several studies suggest that calcium carbonate is absorbed less effectively than the more soluble calcium citrate (422, 438, 439, 789), while others conclude that there is no difference in bioavailability (432, 433, 520, 547, 831, 832, 891, 997, 1038). A detailed analysis of the individual trials is beyond the scope of this review. It suffices to say that there is strong variability in the experimental methods (direct absorption measurements vs. measurement of postprandial serum calcium vs. urine excretion) and design of the studies (populations; administration in the fasting state vs. with a meal). A confounding factor to the results of these studies may be the gastric pH at the time of the measurement. As discussed earlier, calcium carbonate is not very soluble at more alkali pH values, which may have implications for patients using these supplements while on PPI therapy (997). Furthermore, meals dramatically affect gastric pH and may change the bioavailability of the supplements. Indeed, there seems to be a correlation between gastric pH and the absorbability of calcium carbonate. The first observation indicative of this association was made by Ivanovich et al. in the late 1960s (520). The group reported that absorption of calcium carbonate was severely impaired in four male patients suffering from achlorhydria (520). Compared with five control subjects, who absorbed between 9 and 18% of the ingested calcium carbonate, these patients only absorbed 0 –2%. Interestingly, when gastric acid secretion of one of these patients was stimulated by administration of betazol hydrochloride (a histamine analog), calcium carbonate absorption rose from 2 to 10% (520). This investigation somewhat spawned the entire controversy of whether gastric acid is necessary to absorb calcium effectively from the intestine. A similar investigation was conducted later by Recker (891) in a larger sample of achlorhydric patients. The investigator concluded that 1) control subjects absorb calcium carbonate and calcium citrate equally well, 2) but that achlorhydric patients lose their capability to absorb calcium carbonate, while calcium citrate absorption is increased (presumably through compensatory upregulation of the absorption via vitamin D) (891). It is important to mention that these absorption assays were conducted in the fasting state. When the achlorhydric patients ingested the calcium carbonate salt together with a meal, their calcium uptake normalized. It cannot be conclusively answered which factor of the meal was responsible for the increase, as several food components, such as fiber and protein, are known to affect calcium uptake. However, the authors speculated that the pH (5.8) of the meal was sufficiently low to dissolve the ingested calcium carbonate (891). Furthermore, a previously cited study that demonstrated decreased calcium absorption under PPI therapy employed calcium carbonate as source of calcium for the conducted measurements (807). Patients with gastric bypass surgery also absorb calcium carbonate less effectively than calcium citrate (1089). The importance of acid for the absorption of calcium carbonate was also demonstrated in the previously discussed achlorhydric CCK2 (⫺/⫺) mouse model (963). The osteoporotic phenotype and secondary hyperparathyroidism in these achlorhydric mice could only be fully rescued by a high-calcium gluconate (2%) diet, but not by a high-calcium carbonate (2%) diet (963). In the light of these reports, it is evident that although the bioavailability of calcium salts in the healthy individual may be equal, an impairment of acid secretion has a negative effect on the bioavailability of calcium carbonate, presumably because of decreased solubility. Given the fact that calcium carbonate is the most commonly used formulation for calcium substitution therapy, these findings may partially account for the statistical correlation between PPI use and the increased risk of sustaining fractures. Another factor that needs to be taken into consideration when assessing solubility of calcium salts is the PCO2 (390). In the local milieu of the duodenum, the PCO2 can reach values of up to 300 mmHg, resulting from the Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 235 SASCHA KOPIC AND JOHN P. GEIBEL pancreatic secretion of bicarbonate (929). Solubility experiments of calcium carbonate have shown that a high PCO2 negatively affects its solubility, as the carbonate enters equilibrium with CO2 (390). Compared with other calcium salts, the particularly low bioavailability of calcium carbonate in acid suppressed patients may thus be a compounded effect of reduced acid secretion and a high duodenal PCO2. D. The Endocrine Stomach and Calcium Homeostasis Apart from being a mere acid secretory organ, the stomach also plays an important role as an endocrine organ. It should be noted that all of the aforementioned surgical or pharmacological interventions, i.e., gastrectomies, vagotomies, or pharmacological acid suppression therapy, will inevitably impact the endocrine functions of the stomach. It is therefore plausible that not only the changes in intragastric pH affect the absorption of calcium, but that the dysregulation of the endocrine stomach is responsible for changes in calcium homeostasis. The following section will address how hormones that are secreted by the stomach may impact calcium and bone homeostasis. 1. Ghrelin Ghrelin has been discovered fairly recently (1999) by Kojima and colleagues and is mainly implicated in regulating food intake in the hypothalamus (588, 777). Ghrelin levels are inversely correlated with body mass and elevated in conditions of fasting, such as anorexia nervosa (377). Ghrelin is mainly synthesized and secreted in a pulsatile manner by special neuroendocrine cells (P/D1 cells) in the fundic region of the stomach (242, 777). The influence of ghrelin on gastric acid secretion is discussed in a separate section (see section IIB5E). A few years after its discovery, it was shown that ghrelin can also directly affect osteoblasts (254, 357, 576, 691). Ghrelin induces osteoblast proliferation and differentiation and inhibits their apoptosis (357, 576, 691, 1141). It is not entirely clear whether this effect is mediated via the ghrelin surface receptor, the growth hormone secretagogue receptor 1a (GHS-R1a), or not. While the receptor is expressed in rat and murine osteoblasts and its pharmacological inhibition abolishes the effects of ghrelin on differentiation and proliferation, no GHS-R1a mRNA could be detected in a human osteoblast cell line (254, 357, 691). It should be noted that this effect is independent of growth hormone (GH). Ghrelin serves as a potent stimulator of GH secretion from the pituitary gland, which in turn acts as an activator of osteoblasts through the GH/IGF-I axis. However, the observations that 1) pharmacological inhibition of GHS-R1a attenuates the effects of ghrelin and that 2) GH-deficient rats are still sensitive to ghrelin, suggest a direct effect on osteoblasts (357). In vivo, the activation of osteoblasts translates to an increase in 236 bone mineral density (BMD) in rat and murine models (261, 357). Ghrelin also promotes the formation of new bone following injury (261). For example, mice that received a standardized bone injury demonstrated 1.6 times more new bone surface if treated with ghrelin compared with control animals (261). Several studies aimed to identify a link between serum ghrelin levels and BMD in human populations. The most recent, and one of the largest (n ⫽ 707 subjects), investigation assessed BMD with peripheral quantitative computed tomography (pQCT). This technique allows for separate analysis of trabecular and cortical bone. The results showed a positive correlation between ghrelin and trabecular BMD in elderly men and women (775). A different large-scale study, investigating a similar cohort (n ⫽ 977) found no association using dual-energy X-ray and single-photon absorptiometry (1157). These techniques, however, do not permit a discrimination between cortical and trabecular bone. Other small-scale studies also came to contradictory conclusions (388, 811). The reason for these discrepancies is elusive. Since the formation of trabecular bone represents a more dynamic process, its direct measurement may be more sensitive to subtle changes than overall bone density measurement (775). Baseline plasma ghrelin levels were also shown to be inversely correlated to type 1 collagen  C-telopeptide (CTX), a marker for bone resorption (501). The source of ghrelin represents another potential caveat. In vitro studies suggest that osteoblasts can also synthesize ghrelin (254, 357). Ghrelin was identified on the mRNA and protein level by two investigations (254, 357). A different group did not find evidence for ghrelin in osteoblasts (214). This has important implications, as ghrelin may be secreted in an auto-/paracrine fashion, which would make plasma ghrelin levels less significant for osteoblast activation. On the other hand, (partial) gastrectomy significantly decreases plasma ghrelin concentrations, which could contribute to postgastrectomy osteopenia, although these may just be two independent factors. Total gastrectomy causes a drop in plasma ghrelin levels by as much as 70% (528). Partial gastrectomy also severely decreases plasma ghrelin; however, levels normalize depending on the type of resection to 48 – 88% of the preoperative levels due to compensatory production in the remaining gastric mucosa (528). This recovery already occurs after 7days. It thus remains to be elucidated if the slightly decreased ghrelin levels after small gastric resections can account for the long-term phenomenon of postgastrectomy osteopenia. Furthermore, in mice, the reduction of bone mass after gastrectomy cannot be rescued by exogenous administration of ghrelin (277). Ghrelin administration did also not affect markers of bone resorption in gastrectomized patients, although these parameters were only measured very acutely 4 h after ghrelin infusion (501). So far, no data on the effect of chronic ghrelin treatment on BMD are available. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH Although decreased ghrelin levels could in theory be a contributing factor to the reduction of bone mass following gastrectomy, it is unknown if PPIs can directly affect ghrelin levels. A potential link between PPI-related fractures and ghrelin levels remains to be investigated. An indirect association may be present in patients with Helicobacter pylori infections. These infections represent a common indication for PPI intake and were suggested to coincide with reduced ghrelin in plasma and the gastric mucosa (527). Given the recent discovery of ghrelin’s impact on osteoblast function, many questions still remain to be answered. It is, however, clear that our view of the stomach as a mere acid secretory pouch needs to be expanded to a new level. 2. Gastrin Gastrin represents one of the main acid secretagogues (see sect. IIB2). It is secreted by specialized G-cells in the antrum of the stomach and the duodenum. The released gastrin enters the circulation and induces acid secretion in gastric parietal cells via the CCK2 receptor. It has been hypothesized fairly early that plasma gastrin may have an impact on bone metabolism. Injection of gastrin and its synthetic analog pentagastrin was shown to decrease plasma calcium levels in pigs and rats in the 1970s (222, 980). This effect was attributed to gastrin-stimulated release of calcitonin from the thyroid gland. Indeed, pentagastrin was shown to be a potent stimulator of calcitonin secretion in various species and is still in clinical use to evaluate thyroid C-cell hyperplasia and medullary carcinomas (155, 156, 226, 441, 829). Although a clinical correlation between plasma gastrin levels and plasma calcitonin has been demonstrated by one study in patients with Zollinger-Ellison syndrome (hypergastrinemic patients) and in pigs, it is unclear if native gastrin, i.e., not pentagastrin, acts as an important secretagogue for calcitonin in humans (224, 1020). In fact, other investigations found no association between gastrin and calcitonin levels in other cohorts (122, 454). At least in the rat, the hypothesis of the gastrin-calcitonin axis has been severely challenged. Although gastrin decreases plasma calcium levels in rats, the same effect occurs in (para)thyroidectomized animals, suggesting that calcitonin is not involved in this process (980). Furthermore, cultured rat thyroid cells could not be stimulated to release calcitonin if incubated with gastrin (225). Fundectomy- and omeprazole-induced hypergastrinemia also did not affect calcitonin levels in rats (843, 927). Interestingly, hypocalcemia after gastrin injection could not be induced in rats that had been (para)thyroidectomized and gastrectomized (844, 981). This observation led to the conclusion that gastrin may stimulate the release of an unknown substance from the rat stomach, which in turn exerts calcitropic activity. In accordance with this hypothesis, mucosal extracts from rat stomachs were shown to have the same hypocalcemic effects as gastrin and to stimulate uptake of radiolabeled calcium into the bone (844). These findings were also replicated in chicken (842). The unknown hormone was tentatively named ”gastrocalcin“ (844). When ghrelin was discovered, it was speculated that it might represent a candidate hormone for gastrocalcin. However, unlike gastrocalcin, ghrelin is not under gastrin control, making this proposition unlikely (276). Subsequent investigations suggested that the origin of gastrocalcin were the gastric ECL cells. ECL extracts can indeed trigger a calcium second messenger response in osteoblast (629, 630). Yet, functional evidence for gastrocalcin-mediated osteoblast activation is still lacking. A recent report postulates that parathyroid hormone-like hormone (PTHLH) may in fact be gastrocalcin (676). PTHLH exerts similar physiological effects as PTH by sharing a common receptor and is commonly elevated in paraneoplastic syndromes (1056). PTHLH has been identified in ECL cells, and its transcription has been shown to be inducible by gastrin in parietal cells (523, 676). Of note, PTH causes effects opposite to those assigned to gastrin and gastrocalcin, namely, hypercalcemia. Further studies will thus be needed to corroborate this hypothesis. Whatever the exact effector hormone of gastrin may be, changes in gastrin levels cannot entirely explain the clinical phenomenon of postgastrectomy osteopenia and PPI-related fractures. Although vagotomy and PPIs undoubtedly increase serum gastrin levels through a negative-feedback mechanism, most partial and all total gastrectomies result in hypogastrinemia. Yet both hyper- and hypogastrinemic conditions have similar outcomes, i.e., osteopenia and increased risks of fractures. It is, of course, plausible that different factors contribute to this outcome in each individual group. Gastrin may be involved in certain pathologies, but given that its true impact on bone metabolism is somewhat elusive, this assumption remains speculative. Acid suppression Calcium solubility Gastrin ? Intestinal absorption Pancreastatin Calcitonin PTH FIGURE 9. Model summarizing the potential impact of acid suppression on calcium homeostasis. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 237 SASCHA KOPIC AND JOHN P. GEIBEL 3. Pancreastatin Pancreastatin is a cleavage product of chromogranin A that was initially isolated from porcine pancreas (1072). Gastric ECL cells are also known to harbor significant amounts of chromogranin A and pancreastatin. Pancreastatin is secreted together with histamine from ECL cells in response to their neuroendocrine stimulation (see sect. IIB3) (176). In rat, it has been shown that the serum pancreastatin levels correlate with the secretory status of ECL cells. States that enhance ECL cell secretion, such as gastrin infusion, resulted in elevated serum pancreastatin levels (411). In accordance with this hypothesis, and of special relevance for the topic of this review, PPI therapy also resulted in increased serum pancreastatin levels (the ECL is stimulated by gastrin, which in turn is released in response to high gastric pH) (411). These observations led the investigators to conclude that ECL cells are a major contributor to the serum levels of pancreastatin in the rat and that these levels change in parallel with ECL cell secretion (411). This is also corroborated by the observation that gastrectomy reduces pancreastatin levels in rats (644). review we have focused on the important role calcium plays as a first and second messenger in the maintenance of bone health. By relying on a complex series of receptors, channels, and transport proteins, calcium is tightly controlled at the cellular and tissue level to ensure its bioavailability to bone. Modulations to any of these pathways by disease, mutation, or pharmaceutical perturbation can lead to clinical changes in bone health. ACKNOWLEDGMENTS S. Kopic is a Howard Hughes Medical Institute International Student Research Fellow. Special thanks to Sashka Dimitrievska for her untiring support and critical editing of the manuscript. Address for reprint requests and other correspondence: J. P. Geibel, Yale School of Medicine, 310 Cedar St., BML 238, New Haven, CT 06510 (e-mail: John.geibel@yale.edu). DISCLOSURES Pancreastatin exerts a variety of metabolic effects. Apart from influencing energy metabolism, pancreasstatin was shown to affect the secretion of PTH from the parathyroid gland. In isolated bovine and porcine parathyroid cells, pancreastatin has a clear inhibitory effect on PTH secretion (282, 317, 911). The suppression of PTH functions on a transcriptional level (1211). Reduced PTH secretion in turn would have a potential impact on calcium and bone metabolism. Whether the same observations are valid for humans is less clear, as pancreastatin failed to inhibit PTH secretion from isolated human parathyroid cells (911). Regardless, the volume of data on pancreastatin and its influence on the parathyroid gland is very small, and further investigations would be necessary to establish this intriguing link. Apart from potential modulation of parathyroid secretion, pancreastatin has also been shown to have an inhibitory effect on gastric acid secretion (655). In summary, it should be noted that the stomach secretes not only acid, but also hormones that have been shown to directly alter calcium and/or bone homeostasis. The secretion of these hormones depends on the neuroendocrine machinery that also regulates acid secretion. It is therefore plausible that the correlation between states of impaired acid secretion and impaired bone mineralization is multifactorial by depending on intragastric pH and serum levels of gastric hormones (FIGURE 9). No conflicts of interest, financial or otherwise, are declared by the authors. REFERENCES 1. Abe K, Kaya S, Taniguchi K, Hayashi Y, Imagawa T, Kikumoto M, Oiwa K, Sakaguchi K. Evidence for a relationship between activity and the tetraprotomeric assembly of solubilized pig gastric H/K-ATPase. J Biochem 138: 293–301, 2005. 2. Abe K, Tani K, Fujiyoshi Y. Conformational rearrangement of gastric H⫹,K⫹-ATPase induced by an acid suppressant. Nat Commun 2: 155, 2011. 3. Abe K, Tani K, Fujiyoshi Y. Structural and functional characterization of H⫹,K⫹ATPase with bound fluorinated phosphate analogs. J Struct Biol 170: 60 – 68, 2010. 4. Abe K, Tani K, Nishizawa T, Fujiyoshi Y. Inter-subunit interaction of gastric H⫹,K⫹ATPase prevents reverse reaction of the transport cycle. EMBO J 28: 1637–1643, 2009. 5. Abou-Samra AB, Juppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JT Jr. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA 89: 2732–2736, 1992. 6. Abreo K, Adlakha A, Kilpatrick S, Flanagan R, Webb R, Shakamuri S. The milk-alkali syndrome. A reversible form of acute renal failure. Arch Intern Med 153: 1005–1010, 1993. 7. Adams JS, Clemens TL, Parrish JA, Holick MF. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med 306: 722–725, 1982. VI. CONCLUSIONS 8. Agnew JE, Holdsworth CD. The effect of fat on calcium absorption from a mixed meal in normal subjects, patients with malabsorptive disease, and patients with a partial gastrectomy. Gut 12: 973–977, 1971. We set out in this review to demonstrate that gastric and intestinal physiology are intertwined to regulate calcium absorption and secretion to maintain bone health. In this 9. Aihara T, Fujishita T, Kanatani K, Furutani K, Nakamura E, Taketo MM, Matsui M, Chen D, Okabe S. Impaired gastric secretion and lack of trophic responses to hypergastrinemia in M3 muscarinic receptor knockout mice. Gastroenterology 125: 1774 –1784, 2003. 238 Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH 10. Aihara T, Nakamura Y, Taketo MM, Matsui M, Okabe S. Cholinergically stimulated gastric acid secretion is mediated by M3 and M5 but not M1 muscarinic acetylcholine receptors in mice. Am J Physiol Gastrointest Liver Physiol 288: G1199 –G1207, 2005. 30. Anusaksathien O, Laplace C, Li X, Ren Y, Peng L, Goldring SR, Galson DL. Tissuespecific and ubiquitous promoters direct the expression of alternatively spliced transcripts from the calcitonin receptor gene. J Biol Chem 276: 22663–22674, 2001. 11. Akeno N, Saikatsu S, Kawane T, Horiuchi N. Mouse vitamin D-24-hydroxylase: molecular cloning, tissue distribution, transcriptional regulation by 1alpha,25-dihydroxyvitamin D3. Endocrinology 138: 2233–2240, 1997. 31. Ardaillou R. Kidney and calcitonin. Nephron 15: 250 –260, 1975. 12. Akey JM, Swanson WJ, Madeoy J, Eberle M, Shriver MD. TRPV6 exhibits unusual patterns of polymorphism and divergence in worldwide populations. Hum Mol Genet 15: 2106 –2113, 2006. 13. Akhter S, Kutuzova GD, Christakos S, DeLuca HF. Calbindin D9k is not required for 1,25-dihydroxyvitamin D3-mediated Ca2⫹ absorption in small intestine. Arc Biochem Biophys 460: 227–232, 2007. 14. Akiyoshi-Shibata M, Sakaki T, Ohyama Y, Noshiro M, Okuda K, Yabusaki Y. Further oxidation of hydroxycalcidiol by calcidiol 24-hydroxylase. A study with the mature enzyme expressed in Escherichia coli. Eur J Biochem 224: 335–343, 1994. 15. Al-Ansary D, Bogeski I, Disteldorf BMJ, Becherer U, Niemeyer BA. ATP modulates Ca2⫹ uptake by TRPV6 and is counteracted by isoform-specific phosphorylation. FASEB J 24: 425– 435, 2010. 16. Alam AS, Moonga BS, Bevis PJ, Huang CL, Zaidi M. Amylin inhibits bone resorption by a direct effect on the motility of rat osteoclasts. Exp Physiol 78: 183–196, 1993. 32. Ardaillou R, Vuagnat P, Milhaud G, Richet G. Effects of thyrocalcitonin on the renal excretion of phosphates, calcium and hydrogen ions in man. Nephron 4: 298 –314, 1967. 33. Aris RM, Lester GE, Dingman S, Ontjes DA. Altered calcium homeostasis in adults with cystic fibrosis. Osteoporos Int 10: 102–108, 1999. 34. Arman E, Nilsson LH, Reizenstein P. Studies in the dumping syndrome. VI. Calcium deficiency after partial gastrectomy. Am J Dig Dis 15: 455– 462, 1970. 35. Armbrecht HJ, Boltz MA, Kumar VB. Intestinal plasma membrane calcium pump protein and its induction by 1,25(OH)2D3 decrease with age. Am J Physiol Gastrointest Liver Physiol 277: G41–G47, 1999. 36. Armbrecht HJ, Boltz MA, Wongsurawat N. Expression of plasma membrane calcium pump mRNA in rat intestine: effect of age and 1,25-dihydroxyvitamin D. Biochim Biophys Acta 1195: 110 –114, 1994. 37. Armbrecht HJ, Hodam TL, Boltz MA, Partridge NC, Brown AJ, Kumar VB. Induction of the vitamin D 24-hydroxylase (CYP24) by 1,25-dihydroxyvitamin D3 is regulated by parathyroid hormone in UMR106 osteoblastic cells. Endocrinology 139: 3375– 3381, 1998. 17. Albrandt K, Brady EM, Moore CX, Mull E, Sierzega ME, Beaumont K. Molecular cloning and functional expression of a third isoform of the human calcitonin receptor and partial characterization of the calcitonin receptor gene. Endocrinology 136: 5377–5384, 1995. 38. Arnold A, Horst SA, Gardella TJ, Baba H, Levine MA, Kronenberg HM. Mutation of the signal peptide-encoding region of the preproparathyroid hormone gene in familial isolated hypoparathyroidism. J Clin Invest 86: 1084 –1087, 1990. 18. Allen LH. Calcium bioavailability and absorption: a review. Am J Clin Nutr 35: 783– 808, 1982. 39. Arnold R, Koop H, Schwarting H, Tuch K, Willemer B. Effect of acid inhibition on gastric endocrine cells. Scand J Gastroenterol Suppl 125: 14 –19, 1986. 19. Alumets J, Ekelund M, El Munshid HA, Hakanson R, Loren I, Sundler F. Topography of somatostatin cells in the stomach of the rat: possible functional significance. Cell Tissue Res 202: 177–188, 1979. 40. Arthur JM, Collinsworth GP, Gettys TW, Quarles LD, Raymond JR. Specific coupling of a cation-sensing receptor to G protein ␣-subunits in MDCK cells. Am J Physiol Renal Physiol 273: F129 –F135, 1997. 20. Aly A, Shulkes A, Baldwin GS. Short term infusion of glycine-extended gastrin(17) stimulates both proliferation and formation of aberrant crypt foci in rat colonic mucosa. Int J Cancer 94: 307–313, 2001. 41. Asano S, Kawada K, Kimura T, Grishin AV, Caplan MJ, Takeguchi N. The roles of carbohydrate chains of the beta-subunit on the functional expression of gastric H⫹,K⫹-ATPase. J Biol Chem 275: 8324 – 8330, 2000. 21. Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 140: 4982– 4987, 1999. 42. Asano S, Yoshida A, Yashiro H, Kobayashi Y, Morisato A, Ogawa H, Takeguchi N, Morii M. The cavity structure for docking the K⫹-competitive inhibitors in the gastric proton pump. J Biol Chem 279: 13968 –13975, 2004. 22. Andersen BN. Species variation in the tyrosine sulfation of mammalian gastrins. Gen Comp Endocrinol 58: 44 –50, 1985. 23. Anderson NG, Hanson PJ. Involvement of calcium-sensitive phospholipid-dependent protein kinase in control of acid secretion by isolated rat parietal cells. Biochem J 232: 609 – 611, 1985. 24. Andersson K, Cabero JL, Mattsson H, Hakanson R. Gastric acid secretion after depletion of enterochromaffin-like cell histamine. A study with alpha-fluoromethylhistidine in rats. Scand J Gastroenterol 31: 24 –30, 1996. 25. Andersson S, Chang D, Folkers K, Rosell S. Inhibition of gastric acid secretion in dogs by neurotensin. Life Sci 19: 367–370, 1976. 26. Andersson S, Davis DL, Dahlback H, Jornvall H, Russell DW. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem 264: 8222– 8229, 1989. 27. Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1–34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res 14: 960 –968, 1999. 28. Andreotti G, Mendez BL, Amodeo P, Morelli MA, Nakamuta H, Motta A. Structural determinants of salmon calcitonin bioactivity: the role of the Leu-based amphipathic alpha-helix. J Biol Chem 281: 24193–24203, 2006. 29. Anisuzzaman AS, Morishima S, Suzuki F, Tanaka T, Muramatsu I. Identification of M(1) muscarinic receptor subtype in rat stomach using a tissue segment binding method, the effects of immobilization stress on the muscarinic receptors. Eur J Pharmacol 599: 146 –151, 2008. 43. Askew FA, Bruce HM, Callow RK, Philpot JS, Webster TA. Crystalline vitamin D. Nature 128: 758, 1931. 44. Athmann C, Zeng N, Scott DR, Sachs G. Regulation of parietal cell calcium signaling in gastric glands. Am J Physiol Gastrointest Liver Physiol 279: G1048 –G1058, 2000. 45. Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, O’Loughlin PD, Morris HA. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone 40: 1517–1528, 2007. 46. Auchere D, Tardivel S, Gounelle JC, Drueke T, Lacour B. Role of transcellular pathway in ileal Ca2⫹ absorption: stimulation by low-Ca2⫹ diet. Am J Physiol Gastrointest Liver Physiol 275: G951–G956, 1998. 47. Auer J, Reeh PW, Fischer MJ. Acid-induced CGRP release from the stomach does not depend on TRPV1 or ASIC3. Neurogastroenterol Motil 22: 680 – 687, 2010. 48. Awata H, Huang C, Handlogten ME, Miller RT. Interaction of the calcium-sensing receptor and filamin, a potential scaffolding protein. J Biol Chem 276: 34871–34879, 2001. 49. Axelson J, Persson P, Gagnemo-Persson R, Hakanson R. Importance of the stomach in maintaining calcium homoeostasis in the rat. Gut 32: 1298 –1302, 1991. 50. Axen E, Postlind H, Sjoberg H, Wikvall K. Liver mitochondrial cytochrome P450 CYP27 and recombinant-expressed human CYP27 catalyze 1␣-hydroxylation of 25-hydroxyvitamin D3. Proc Natl Acad Sci USA 91: 10014 –10018, 1994. 51. Axen E, Postlind H, Wikvall K. Effects on CYP27 mRNA expression in rat kidney and liver by 1␣,25-dihydroxyvitamin D3, a suppressor of renal 25-hydroxyvitamin D3 1␣-hydroxylase activity. Biochem Biophys Res Commun 215: 136 –141, 1995. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 239 SASCHA KOPIC AND JOHN P. GEIBEL 52. Ba J, Brown D, Friedman PA. Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. Am J Physiol Renal Physiol 285: F1233– F1243, 2003. 74. Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion 51 Suppl 1: 59 – 67, 1992. 53. Bado A, Cloarec D, Moizo L, Laigneau JP, Bataille D, Lewin MJ. Neurotensin and oxyntomodulin-(30 –37) potentiate PYY regulation of gastric acid and somatostatin secretions. Am J Physiol Gastrointest Liver Physiol 265: G113–G117, 1993. 75. Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O’Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem 278: 50259 –50272, 2003. 54. Bai M, Trivedi S, Brown EM. Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J Biol Chem 273: 23605–23610, 1998. 55. Bai M, Trivedi S, Kifor O, Quinn SJ, Brown EM. Intermolecular interactions between dimeric calcium-sensing receptor monomers are important for its normal function. Proc Natl Acad Sci USA 96: 2834 –2839, 1999. 56. Bai M, Trivedi S, Lane CR, Yang Y, Quinn SJ, Brown EM. Protein kinase C phosphorylation of threonine at position 888 in Ca2⫹o-sensing receptor (CaR) inhibits coupling to Ca2⫹ store release. J Biol Chem 273: 21267–21275, 1998. 57. Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr 140: 817– 822, 2010. 58. Baird IM, Oleesky S. Osteomalacia following gastric surgery. Gastroenterology 33: 284 –292, 1957. 59. Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, Pike JW, Shine J, O’Malley BW. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci USA 85: 3294 –3298, 1988. 60. Bakke I, Qvigstad G, Brenna E, Sandvik AK, Waldum HL. Gastrin has a specific proliferative effect on the rat enterochromaffin-like cell, but not on the parietal cell: a study by elutriation centrifugation. Acta Physiol Scand 169: 29 –37, 2000. 61. Baldissera FG, Nielsen OV, Holst JJ. The intestinal mucosa preferentially releases somatostatin-28 in pigs. Regul Pept 11: 251–262, 1985. 62. Balesaria S, Sangha S, Walters JR. Human duodenum responses to vitamin D metabolites of TRPV6 and other genes involved in calcium absorption. Am J Physiol Gastrointest Liver Physiol 297: G1193–G1197, 2009. 63. Barger G, Dale HH. The presence in ergot and physiological activity of beta-imidazolylethylamine. J Physiol 40: xxxviii-xl, 1910. 76. Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindinD9k. Endocrinology 149: 3196 –3205, 2008. 77. Benoit R, Ling N, Esch F. A new prosomatostatin-derived peptide reveals a pattern for prohormone cleavage at monobasic sites. Science 238: 1126 –1129, 1987. 78. Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci 102: 341–351, 1992. 79. Beresford JN, Joyner CJ, Devlin C, Triffitt JT. The effects of dexamethasone and 1,25-dihydroxyvitamin D3 on osteogenic differentiation of human marrow stromal cells in vitro. Arch Oral Biol 39: 941–947, 1994. 80. Berg A, Kechagias S, Sjostrand SE, Ericson AC. Morphological support for paracrine inhibition of gastric acid secretion by nitric oxide in humans. Scand J Gastroenterol 36: 1016 –1021, 2001. 81. Berg A, Redeen S, Ericson AC, Sjostrand SE. Nitric oxide-an endogenous inhibitor of gastric acid secretion in isolated human gastric glands. BMC Gastroenterol 4: 16, 2004. 82. Berg A, Redeen S, Grenegard M, Ericson AC, Sjostrand SE. Nitric oxide inhibits gastric acid secretion by increasing intraparietal cell levels of cGMP in isolated human gastric glands. Am J Physiol Gastrointest Liver Physiol 289: G1061–G1066, 2005. 83. Berginer VM, Shany S, Alkalay D, Berginer J, Dekel S, Salen G, Tint GS, Gazit D. Osteoporosis and increased bone fractures in cerebrotendinous xanthomatosis. Metabolism 42: 69 –74, 1993. 84. Berndt TJ, Knox FG. Effects of parathyroid hormone and calcitonin on electrolyte excretion in the rabbit. Kidney Int 17: 473– 478, 1980. 64. Barley NF, Howard A, O’Callaghan D, Legon S, Walters JR. Epithelial calcium transporter expression in human duodenum. Am J Physiol Gastrointest Liver Physiol 280: G285–G290, 2001. 85. Bers DM, Ginsburg KS. Na:Ca stoichiometry and cytosolic Ca-dependent activation of NCX in intact cardiomyocytes. Ann NY Acad Sci 1099: 326 –338, 2007. 65. Basso D, Scrigner M, Toma A, Navaglia F, Di Mario F, Rugge M, Plebani M. Helicobacter pylori infection enhances mucosal interleukin-1 beta, interleukin-6, the soluble receptor of interleukin-2. Int J Clin Lab Res 26: 207–210, 1996. 86. Berthoud HR. Morphological analysis of vagal input to gastrin releasing peptide and vasoactive intestinal peptide containing neurons in the rat glandular stomach. J Comp Neurol 370: 61–70, 1996. 66. Bataille D, Blache P, Mercier F, Jarrousse C, Kervran A, Dufour M, Mangeat P, Dubrasquet M, Mallat A, Lotersztajn S. Glucagon and related peptides Molecular structure and biological specificity. Ann NY Acad Sci 527: 168 –185, 1988. 87. Bertrand C, Kowalski-Chauvel A, Do C, Resa C, Najib S, Daulhac L, Wang TC, Ferrand A, Seva C. A gastrin precursor, gastrin-gly, upregulates VEGF expression in colonic epithelial cells through an HIF-1-independent mechanism. Int J Cancer 126: 2847–2857, 2010. 67. Batzri S, Gardner JD. Cellular cyclic AMP in dispersed mucosal cells from guinea pig stomach. Biochim Biophys Acta 541: 181–189, 1978. 68. Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol 28: 325– 353, 1902. 69. Beales IL, Calam J. Inhibition of carbachol stimulated acid secretion by interleukin 1beta in rabbit parietal cells requires protein kinase C. Gut 48: 782–789, 2001. 70. Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut 42: 227–234, 1998. 71. Beales IL, Ogunwobi OO. Glycine-extended gastrin inhibits apoptosis in Barrett’s oesophageal and oesophageal adenocarcinoma cells through JAK2/STAT3 activation. J Mol Endocrinol 42: 305–318, 2009. 88. Bertrand CA, Zhang R, Pilewski JM, Frizzell RA. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J Gen Physiol 133: 421– 438, 2009. 89. Besancon M, Shin JM, Mercier F, Munson K, Miller M, Hersey S, Sachs G. Membrane topology and omeprazole labeling of the gastric H⫹,K⫹-adenosinetriphosphatase. Biochemistry 32: 2345–2355, 1993. 90. Besancon M, Simon A, Sachs G, Shin JM. Sites of reaction of the gastric H⫹-K⫹ATPase with extracytoplasmic thiol reagents. J Biol Chem 272: 22438 –22446, 1997. 91. Bhattacharyya MH, DeLuca HF. The regulation of calciferol-25-hydroxylase in the chick. Biochem Biophys Res Commun 59: 734 –741, 1974. 92. Bhattacharyya MH, DeLuca HF. The regulation of rat liver calciferol-25-hydroxylase. J Biol Chem 248: 2969 –2973, 1973. 72. Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA 95: 5372–5377, 1998. 93. Bhattacharyya MH, DeLuca HF. Subcellular location of rat liver calciferol-25-hydroxylase. Arch Biochem Biophys 160: 58 – 62, 1974. 73. Beil W, Mannschedel W, Sewing KF. Protein kinase C and parietal cell function. Biochem Biophys Res Commun 149: 720 –728, 1987. 94. Bianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, Zhuang L, Freeman MR, Gouveia CH, Wu J, Luo H, Mauro T, Brown EM, Hediger MA. Marked distur- 240 Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH bance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res 22: 274 –285, 2007. 95. Bieglmayer C, Prager G, Niederle B. Kinetic analyses of parathyroid hormone clearance as measured by three rapid immunoassays during parathyroidectomy. Clin Chem 48: 1731–1738, 2002. 96. Bielefeldt K, Davis BM. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am J Physiol Gastrointest Liver Physiol 294: G130 –G138, 2008. 97. Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest 74: 1966 –1971, 1984. 98. Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab 63: 954 –959, 1986. 99. Bindels RJ, Hartog A, Timmermans J, Van Os CH. Active Ca2⫹ transport in primary cultures of rabbit kidney CCD: stimulation by 1,25-dihydroxyvitamin D3 and PTH. Am J Physiol Renal Fluid Electrolyte Physiol 261: F799 –F807, 1991. 100. Birge SJ, Gilbert HR. Indentification of an intestinal sodium and calcium-dependent phosphatase stimulated by parathyroid hormone. J Clin Invest 54: 710 –717, 1974. 101. Bisballe S, Eriksen EF, Melsen F, Mosekilde L, Sorensen OH, Hessov I. Osteopenia and osteomalacia after gastrectomy: interrelations between biochemical markers of bone remodelling, vitamin D metabolites, and bone histomorphometry. Gut 32: 1303–1307, 1991. 102. Bjorkhem I, Hansson R, Holmberg I, Wikvall K. 25-Hydroxylation of vitamin D3 by a reconstituted system from rat liver microsomes. Biochem Biophys Res Commun 90: 615– 622, 1979. 103. Bjorkhem I, Holmberg I. Assay and properties of a mitochondrial 25-hydroxylase active on vitamine D3. J Biol Chem 253: 842– 849, 1978. 104. Bjorkhem I, Holmberg I, Oftebro H, Pedersen JI. Properties of a reconstituted vitamin D3 25-hydroxylase from rat liver mitochondria. J Biol Chem 255: 5244 –5249, 1980. 105. Bjorkqvist M, Bernsand M, Eliasson L, Hakanson R, Lindstrom E. Somatostatin, misoprostol and galanin inhibit gastrin- and PACAP-stimulated secretion of histamine and pancreastatin from ECL cells by blocking specific Ca2⫹ channels. Regul Pept 130: 81–90, 2005. 106. Black JW, Duncan WA, Durant CJ, Ganellin CR, Parsons EM. Definition and antagonism of histamine H2-receptors. Nature 236: 385–390, 1972. 107. Black JW, Fisher EW, Smith AN. The effects of 5-hydroxytryptamine on gastric secretion in anaesthetized dogs. J Physiol 141: 27–34, 1958. 108. Blackburn AM, Fletcher DR, Bloom SR, Christofides ND, Long RG, Fitzpatrick ML, Baron JH. Effect of neurotensin on gastric function in man. Lancet 1: 987–989, 1980. 109. Bland R, Walker EA, Hughes SV, Stewart PM, Hewison M. Constitutive expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in a transformed human proximal tubule cell line: evidence for direct regulation of vitamin D metabolism by calcium. Endocrinology 140: 2027–2034, 1999. 110. Blichert-Toft M, Beck A, Christiansen C, Transbol I. Effects of gastric resection and vagotomy on blood and bone mineral content. World J Surg 3: 99 –102, 133–105, 1979. 111. Blunt JW, DeLuca HF. The synthesis of 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry 8: 671– 675, 1969. 115. Boivin G, Mesguich P, Pike JW, Bouillon R, Meunier PJ, Haussler MR, Dubois PM, Morel G. Ultrastructural immunocytochemical localization of endogenous 1,25-dihydroxyvitamin D3 and its receptors in osteoblasts and osteocytes from neonatal mouse and rat calvaria. Bone Miner 3: 125–136, 1987. 116. Booth BE, Tsai HC, Morris RC Jr. Vitamin D status regulates 25-hydroxyvitamin D3-1 alpha-hydroxylase and its responsiveness to parathyroid hormone in the chick. J Clin Invest 75: 155–161, 1985. 117. Borthwick LA, Neal A, Hobson L, Gerke V, Robson L, Muimo R. The annexin 2–S100A10 complex and its association with TRPV6 is regulated by cAMP/PKA/CnA in airway and gut epithelia. Cell Calcium 44: 147–157, 2008. 118. Bosl MR, Stein V, Hubner C, Zdebik AA, Jordt SE, Mukhopadhyay AK, Davidoff MS, Holstein AF, Jentsch TJ. Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2 Cl⫺ channel disruption. EMBO J 20: 1289 –1299, 2001. 119. Bouley R, Lu HA, Nunes P, Da Silva N, McLaughlin M, Chen Y, Brown D. Calcitonin has a vasopressin-like effect on aquaporin-2 trafficking and urinary concentration. J Am Soc Nephrol 22: 59 –72, 2011. 120. Bourdeau A, Moutahir M, Souberbielle JC, Bonnet P, Herviaux P, Sachs C, Lieberherr M. Effects of lipoxygenase products of arachidonate metabolism on parathyroid hormone secretion. Endocrinology 135: 1109 –1112, 1994. 121. Bourdeau A, Souberbielle JC, Bonnet P, Herviaux P, Sachs C, Lieberherr M. Phospholipase-A2 action and arachidonic acid metabolism in calcium-mediated parathyroid hormone secretion. Endocrinology 130: 1339 –1344, 1992. 122. Brandsborg M, Nielsen HE, Brandsborg O, Olsen KJ, Lovgreen NA. The role of serum gastrin in the secretion of calcitonin: studies in patients with pernicious anaemia and in healthy subjects. Scand J Gastroenterol 15: 23–28, 1980. 123. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA 302: 1573–1579, 2009. 124. Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179: 77–79, 1973. 125. Brenza HL, DeLuca HF. Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys 381: 143–152, 2000. 126. Brewer HB Jr, Ronan R. Bovine parathyroid hormone: amino acid sequence. Proc Natl Acad Sci USA 67: 1862–1869, 1970. 127. Bringhurst FR, Stern AM, Yotts M, Mizrahi N, Segre GV, Potts JT Jr. Peripheral metabolism of PTH: fate of biologically active amino terminus in vivo. Am J Physiol Endocrinol Metab 255: E886 –E893, 1988. 128. Brommage R. Measurement of calcium and phosphorus fluxes during lactation in the rat. J Nutr 119: 428 – 438, 1989. 129. Bronner F, Pansu D, Stein WD. An analysis of intestinal calcium transport across the rat intestine. Am J Physiol Gastrointest Liver Physiol 250: G561–G569, 1986. 130. Brossard JH, Cloutier M, Roy L, Lepage R, Gascon-Barre M, D’Amour P. Accumulation of a non-(1– 84) molecular form of parathyroid hormone (PTH) detected by intact PTH assay in renal failure: importance in the interpretation of PTH values. J Clin Endocrinol Metab 81: 3923–3929, 1996. 131. Brossard JH, Whittom S, Lepage R, D’Amour P. Carboxyl-terminal fragments of parathyroid hormone are not secreted preferentially in primary hyperparathyroidism as they are in other hypercalcemic conditions. J Clin Endocrinol Metab 77: 413– 419, 1993. 112. Blunt JW, DeLuca HF, Schnoes HK. 25-Hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry 7: 3317–3322, 1968. 132. Brown AJ, Krits I, Armbrecht HJ. Effect of age, vitamin D, and calcium on the regulation of rat intestinal epithelial calcium channels. Arch Biochem Biophys 437: 51–58, 2005. 113. Boel E, Vuust J, Norris F, Norris K, Wind A, Rehfeld JF, Marcker KA. Molecular cloning of human gastrin cDNA: evidence for evolution of gastrin by gene duplication. Proc Natl Acad Sci USA 80: 2866 –2869, 1983. 133. Brown E, Enyedi P, LeBoff M, Rotberg J, Preston J, Chen C. High extracellular Ca2⫹ and Mg2⫹ stimulate accumulation of inositol phosphates in bovine parathyroid cells. FEBS Lett 218: 113–118, 1987. 114. Bohmer C, Palmada M, Kenngott C, Lindner R, Klaus F, Laufer J, Lang F. Regulation of the epithelial calcium channel TRPV6 by the serum and glucocorticoid-inducible kinase isoforms SGK1 and SGK3. FEBS Lett 581: 5586 –5590, 2007. 134. Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2⫹sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 241 SASCHA KOPIC AND JOHN P. GEIBEL 135. Brown J 3rd, Theisler C, Silberman S, Magnuson D, Gottardi-Littell N, Lee JM, Yager D, Crowley J, Sambamurti K, Rahman MM, Reiss AB, Eckman CB, Wolozin B. Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J Biol Chem 279: 34674 –34681, 2004. 136. Brown MR, Chew CS. Multiple effects of phorbol ester on secretory activity in rabbit gastric glands and parietal cells. Can J Physiol Pharmacol 65: 1840 –1847, 1987. 137. Bruley des Varannes S, Levy P, Lartigue S, Dellatolas F, Lemaire M, Galmiche JP. Comparison of lansoprazole with omeprazole on 24-hour intragastric pH, acid secretion and serum gastrin in healthy volunteers. Aliment Pharmacol Ther 8: 309 –314, 1994. 138. Bruns ME, Fleisher EB, Avioli LV. Control of vitamin D-dependent calcium-binding protein in rat intestine by growth and fasting. J Biol Chem 252: 4145– 4150, 1977. 156. Care AD, Bruce JB, Boelkins J, Kenny AD, Conaway H, Anast CS. Role of pancreozymin-cholecystokinin and structurally related compounds as calcitonin secretogogues. Endocrinology 89: 262–271, 1971. 157. Carles-Bonnet C, Jarrousse C, Niel H, Martinez J, Bataille D. Oxyntomodulin and its (19 –37) and (30 –37) fragments inhibit histamine-stimulated gastric acid secretion in the conscious rat. Eur J Pharmacol 203: 245–252, 1991. 158. Carney S, Thompson L. Acute effect of calcitonin on rat renal electrolyte transport. Am J Physiol Renal Fluid Electrolyte Physiol 240: F12–F16, 1981. 159. Carney SL. Acute effect of endogenous calcitonin on rat renal function. Miner Electrolyte Metab 21: 411– 416, 1995. 160. Carney SL. Calcitonin and human renal calcium and electrolyte transport. Miner Electrolyte Metab 23: 43– 47, 1997. 139. Bu FX, Armas L, Lappe J, Zhou Y, Gao G, Wang HW, Recker R, Zhao LJ. Comprehensive association analysis of nine candidate genes with serum 25-hydroxy vitamin D levels among healthy Caucasian subjects. Hum Genet 128: 549 –556, 2010. 161. Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem 248: 6854 – 6861, 1973. 140. Buchan AM, MacLeod MD, Meloche RM, Kwok YN. Muscarinic regulation of somatostatin release from primary cultures of human antral epithelial cells. Pharmacology 44: 33– 40, 1992. 162. Carrillo-Lopez N, Alvarez-Hernandez D, Gonzalez-Suarez I, Roman-Garcia P, Valdivielso JM, Fernandez-Martin JL, Cannata-Andia JB. Simultaneous changes in the calcium-sensing receptor and the vitamin D receptor under the influence of calcium and calcitriol. Nephrol Dial Transplant 23: 3479 –3484, 2008. 141. Buchan AM, Meloche RM, Kwok YN, Kofod H. Effect of cholecystokinin and secretin on somatostatin release from cultured antral cells. Gastroenterology 104: 1414 – 1419, 1993. 163. Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398: 436 – 441, 1999. 142. Buchan AM, Squires PE, Ring M, Meloche RM. Mechanism of action of the calciumsensing receptor in human antral gastrin cells. Gastroenterology 120: 1128 –1139, 2001. 164. Catlow K, Ashurst HL, Varro A, Dimaline R. Identification of a gastrin response element in the vesicular monoamine transporter type 2 promoter and requirement of 20 S proteasome subunits for transcriptional activity. J Biol Chem 282: 17069 – 17077, 2007. 143. Buclin T, Cosma Rochat M, Burckhardt P, Azria M, Attinger M. Bioavailability and biological efficacy of a new oral formulation of salmon calcitonin in healthy volunteers. J Bone Miner Res 17: 1478 –1485, 2002. 144. Bugrim AE. Regulation of Ca2⫹ release by cAMP-dependent protein kinase. A mechanism for agonist-specific calcium signaling? Cell Calcium 25: 219 –226, 1999. 145. Busque SM, Kerstetter JE, Geibel JP, Insogna K. L-type amino acids stimulate gastric acid secretion by activation of the calcium-sensing receptor in parietal cells. Am J Physiol Gastrointest Liver Physiol 289: G664 –G669, 2005. 146. Cai Q, Chandler JS, Wasserman RH, Kumar R, Penniston JT. Vitamin D and adaptation to dietary calcium and phosphate deficiencies increase intestinal plasma membrane calcium pump gene expression. Proc Natl Acad Sci USA 90: 1345–1349, 1993. 147. Calhoun BC, Lapierre LA, Chew CS, Goldenring JR. Rab11a redistributes to apical secretory canaliculus during stimulation of gastric parietal cells. Am J Physiol Cell Physiol 275: C163–C170, 1998. 148. Cali JJ, Hsieh CL, Francke U, Russell DW. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem 266: 7779 –7783, 1991. 149. Cali JJ, Russell DW. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J Biol Chem 266: 7774 –7778, 1991. 150. Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: a global perspective of current status. J Nutr 135: 310 –316, 2005. 151. Canadillas S, Canalejo R, Rodriguez-Ortiz ME, Martinez-Moreno JM, Estepa JC, Zafra R, Perez J, Munoz-Castaneda JR, Canalejo A, Rodriguez M, Almaden Y. The up-regulation of the parathyroid VDR expression by extracellular calcium is mediated by the ERK1/2-MAPK signaling pathway. Am J Physiol Renal Physiol 2010. 152. Canalejo A, Canadillas S, Ballesteros E, Rodriguez M, Almaden Y. Importance of arachidonic acid as a mediator of parathyroid gland response. Kidney Int Suppl S10 – S13, 2003. 153. Canfield SP, Spencer JE. The inhibitory effects of 5-hydroxytryptamine on gastric acid secretion by the rat isolated stomach. Br J Pharmacol 78: 123–129, 1983. 165. Ceglia L, Harris SS, Rasmussen HM, Dawson-Hughes B. Activation of the calcium sensing receptor stimulates gastrin and gastric acid secretion in healthy participants. Osteoporos Int 20: 71–78, 2009. 166. Chabardes D, Gagnan-Brunette M, Imbert-Teboul M, Gontcharevskaia O, Montegut M, Clique A, Morel F. Adenylate cyclase responsiveness to hormones in various portions of the human nephron. J Clin Invest 65: 439 – 448, 1980. 167. Chabre O, Conklin BR, Lin HY, Lodish HF, Wilson E, Ives HE, Catanzariti L, Hemmings BA, Bourne HR. A recombinant calcitonin receptor independently stimulates 3=,5=-cyclic adenosine monophosphate and Ca2⫹/inositol phosphate signaling pathways. Mol Endocrinol 6: 551–556, 1992. 168. Chai SY, Christopoulos G, Cooper ME, Sexton PM. Characterization of binding sites for amylin, calcitonin, and CGRP in primate kidney. Am J Physiol Renal Physiol 274: F51–F62, 1998. 169. Chambers TJ, Magnus CJ. Calcitonin alters behaviour of isolated osteoclasts. J Pathol 136: 27–39, 1982. 170. Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310: 490 – 493, 2005. 171. Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal 1: ra1, 2008. 172. Chang W, Tu C, Chen TH, Komuves L, Oda Y, Pratt SA, Miller S, Shoback D. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology 140: 5883–5893, 1999. 173. Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, Hebert SC, Soybel DI, Brown EM. Identification and localization of extracellular Ca2⫹-sensing receptor in rat intestine. Am J Physiol Gastrointest Liver Physiol 274: G122–G130, 1998. 174. Chattopadhyay N, Yano S, Tfelt-Hansen J, Rooney P, Kanuparthi D, Bandyopadhyay S, Ren X, Terwilliger E, Brown EM. Mitogenic action of calcium-sensing receptor on rat calvarial osteoblasts. Endocrinology 145: 3451–3462, 2004. 154. Cantley LK, Russell J, Lettieri D, Sherwood LM. 1,25-Dihydroxyvitamin D3 suppresses parathyroid hormone secretion from bovine parathyroid cells in tissue culture. Endocrinology 117: 2114 –2119, 1985. 175. Chen CJ, Barnett JV, Congo DA, Brown EM. Divalent cations suppress 3=,5=-adenosine monophosphate accumulation by stimulating a pertussis toxin-sensitive guanine nucleotide-binding protein in cultured bovine parathyroid cells. Endocrinology 124: 233–239, 1989. 155. Care AD, Bates RF, Swaminathan R, Ganguli PC. The role of gastrin as a calcitonin secretagogue. J Endocrinol 51: 735–744, 1971. 176. Chen D, Marvik R, Ronning K, Andersson K, Waldum HL, Hakanson R. Gastrinevoked secretion of pancreastatin and histamine from ECL cells and of acid from 242 Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH parietal cells in isolated, vascularly perfused rat stomach. Effects of isobutyl methylxanthin and alpha-fluoromethylhistidine. Regul Pept 65: 133–138, 1996. 177. Chen D, Zhao CM, Al-Haider W, Hakanson R, Rehfeld JF, Kopin AS. Differentiation of gastric ECL cells is altered in CCK(2) receptor-deficient mice. Gastroenterology 123: 577–585, 2002. 178. Chen D, Zhao CM, Dockray GJ, Varro A, Van Hoek A, Sinclair NF, Wang TC, Koh TJ. Glycine-extended gastrin synergizes with gastrin 17 to stimulate acid secretion in gastrin-deficient mice. Gastroenterology 119: 756 –765, 2000. 197. Chiba T, Fujita T, Yamada T. Carbachol inhibits stimulant-induced increases in fundic D-cell cytosolic Ca2⫹ concentration. Am J Physiol Gastrointest Liver Physiol 257: G308 –G312, 1989. 198. Chiba T, Park J, Yamada T. Biosynthesis of somatostatin in canine fundic D cells. J Clin Invest 81: 282–287, 1988. 199. Chiba T, Taminato T, Kadowaki S, Abe H, Chihara K, Seino Y, Matsukura S, Fujita T. Effects of glucagon, secretin, and vasoactive intestinal polypeptide on gastric somatostatin and gastrin release from isolated perfused rat stomach. Gastroenterology 79: 67–71, 1980. 179. Chen D, Zhao CM, Hakanson R, Samuelson LC, Rehfeld JF, Friis-Hansen L. Altered control of gastric acid secretion in gastrin-cholecystokinin double mutant mice. Gastroenterology 126: 476 – 487, 2004. 200. Chiba T, Yamada T. Mechanisms for muscarinic inhibition of somatostatin release from canine fundic D cells. Metabolism 39: 122–124, 1990. 180. Chen KS, DeLuca HF. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim Biophys Acta 1263: 1–9, 1995. 201. Chirayath MV, Gajdzik L, Hulla W, Graf J, Cross HS, Peterlik M. Vitamin D increases tight-junction conductance and paracellular Ca2⫹ transport in Caco-2 cell cultures. Am J Physiol Gastrointest Liver Physiol 274: G389 –G396, 1998. 181. Chen S, Glenn DJ, Ni W, Grigsby CL, Olsen K, Nishimoto M, Law CS, Gardner DG. Expression of the vitamin d receptor is increased in the hypertrophic heart. Hypertension 52: 1106 –1112, 2008. 202. Chiu HF, Huang YW, Chang CC, Yang CY. Use of proton pump inhibitors increased the risk of hip fracture: a population-based case-control study. Pharmacoepidemiol Drug Safety 19: 1131–1136, 2010. 182. Cheng I, Qureshi I, Chattopadhyay N, Qureshi A, Butters RR, Hall AE, Cima RR, Rogers KV, Hebert SC, Geibel JP, Brown EM, Soybel DI. Expression of an extracellular calcium-sensing receptor in rat stomach. Gastroenterology 116: 118 –126, 1999. 203. Christakos S, Brunette MG, Norman AW. Localization of immunoreactive vitamin D-dependent calcium binding protein in chick nephron. Endocrinology 109: 322–324, 1981. 183. Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA 101: 7711–7715, 2004. 204. Chuang CN, Tanner M, Lloyd KC, Wong H, Soll AH. Endogenous somatostatin inhibits histamine release from canine gastric mucosal cells in primary culture. Am J Physiol Gastrointest Liver Physiol 265: G521–G525, 1993. 184. Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. J Biol Chem 278: 38084 – 38093, 2003. 205. Chung I, Li P, Lee K, Chang T, Chey WY. Dual inhibitory mechanism of secretin action on acid secretion in totally isolated, vascularly perfused rat stomach. Gastroenterology 107: 1751–1758, 1994. 185. Cheng SX, Geibel JP, Hebert SC. Extracellular polyamines regulate fluid secretion in rat colonic crypts via the extracellular calcium-sensing receptor. Gastroenterology 126: 148 –158, 2004. 206. Chung MK, Guler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci 11: 555–564, 2008. 186. Cheng SX, Okuda M, Hall AE, Geibel JP, Hebert SC. Expression of calcium-sensing receptor in rat colonic epithelium: evidence for modulation of fluid secretion. Am J Physiol Gastrointest Liver Physiol 283: G240 –G250, 2002. 187. Chesney RW, Rosen JF, Hamstra AJ, Smith C, Mahaffey K, DeLuca HF. Absence of seasonal variation in serum concentrations of 1,25-dihydroxyvitamin D despite a rise in 25-hydroxyvitamin D in summer. J Clin Endocrinol Metab 53: 139 –142, 1981. 188. Cheung R, Erclik MS, Mitchell J. Increased expression of G11alpha in osteoblastic cells enhances parathyroid hormone activation of phospholipase C and AP-1 regulation of matrix metalloproteinase-13 mRNA. J Cell Physiol 204: 336 –343, 2005. 189. Chew CS. Cholecystokinin, carbachol, gastrin, histamine, and forskolin increase [Ca2⫹]i in gastric glands. Am J Physiol Gastrointest Liver Physiol 250: G814 –G823, 1986. 190. Chew CS, Brown MR. Release of intracellular Ca2⫹ and elevation of inositol trisphosphate by secretagogues in parietal and chief cells isolated from rabbit gastric mucosa. Biochim Biophys Acta 888: 116 –125, 1986. 191. Chey WY, Chang CH, Pan HJ, Chang C, Kim BM, Park IS, Chang TM. Evidence on the presence of secretin cells in the gastric antral and oxyntic mucosa. Regul Pept 111: 183–190, 2003. 192. Chey WY, Chang TM, Park HJ, Lee KY, Escoffery R. Secretin-like immunoreactivity and biological activity in the antral mucosa. Endocrinology 113: 651– 656, 1983. 193. Chey WY, Escoffery R. Secretion cells in the gastrointestinal tract. Endocrinology 98: 1390 –1395, 1976. 194. Chey WY, Hitanant S, Hendricks J, Lorber SH. Effect of secretin and cholecystokinin on gastric emptying and gastric secretion in man. Gastroenterology 58: 820 – 827, 1970. 207. Cid LP, Montrose-Rafizadeh C, Smith DI, Guggino WB, Cutting GR. Cloning of a putative human voltage-gated chloride channel (CIC-2) cDNA widely expressed in human tissues. Hum Mol Genet 4: 407– 413, 1995. 208. Clark CG, Crooks J, Dawson AA, Mitchell PE. Disordered calcium metabolism after polya partial gastrectomy. Lancet 1: 734 –738, 1964. 209. Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1: 74 –76, 1982. 210. Clements MR, Davies M, Fraser DR, Lumb GA, Mawer EB, Adams PH. Metabolic inactivation of vitamin D is enhanced in primary hyperparathyroidism. Clin Sci 73: 659 – 664, 1987. 211. Clements MR, Davies M, Hayes ME, Hickey CD, Lumb GA, Mawer EB, Adams PH. The role of 1,25-dihydroxyvitamin D in the mechanism of acquired vitamin D deficiency. Clin Endocrinol 37: 17–27, 1992. 212. Clements MR, Johnson L, Fraser DR. A new mechanism for induced vitamin D deficiency in calcium deprivation. Nature 325: 62– 65, 1987. 213. Cleve H. The variants of the group-specific component. A review of their distribution in human populations. Isr J Med Sci 9: 1133–1146, 1973. 214. Cocchi D, Maccarinelli G, Sibilia V, Tulipano G, Torsello A, Pazzaglia UE, Giustina A, Netti C. GH-releasing peptides and bone. J Endocrinol Invest 28: 11–14, 2005. 215. Cochran M, Peacock M, Sachs G, Nordin BE. Renal effects of calcitonin. Br Med J 1: 135–137, 1970. 216. Collip JB. The extraction of a parathyroid hormone which will prevent or control parathyroid tetany and which regulates the level of blood calcium. J Biol Chem 63: 395– 438, 1925. 195. Chey WY, Lee YH, Hendricks JG, Rhodes RA, Tai HH. Plasma secretin concentrations in fasting and postprandial state in man. Am J Dig Dis 23: 981–988, 1978. 217. Colnot S, Ovejero C, Romagnolo B, Porteu A, Lacourte P, Thomasset M, Perret C. Transgenic analysis of the response of the rat calbindin-D 9k gene to vitamin D. Endocrinology 141: 2301–2308, 2000. 196. Chiba T, Fisher SK, Agranoff BW, Yamada T. Autoregulation of muscarinic and gastrin receptors on gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 256: G356 –G363, 1989. 218. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 243 SASCHA KOPIC AND JOHN P. GEIBEL 219. Compston JE, Thompson RP. Intestinal absorption of 25-hydroxyvitamin D and osteomalacia in primary biliary cirrhosis. Lancet 1: 721–724, 1977. 220. Conigrave AD, Quinn SJ, Brown EM. L-Amino acid sensing by the extracellular Ca2⫹-sensing receptor. Proc Natl Acad Sci USA 97: 4814 – 4819, 2000. 221. Cooke NE, David EV. Serum vitamin D-binding protein is a third member of the albumin and alpha fetoprotein gene family. J Clin Invest 76: 2420 –2424, 1985. 222. Cooper CW, Biggerstaff CR, Wiseman CW, Carlone MF. Hypocalcemic effect of pentagastrin and related gastrointestinal hormnal peptides in the rat. Endocrinology 91: 1455–1461, 1972. 223. Cooper CW, Hirsch PF, Munson PL. Importance of endogenous thyrocalcitonin for protection against hypercalcemia in the rat. Endocrinology 86: 406 – 415, 1970. 240. Darwish HM, DeLuca HF. Identification of a 1,25-dihydroxyvitamin D3-response element in the 5=-flanking region of the rat calbindin D-9k gene. Proc Natl Acad Sci USA 89: 603– 607, 1992. 241. Database Csr. http://www.casrdb.mcgill.ca/ [retrieved September 2011]. 242. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141: 4255– 4261, 2000. 243. Datta R, Waheed A, Shah GN, Sly WS. Signal sequence mutation in autosomal dominant form of hypoparathyroidism induces apoptosis that is corrected by a chemical chaperone. Proc Natl Acad Sci USA 104: 19989 –19994, 2007. 224. Cooper CW, McGuigan JE, Schwesinger WH, Brubaker RL, Munson PL. Correlation between levels of gastrin and thyrocalcitonin in pig thyroid venous blood. Endocrinology 95: 302–307, 1974. 244. Davies M, Heys SE, Selby PL, Berry JL, Mawer EB. Increased catabolism of 25hydroxyvitamin D in patients with partial gastrectomy and elevated 1,25-dihydroxyvitamin D levels. Implications for metabolic bone disease. J Clin Endocrinol Metab 82: 209 –212, 1997. 225. Cooper CW, Ramp WK, Becker DI, Ontjes DA. In vitro secretion of immunoreactive rat thyrocalcitonin. Endocrinology 101: 304 –311, 1977. 245. Davies M, Mawer EB, Krawitt EL. Comparative absorption of vitamin D3 and 25hydroxyvitamin D3 in intestinal disease. Gut 21: 287–292, 1980. 226. Cooper CW, Schwesinger WH, Mahgoub AM, Ontjes DA. Thyrocalcitonin: stimulation of secretion by pentagastrin. Science 172: 1238 –1240, 1971. 227. Copp DH, Cameron EC, Cheney BA, Davidson AG, Henze KG. Evidence for calcitonin: a new hormone from the parathyroid that lowers blood calcium. Endocrinology 70: 638 – 649, 1962. 228. Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 139: 93–101, 2010. 229. Cornish J, Callon KE, Bava U, Kamona SA, Cooper GJ, Reid IR. Effects of calcitonin, amylin, calcitonin gene-related peptide on osteoclast development. Bone 29: 162– 168, 2001. 230. Couper RT, Corey M, Moore DJ, Fisher LJ, Forstner GG, Durie PR. Decline of exocrine pancreatic function in cystic fibrosis patients with pancreatic sufficiency. Pediatr Res 32: 179 –182, 1992. 231. Cramer T, Juttner S, Plath T, Mergler S, Seufferlein T, Wang TC, Merchant J, Hocker M. Gastrin transactivates the chromogranin A gene through MEK-1/ERK- and PKCdependent phosphorylation of Sp1 and CREB. Cell Signal 20: 60 –72, 2008. 232. Cuppoletti J, Sachs G. Regulation of gastric acid secretion via modulation of a chloride conductance. J Biol Chem 259: 14952–14959, 1984. 233. D’Amour P, Brossard JH, Rousseau L, Nguyen-Yamamoto L, Nassif E, Lazure C, Gauthier D, Lavigne JR, Zahradnik RJ. Structure of non-(1– 84) PTH fragments secreted by parathyroid glands in primary and secondary hyperparathyroidism. Kidney Int 68: 998 –1007, 2005. 234. D’Amour P, Segre GV, Roth SI, Potts JT Jr. Analysis of parathyroid hormone and its fragments in rat tissues: chemical identification and microscopical localization. J Clin Invest 63: 89 –98, 1979. 235. Dahlback H, Wikvall K. 25-Hydroxylation of vitamin D3 by a cytochrome P-450 from rabbit liver mitochondria. Biochem J 252: 207–213, 1988. 236. Daiger SP, Schanfield MS, Cavalli-Sforza LL. Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D. Proc Natl Acad Sci USA 72: 2076 – 2080, 1975. 237. Dale HH, Laidlow PP. The physiological action of beta-iminazolylethylamine. J Physiol 41: 318 –344, 1910. 238. Dardenne O, Prud’homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology 142: 3135–3141, 2001. 239. Dardenne O, Prud’homme J, Hacking SA, Glorieux FH, St-Arnaud R. Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, highlactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1). Bone 32: 332–340, 2003. 244 246. Davies SL, Ozawa A, McCormick WD, Dvorak MM, Ward DT. Protein kinase C-mediated phosphorylation of the calcium-sensing receptor is stimulated by receptor activation and attenuated by calyculin-sensitive phosphatase activity. J Biol Chem 282: 15048 –15056, 2007. 247. Davis WL, Jones RG. Lysosomal proliferation in rachitic avian intestinal absorptive cells following 1,25-dihydroxycholecalciferol. Tissue Cell 14: 585–595, 1982. 248. De Groot T, Kovalevskaya NV, Verkaart S, Schilderink N, Felici M, van der Hagen EA, Bindels RJ, Vuister GW, Hoenderop JG. Molecular mechanisms of calmodulin action on TRPV5 and modulation by parathyroid hormone. Mol Cell Biol 31: 2845– 2853, 2011. 249. De Groot T, Lee K, Langeslag M, Xi Q, Jalink K, Bindels RJ, Hoenderop JG. Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol 20: 1693–1704, 2009. 250. De Jesus Ferreira MC, Helies-Toussaint C, Imbert-Teboul M, Bailly C, Verbavatz JM, Bellanger AC, Chabardes D. Co-expression of a Ca2⫹-inhibitable adenylyl cyclase and of a Ca2⫹-sensing receptor in the cortical thick ascending limb cell of the rat kidney. Inhibition of hormone-dependent cAMP accumulation by extracellular Ca2⫹. J Biol Chem 273: 15192–15202, 1998. 251. De Rouffignac C, Elalouf JM. Effects of calcitonin on the renal concentrating mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 245: F506 –F511, 1983. 252. De Vries F, Cooper AL, Cockle SM, Van Staa TP, Cooper C. Fracture risk in patients receiving acid-suppressant medication alone and in combination with bisphosphonates. Osteoporosis Int 20: 1989 –1998, 2009. 253. Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflügers Arch 442: 896 –902, 2001. 254. Delhanty PJ, van der Eerden BC, van der Velde M, Gauna C, Pols HA, Jahr H, Chiba H, van der Lely AJ, van Leeuwen JP. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J Endocrinol 188: 37– 47, 2006. 255. Deller DJ. Radiocalcium absorption after partial gastrectomy. Am J Dig Dis 11: 10 –19, 1966. 256. DeLuca HF, Lund J, Rosenbloom A, Lobeck CC. Metabolism of tritiated vitamin D3 in familial vitamin D-resistant rickets with hypophosphatemia. J Pediatr 70: 828 – 832, 1967. 257. DelValle J, Yamada T. Amino acids and amines stimulate gastrin release from canine antral G-cells via different pathways. J Clin Invest 85: 139 –143, 1990. 258. Delvin EE, Arabian A, Glorieux FH. Kinetics of liver microsomal cholecalciferol 25-hydroxylase in vitamin D-depleted and -repleated rats. Biochem J 172: 417– 422, 1978. 259. Demarest JR, Loo DD, Sachs G. Activation of apical chloride channels in the gastric oxyntic cell. Science 245: 402– 404, 1989. 260. Dempster DW, Hughes-Begos CE, Plavetic-Chee K, Brandao-Burch A, Cosman F, Nieves J, Neubort S, Lu SS, Iida-Klein A, Arnett T, Lindsay R. Normal human oste- Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH oclasts formed from peripheral blood monocytes express PTH type 1 receptors and are stimulated by PTH in the absence of osteoblasts. J Cell Biochem 95: 139 –148, 2005. 280. Dorrington KJ, Hui A, Hofmann T, Hitchman AJ, Harrison JE. Porcine intestinal calcium-binding protein. Molecular properties and the effect of binding calcium ions. J Biol Chem 249: 199 –204, 1974. 261. Deng F, Ling J, Ma J, Liu C, Zhang W. Stimulation of intramembranous bone repair in rats by ghrelin. Exp Physiol 93: 872– 879, 2008. 281. Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl⫺ channel regulated by the WNK kinases. J Physiol 584: 333–345, 2007. 262. Dent J, Kahrilas PJ, Hatlebakk J, Vakil N, Denison H, Franzen S, Lundborg P. A randomized, comparative trial of a potassium-competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol 103: 20 –26, 2008. 282. Drees BM, Hamilton JW. Pancreastatin and bovine parathyroid cell secretion. Bone Miner 17: 335–346, 1992. 263. Derler I, Hofbauer M, Kahr H, Fritsch R, Muik M, Kepplinger K, Hack ME, Moritz S, Schindl R, Groschner K, Romanin C. Dynamic but not constitutive association of calmodulin with rat TRPV6 channels enables fine tuning of Ca2⫹-dependent inactivation. J Physiol 577: 31– 44, 2006. 264. Desfleurs E, Wittner M, Simeone S, Pajaud S, Moine G, Rajerison R, Di Stefano A. Calcium-sensing receptor: regulation of electrolyte transport in the thick ascending limb of Henle’s loop. Kidney Blood Press Res 21: 401– 412, 1998. 283. Drescher D, DeLuca HF. Vitamin D stimulated calcium binding protein from rat intestinal mucosa. Purification and some properties. Biochemistry 10: 2302–2307, 1971. 284. Dressman JB, Berardi RR, Dermentzoglou LC, Russell TL, Schmaltz SP, Barnett JL, Jarvenpaa KM. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm Res 7: 756 –761, 1990. 285. Dubrasquet M, Bataille D, Gespach C. Oxyntomodulin (glucagon-37 or bioactive enteroglucagon): a potent inhibitor of pentagastrin-stimulated acid secretion in rats. Biosci Rep 2: 391–395, 1982. 265. Di Stefano A, Wittner M, Nitschke R, Braitsch R, Greger R, Bailly C, Amiel C, Roinel N, de Rouffignac C. Effects of parathyroid hormone and calcitonin on Na⫹, Cl⫺, K⫹, Mg2⫹ and Ca2⫹ transport in cortical and medullary thick ascending limbs of mouse kidney. Pflügers Arch 417: 161–167, 1990. 286. Dueland S, Helgerud P, Pedersen JI, Berg T, Drevon CA. Plasma clearance, transfer, and distribution of vitamin D3 from intestinal lymph. Am J Physiol Endocrinol Metab 245: E326 –E331, 1983. 266. Diamond JM, Bossert WH. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol 50: 2061–2083, 1967. 287. Dueland S, Pedersen JI, Helgerud P, Drevon CA. Absorption, distribution, transport of vitamin D3 and 25-hydroxyvitamin D3 in the rat. Am J Physiol Endocrinol Metab 245: E463–E467, 1983. 267. Dicker F, Quitterer U, Winstel R, Honold K, Lohse MJ. Phosphorylation-independent inhibition of parathyroid hormone receptor signaling by G protein-coupled receptor kinases. Proc Natl Acad Sci USA 96: 5476 –5481, 1999. 268. Dimaline R, Sandvik AK. Histidine decarboxylase gene expression in rat fundus is regulated by gastrin. FEBS Lett 281: 20 –22, 1991. 269. Dinoso V, Chey WY, Hendricks J, Lorber SH. Intestinal mucosal hormones and motor function of the stomach in man. J Appl Physiol 26: 326 –329, 1969. 270. Divieti P, Geller AI, Suliman G, Juppner H, Bringhurst FR. Receptors specific for the carboxyl-terminal region of parathyroid hormone on bone-derived cells: determinants of ligand binding and bioactivity. Endocrinology 146: 1863–1870, 2005. 271. Divieti P, Inomata N, Chapin K, Singh R, Juppner H, Bringhurst FR. Receptors for the carboxyl-terminal region of pth(1– 84) are highly expressed in osteocytic cells. Endocrinology 142: 916 –925, 2001. 272. Divieti P, John MR, Juppner H, Bringhurst FR. Human PTH-(7– 84) inhibits bone resorption in vitro via actions independent of the type 1 PTH/PTHrP receptor. Endocrinology 143: 171–176, 2002. 273. Dixon KM, Deo SS, Norman AW, Bishop JE, Halliday GM, Reeve VE, Mason RS. In vivo relevance for photoprotection by the vitamin D rapid response pathway. J Steroid Biochem Mol Biol 103: 451– 456, 2007. 274. Dobnig H, Turner RT. The effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology 138: 4607– 4612, 1997. 275. Dong H, Sellers ZM, Smith A, Chow JY, Barrett KE. Na⫹/Ca2⫹ exchange regulates Ca2⫹-dependent duodenal mucosal ion transport and HCO3⫺ secretion in mice. Am J Physiol Gastrointest Liver Physiol 288: G457–G465, 2005. 276. Dornonville de la Cour C, Bjorkqvist M, Sandvik AK, Bakke I, Zhao CM, Chen D, Hakanson R. A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul Pept 99: 141–150, 2001. 277. Dornonville de la Cour C, Lindqvist A, Egecioglu E, Tung YC, Surve V, Ohlsson C, Jansson JO, Erlanson-Albertsson C, Dickson SL, Hakanson R. Ghrelin treatment reverses the reduction in weight gain and body fat in gastrectomised mice. Gut 54: 907–913, 2005. 288. Dueland S, Pedersen JI, Helgerud P, Drevon CA. Transport of vitamin D3 from rat intestine. Evidence for transfer of vitamin D3 from chylomicrons to alpha-globulins. J Biol Chem 257: 146 –150, 1982. 289. Duflos C, Bellaton C, Baghdassarian N, Gadoux M, Pansu D, Bronner F. 1,25Dihydroxycholecalciferol regulates rat intestinal calbindin D9k posttranscriptionally. J Nutr 126: 834 – 841, 1996. 290. Duflos C, Bellaton C, Pansu D, Bronner F. Calcium solubility, intestinal sojourn time and paracellular permeability codetermine passive calcium absorption in rats. J Nutr 125: 2348 –2355, 1995. 291. Dufner MM, Kirchhoff P, Remy C, Hafner P, Muller MK, Cheng SX, Tang LQ, Hebert SC, Geibel JP, Wagner CA. The calcium-sensing receptor acts as a modulator of gastric acid secretion in freshly isolated human gastric glands. Am J Physiol Gastrointest Liver Physiol 289: G1084 –G1090, 2005. 292. Duman JG, Pathak NJ, Ladinsky MS, McDonald KL, Forte JG. Three-dimensional reconstruction of cytoplasmic membrane networks in parietal cells. J Cell Sci 115: 1251–1258, 2002. 293. Duman JG, Tyagarajan K, Kolsi MS, Moore HP, Forte JG. Expression of rab11a N124I in gastric parietal cells inhibits stimulatory recruitment of the H⫹-K⫹-ATPase. Am J Physiol Cell Physiol 277: C361–C372, 1999. 294. Durr KL, Abe K, Tavraz NN, Friedrich T. E2P state stabilization by the N-terminal tail of the H⫹-K⫹-ATPase beta-subunit is critical for efficient proton pumping under in vivo conditions. J Biol Chem 284: 20147–20154, 2009. 295. DuVal JW, Saffouri B, Weir GC, Walsh JH, Arimura A, Makhlouf GM. Stimulation of gastrin and somatostatin secretion from the isolated rat stomach by bombesin. Am J Physiol Gastrointest Liver Physiol 241: G242–G247, 1981. 296. Dvorak MM, Chen TH, Orwoll B, Garvey C, Chang W, Bikle DD, Shoback DM. Constitutive activity of the osteoblast Ca2⫹-sensing receptor promotes loss of cancellous bone. Endocrinology 148: 3156 –3163, 2007. 297. Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, Riccardi D. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci USA 101: 5140 –5145, 2004. 298. Edkins JS. The chemical mechanism of gastric secretion. J Physiol 34: 133–144, 1906. 278. Dornonville de la Cour C, Lindstrom E, Norlen P, Hakanson R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept 120: 23–32, 2004. 279. Doroszewicz J, Waldegger P, Jeck N, Seyberth H, Waldegger S. pH dependence of extracellular calcium sensing receptor activity determined by a novel technique. Kidney Int 67: 187–192, 2005. 299. Edwards BR, Baer PG, Sutton RA, Dirks JH. Micropuncture study of diuretic effects on sodium and calcium reabsorption in the dog nephron. J Clin Invest 52: 2418 –2427, 1973. 300. Edwards BR, Sutton RA, Dirks JH. Effect of calcium infusion on renal tubular reabsorption in the dog. Am J Physiol 227: 13–18, 1974. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 245 SASCHA KOPIC AND JOHN P. GEIBEL 301. Edwards LW, Herrington JL Jr. Vagotomy and gastro-enterostomy; vagotomy and conservative gastrectomy; a comparative study. Ann Surg 137: 873– 883, 1953. 302. Ekblad E, Ekelund M, Graffner H, Hakanson R, Sundler F. Peptide-containing nerve fibers in the stomach wall of rat and mouse. Gastroenterology 89: 73– 85, 1985. 303. El Hag AI, Karrar ZA. Nutritional vitamin D deficiency rickets in Sudanese children. Ann Trop Paediatr 15: 69 –76, 1995. 304. Elalouf JM, Roinel N, de Rouffignac C. ADH-like effects of calcitonin on electrolyte transport by Henle’s loop of rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 246: F213–F220, 1984. 305. Ellman P, Irwin DB. Osteomalacia following gastrectomy. Postgrad Med J 35: 358 – 361, 1959. 306. Emmelin N, Kahlson GS. Histamine as a physiological excitant of acid gastric secretion. Acta Physiol Scand 8: 289 –304, 1944. 307. Engelhardt W, Grohmann C, Schwille PO, Geus A. Calcium absorption in the rat as influenced by highly selective vagotomy with special regard to endogenous gastrin. Res Exp Med 180: 1–9, 1982. 308. Engelhardt W, Rumenapf G, Schwille PO. Effects of highly selective vagotomy on small intestinal calcium transport in the rat. Miner Electrolyte Metab 10: 239 –243, 1984. 309. Engqvist-Goldstein AE, Warren RA, Kessels MM, Keen JH, Heuser J, Drubin DG. The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. J Cell Biol 154: 1209 –1223, 2001. 310. Ericsson P, Hakanson R, Rehfeld JF, Norlen P. Gastrin release: antrum microdialysis reveals a complex neural control. Regul Pept 161: 22–32, 2010. 311. Erler I, Hirnet D, Wissenbach U, Flockerzi V, Niemeyer BA. Ca2⫹-selective transient receptor potential V channel architecture and function require a specific ankyrin repeat. J Biol Chem 279: 34456 –34463, 2004. 322. Feher JJ, Fullmer CS, Wasserman RH. Role of facilitated diffusion of calcium by calbindin in intestinal calcium absorption. Am J Physiol Cell Physiol 262: C517–C526, 1992. 323. Feldberg W, Toh CC. Distribution of 5-hydroxytryptamine (serotonin, enteramine) in the wall of the digestive tract. J Physiol 119: 352–362, 1953. 324. Fellenius E, Berglindh T, Sachs G, Olbe L, Elander B, Sjostrand SE, Wallmark B. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H⫹ ⫹ K⫹)ATPase. Nature 290: 159 –161, 1981. 325. Feng J, Petersen CD, Coy DH, Jiang JK, Thomas CJ, Pollak MR, Wank SA. Calciumsensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion. Proc Natl Acad Sci USA 107: 17791–17796, 2010. 326. Ferrari SL, Behar V, Chorev M, Rosenblatt M, Bisello A. Endocytosis of ligand-human parathyroid hormone receptor 1 complexes is protein kinase C-dependent and involves beta-arrestin2. Real-time monitoring by fluorescence microscopy. J Biol Chem 274: 29968 –29975, 1999. 327. Ferrari SL, Pierroz DD, Glatt V, Goddard DS, Bianchi EN, Lin FT, Manen D, Bouxsein ML. Bone response to intermittent parathyroid hormone is altered in mice null for -Arrestin2. Endocrinology 146: 1854 –1862, 2005. 328. Findlay DM, Michelangeli VP, Orlowski RC, Martin TJ. Biological activities and receptor interactions of des-Leu16 salmon and des-Phe16 human calcitonin. Endocrinology 112: 1288 –1291, 1983. 329. Firsov D, Bellanger AC, Marsy S, Elalouf JM. Quantitative RT-PCR analysis of calcitonin receptor mRNAs in the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 269: F702–F709, 1995. 330. Flannery PJ, Spurney RF. Domains of the parathyroid hormone (PTH) receptor required for regulation by G protein-coupled receptor kinases (GRKs). Biochem Pharmacol 62: 1047–1058, 2001. 331. Fleming JV, Wang TC. Amino- and carboxy-terminal PEST domains mediate gastrin stabilization of rat L-histidine decarboxylase isoforms. Mol Cell Biol 20: 4932– 4947, 2000. 312. Esplugues JV, Barrachina MD, Calatayud S, Pique JM, Whittle BJ. Nitric oxide mediates the inhibition by interleukin-1 beta of pentagastrin-stimulated rat gastric acid secretion. Br J Pharmacol 108: 9 –10, 1993. 332. Force T, Bonventre JV, Flannery MR, Gorn AH, Yamin M, Goldring SR. A cloned porcine renal calcitonin receptor couples to adenylyl cyclase and phospholipase C. Am J Physiol Renal Fluid Electrolyte Physiol 262: F1110 –F1115, 1992. 313. Fahrmann M, Kaufhold M, Pfeiffer AF, Seidler U. Protein kinase C-alpha attenuates cholinergically stimulated gastric acid secretion of rabbit parietal cells. Br J Pharmacol 139: 545–554, 2003. 333. Foresta C, Strapazzon G, De Toni L, Perilli L, Di Mambro A, Muciaccia B, Sartori L, Selice R. Bone mineral density and testicular failure: evidence for a role of vitamin D 25-hydroxylase in human testis. J Clin Endocrinol Metab 96: E646 –E652, 2011. 314. Fahrmann M, Kaufhold M, Rieg T, Seidler U. Different actions of protein kinase C isoforms alpha and epsilon on gastric acid secretion. Br J Pharmacol 136: 938 –946, 2002. 334. Forster IC, Hernando N, Biber J, Murer H. Proximal tubular handling of phosphate: a molecular perspective. Kidney Int 70: 1548 –1559, 2006. 315. Fahrmann M, Mohlig M, Schatz H, Pfeiffer A. Purification and characterization of a Ca2⫹/calmodulin-dependent protein kinase II from hog gastric mucosa using a protein-protein affinity chromatographic technique. Eur J Biochem 255: 516 –525, 1998. 316. Fahrmann M, Pfeiffer A. Copurification of two holoenzyme-forming calcium/calmodulin-dependent protein kinase II isoforms as holoenzyme from porcine stomach. Arch Biochem Biophys 380: 151–158, 2000. 317. Fasciotto BH, Gorr SU, DeFranco DJ, Levine MA, Cohn DV. Pancreastatin, a presumed product of chromogranin-A (secretory protein-I) processing, inhibits secretion from porcine parathyroid cells in culture. Endocrinology 125: 1617–1622, 1989. 318. Favus MJ, Angeid-Backman E. Effects of 1,25(OH)2D3 and calcium channel blockers on cecal calcium transport in the rat. Am J Physiol Gastrointest Liver Physiol 248: G676 –G681, 1985. 335. Forte JG, Forte GM, Saltman P. K⫹-stimulated phosphatase of microsomes from gastric mucosa. J Cell Physiol 69: 293–304, 1967. 336. Forte JG, Zhu L. Apical recycling of the gastric parietal cell H⫹-K⫹-ATPase. Annu Rev Physiol 72: 273–296, 2010. 337. Fox J, Green DT. Direct effects of calcium channel blockers on duodenal calcium transport in vivo. Eur J Pharmacol 129: 159 –164, 1986. 338. Fraser DR, Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nat New Biol 241: 163–166, 1973. 339. Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature 228: 764 –766, 1970. 340. Freeman MW, Wiren KM, Rapoport A, Lazar M, Potts JT Jr, Kronenberg HM. Consequences of amino-terminal deletions of preproparathyroid hormone signal sequence. Mol Endocrinol 1: 628 – 638, 1987. 319. FDA. FDA Drug Safety Communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors Food and Drug Administration. http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformation forpatientsandproviders/ucm213206.htm [retrieved July 2011]. 341. Freeman TC, Howard A, Bentsen BS, Legon S, Walters JR. Cellular and regional expression of transcripts of the plasma membrane calcium pump PMCA1 in rabbit intestine. Am J Physiol Gastrointest Liver Physiol 269: G126 –G131, 1995. 320. Federico A, Dotti MT, Lore F, Nuti R. Cerebrotendinous xanthomatosis: pathophysiological study on bone metabolism. J Neurol Sci 115: 67–70, 1993. 342. Friedman J, Au WY, Raisz LG. Responses of fetal rat bone to thyrocalcitonin in tissue culture. Endocrinology 82: 149 –156, 1968. 321. Feher JJ. Facilitated calcium diffusion by intestinal calcium-binding protein. Am J Physiol Cell Physiol 244: C303–C307, 1983. 343. Friedman J, Raisz LG. Thyrocalcitonin: inhibitor of bone resorption in tissue culture. Science 150: 1465–1467, 1965. 246 Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH 344. Friedman PA. Basal and hormone-activated calcium absorption in mouse renal thick ascending limbs. Am J Physiol Renal Fluid Electrolyte Physiol 254: F62–F70, 1988. 345. Fries W, Rumenapf G, Schwille PO. Disturbances of mineral and bone metabolism following gastric antrectomy in the rat. Bone Miner 19: 245–256, 1992. 346. Friis-Hansen L, Schjerling CK, de la Cour CD, Hakanson R, Rehfeld JF. Characteristics of gastrin controlled ECL cell specific gene expression. Regul Pept 140: 153–161, 2007. 347. Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 274: G561–G568, 1998. 348. Frolik CA, Black EC, Cain RL, Satterwhite JH, Brown-Augsburger PL, Sato M, Hock JM. Anabolic and catabolic bone effects of human parathyroid hormone (1–34) are predicted by duration of hormone exposure. Bone 33: 372–379, 2003. 349. Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, Portale AA. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol 11: 1961–1970, 1997. 364. Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med 19: 614 – 622, 1990. 365. Garner SC, Pi M, Tu Q, Quarles LD. Rickets in cation-sensing receptor-deficient mice: an unexpected skeletal phenotype. Endocrinology 142: 3996 – 4005, 2001. 366. Garrett JE, Capuano IV, Hammerland LG, Hung BC, Brown EM, Hebert SC, Nemeth EF, Fuller F. Molecular cloning and functional expression of human parathyroid calcium receptor cDNAs. J Biol Chem 270: 12919 –12925, 1995. 367. Garrett JE, Tamir H, Kifor O, Simin RT, Rogers KV, Mithal A, Gagel RF, Brown EM. Calcitonin-secreting cells of the thyroid express an extracellular calcium receptor gene. Endocrinology 136: 5202–5211, 1995. 368. Garrick R, Ireland AW, Posen S. Bone abnormalities after gastric surgery. A prospective histological study. Ann Intern Med 75: 221–225, 1971. 369. Garuti R, Croce MA, Piccinini L, Tiozzo R, Bertolini S, Calandra S. Functional analysis of the promoter of human sterol 27-hydroxylase gene in HepG2 cells. Gene 283: 133–143, 2002. 350. Fu Q, Jilka RL, Manolagas SC, O’Brien CA. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem 277: 48868 – 48875, 2002. 370. Gascon-Barre M, Demers C, Ghrab O, Theodoropoulos C, Lapointe R, Jones G, Valiquette L, Menard D. Expression of CYP27A, a gene encoding a vitamin D-25 hydroxylase in human liver and kidney. Clin Endocrinol 54: 107–115, 2001. 351. Fudge NJ, Kovacs CS. Physiological studies in heterozygous calcium sensing receptor (CaSR) gene-ablated mice confirm that the CaSR regulates calcitonin release in vivo. BMC Physiol 4: 5, 2004. 371. Geibel J, Sritharan K, Geibel R, Geibel P, Persing JS, Seeger A, Roepke TK, Deichstetter M, Prinz C, Cheng SX, Martin D, Hebert SC. Calcium-sensing receptor abrogates secretagogue-induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc Natl Acad Sci USA 103: 9390 –9397, 2006. 352. Fujii T, Takahashi Y, Ikari A, Morii M, Tabuchi Y, Tsukada K, Takeguchi N, Sakai H. Functional association between K⫹-Cl⫺ cotransporter-4 and H⫹,K⫹-ATPase in the apical canalicular membrane of gastric parietal cells. J Biol Chem 284: 619 – 629, 2009. 372. Geibel JP, Hebert SC. The functions and roles of the extracellular Ca2⫹-sensing receptor along the gastrointestinal tract. Annu Rev Physiol 71: 205–217, 2009. 353. Fujita A, Horio Y, Higashi K, Mouri T, Hata F, Takeguchi N, Kurachi Y. Specific localization of an inwardly rectifying K(⫹) channel, Kir4.1, at the apical membrane of rat gastric parietal cells; its possible involvement in K(⫹) recycling for the H(⫹)K(⫹)-pump. J Physiol 540: 85–92, 2002. 354. Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2⫹ absorption between enterocytes. Mol Biol Cell 19: 1912–1921, 2008. 355. Fukumoto K, Nakahara K, Katayama T, Miyazatao M, Kangawa K, Murakami N. Synergistic action of gastrin and ghrelin on gastric acid secretion in rats. Biochem Biophys Res Commun 374: 60 – 63, 2008. 356. Fukushima M, Suzuki Y, Tohira Y, Nishii Y, Suzuki M. 25-Hydroxylation of 1alphahydroxyvitamin D3 in vivo and in perfused rat liver. FEBS Lett 65: 211–214, 1976. 357. Fukushima N, Hanada R, Teranishi H, Fukue Y, Tachibana T, Ishikawa H, Takeda S, Takeuchi Y, Fukumoto S, Kangawa K, Nagata K, Kojima M. Ghrelin directly regulates bone formation. J Bone Miner Res 20: 790 –798, 2005. 358. Fullmer CS, Wasserman RH. Isolation and partial characterization of intestinal calcium-binding proteins from the cow, pig, horse, guinea pig, and chick. Biochim Biophys Acta 393: 134 –142, 1975. 359. Gadelle D, Raibaud P, Sacquet E. beta-Glucuronidase activities of intestinal bacteria determined both in vitro and in vivo in gnotobiotic rats. Appl Environ Microbiol 49: 682– 685, 1985. 373. Geibel JP, Wagner CA, Caroppo R, Qureshi I, Gloeckner J, Manuelidis L, Kirchhoff P, Radebold K. The stomach divalent ion-sensing receptor scar is a modulator of gastric acid secretion. J Biol Chem 276: 39549 –39552, 2001. 374. Gerber JG, Payne NA. Secretin inhibits canine gastric acid secretion in response to pentagastrin by modulating gastric histamine release. J Pharmacol Exp Ther 279: 718 –723, 1996. 375. Gerbino A, Ruder WC, Curci S, Pozzan T, Zaccolo M, Hofer AM. Termination of cAMP signals by Ca2⫹ and G␣i via extracellular Ca2⫹ sensors: a link to intracellular Ca2⫹ oscillations. J Cell Biol 171: 303–312, 2005. 376. Gerhard M, Neumayer N, Presecan-Siedel E, Zanner R, Lengyel E, Cramer T, Hocker M, Prinz C. Gastrin induces expression and promoter activity of the vesicular monoamine transporter subtype 2. Endocrinology 142: 3663–3672, 2001. 377. Germain N, Galusca B, Le Roux CW, Bossu C, Ghatei MA, Lang F, Bloom SR, Estour B. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. Am J Clin Nutr 85: 967– 971, 2007. 378. Gertner JM, Lilburn M, Domenech M. 25-Hydroxycholecalciferol absorption in steatorrhoea and postgastrectomy osteomalacia. Br Med J 1: 1310 –1312, 1977. 379. Gesek FA, Friedman PA. On the mechanism of parathyroid hormone stimulation of calcium uptake by mouse distal convoluted tubule cells. J Clin Invest 90: 749 –758, 1992. 360. Gama L, Baxendale-Cox LM, Breitwieser GE. Ca2⫹-sensing receptors in intestinal epithelium. Am J Physiol Cell Physiol 273: C1168 –C1175, 1997. 380. Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct beta-arrestin- and G proteindependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem 281: 10856 –10864, 2006. 361. Gao XH, Dwivedi PP, Omdahl JL, Morris HA, May BK. Calcitonin stimulates expression of the rat 25-hydroxyvitamin D3-24-hydroxylase (CYP24) promoter in HEK293 cells expressing calcitonin receptor: identification of signaling pathways. J Mol Endocrinol 32: 87–98, 2004. 381. Gesty-Palmer D, Flannery P, Yuan L, Corsino L, Spurney R, Lefkowitz RJ, Luttrell LM. A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med 1: 1ra1, 2009. 362. Garfia B, Canadillas S, Canalejo A, Luque F, Siendones E, Quesada M, Almaden Y, Aguilera-Tejero E, Rodriguez M. Regulation of parathyroid vitamin D receptor expression by extracellular calcium. J Am Soc Nephrol 13: 2945–2952, 2002. 382. Ghijsen WE, De Jong MD, Van Os CH. Kinetic properties of Na⫹/Ca2⫹ exchange in basolateral plasma membranes of rat small intestine. Biochim Biophys Acta 730: 85– 94, 1983. 363. Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet 1: 307–309, 1985. 383. Gilardi F, Viviani B, Galmozzi A, Boraso M, Bartesaghi S, Torri A, Caruso D, Crestani M, Marinovich M, de Fabiani E. Expression of sterol 27-hydroxylase in glial cells and its regulation by liver X receptor signaling. Neuroscience 164: 530 –540, 2009. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 247 SASCHA KOPIC AND JOHN P. GEIBEL 384. Gisbert JP, Gonzalez L, Calvet X, Roque M, Gabriel R, Pajares JM. Proton pump inhibitors versus H2-antagonists: a meta-analysis of their efficacy in treating bleeding peptic ulcer. Aliment Pharmacol Ther 15: 917–926, 2001. 385. Goldblatt H, Soames KM. Studies on the fat-soluble growth-promoting factor. Biochem J 17: 446, 1923. 386. Goldenring JR, Shen KR, Vaughan HD, Modlin IM. Identification of a small GTPbinding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, and lung. J Biol Chem 268: 18419 –18422, 1993. 387. Goldenring JR, Soroka CJ, Shen KR, Tang LH, Rodriguez W, Vaughan HD, Stoch SA, Modlin IM. Enrichment of rab11, a small GTP-binding protein, in gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 267: G187–G194, 1994. 388. Gonnelli S, Caffarelli C, Del Santo K, Cadirni A, Guerriero C, Lucani B, Franci B, Nuti R. The relationship of ghrelin and adiponectin with bone mineral density and bone turnover markers in elderly men. Calcif Tissue Int 83: 55– 60, 2008. 389. Gorn AH, Rudolph SM, Flannery MR, Morton CC, Weremowicz S, Wang TZ, Krane SM, Goldring SR. Expression of two human skeletal calcitonin receptor isoforms cloned from a giant cell tumor of bone. The first intracellular domain modulates ligand binding and signal transduction. J Clin Invest 95: 2680 –2691, 1995. 390. Goss SL, Lemons KA, Kerstetter JE, Bogner RH. Determination of calcium salt solubility with changes in pH and PCO2, simulating varying gastrointestinal environments. J Pharm Pharmacol 59: 1485–1492, 2007. 391. Grahammer F, Herling AW, Lang HJ, Schmitt-Graff A, Wittekindt OH, Nitschke R, Bleich M, Barhanin J, Warth R. The cardiac K⫹ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology 120: 1363–1371, 2001. 392. Grauschopf U, Lilie H, Honold K, Wozny M, Reusch D, Esswein A, Schafer W, Rucknagel KP, Rudolph R. The N-terminal fragment of human parathyroid hormone receptor 1 constitutes a hormone binding domain and reveals a distinct disulfide pattern. Biochemistry 39: 8878 – 8887, 2000. 393. Gray RW, Omdahl JL, Ghazarian JG, DeLuca HF Hydroxycholecalciferol-1-hydroxylase. Subcellular location and properties. J Biol Chem 247: 7528 –7532, 1972. 394. Gray SL, Lacroix AZ, Larson J, Robbins J, Cauley JA, Manson JE, Chen Z. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the women’s health initiative. Arch Internal Med 170: 765–771, 2010. 395. Graziani G, Badalamenti S, Como G, Gallieni M, Finazzi S, Angelini C, Brancaccio D, Ponticelli C. Calcium and phosphate plasma levels in dialysis patients after dietary Ca-P overload: Role of gastric acid secretion. Nephron 91: 474 – 479, 2002. 405. Habener JF, Singer FR, Deftos LJ, Neer RM, Potts JT Jr. Explanation for unusual potency of salmon calcitonin. Nat New Biol 232: 91–92, 1971. 406. Haddad JG, Hillman L, Rojanasathit S. Human serum binding capacity and affinity for 25-hydroxyergocalciferol and 25-hydroxycholecalciferol. J Clin Endocrinol Metab 43: 86 –91, 1976. 407. Haddad JG, Jennings AS, Aw TC. Vitamin D uptake and metabolism by perfused rat liver: influences of carrier proteins. Endocrinology 123: 498 –504, 1988. 408. Haddad JG Jr, Walgate J. 25-Hydroxyvitamin D transport in human plasma. Isolation and partial characterization of calcifidiol-binding protein. J Biol Chem 251: 4803– 4809, 1976. 409. Haddad JG Jr, Walgate J. Radioimmunoassay of the binding protein for vitamin D and its metabolites in human serum: concentrations in normal subjects and patients with disorders of mineral homeostasis. J Clin Invest 58: 1217–1222, 1976. 410. Hakanson R, Blom H, Carlsson E, Larsson H, Ryberg B, Sundler F. Hypergastrinaemia produces trophic effects in stomach but not in pancreas and intestines. Regul Pept 13: 225–233, 1986. 411. Hakanson R, Ding XQ, Norlen P, Chen D. Circulating pancreastatin is a marker for the enterochromaffin-like cells of the rat stomach. Gastroenterology 108: 1445– 1452, 1995. 412. Hakanson R, Liedberg G. The role of endogenous gastrin in the activation of gastric histidine decarboxylase in the rat. Effect of antrectomy and vagal denervation. Eur J Pharmacol 12: 94 –103, 1970. 413. Hakanson R, Owman C. Concomitant histochemical demonstration of histamine and catecholamines in enterochromaffin-like cells of gastric mucosa. Life Sci 6: 759 – 766, 1967. 414. Hammer RA, Fernandez C, Ertan A, Arimura A. Anesthetic dependence of the inhibitory effect of neurotensin on pentagastrin-stimulated acid secretion in rats. A possible role for somatostatin. Life Sci 48: 333–339, 1991. 415. Handlogten ME, Huang C, Shiraishi N, Awata H, Miller RT. The Ca2⫹-sensing receptor activates cytosolic phospholipase A2 via a Gqalpha-dependent ERK-independent pathway. J Biol Chem 276: 13941–13948, 2001. 416. Handlogten ME, Shiraishi N, Awata H, Huang C, Miller RT. Extracellular Ca2⫹sensing receptor is a promiscuous divalent cation sensor that responds to lead. Am J Physiol Renal Physiol 279: F1083–F1091, 2000. 417. Hanley DA, Ayer LM. Calcium-dependent release of carboxyl-terminal fragments of parathyroid hormone by hyperplastic human parathyroid tissue in vitro. J Clin Endocrinol Metab 63: 1075–1079, 1986. 396. Graziani G, Como G, Badalamenti S, Finazzi S, Malesci A, Gallieni M, Brancaccio D, Ponticelli C. Effect of gastric acid secretion on intestinal phosphate and calcium absorption in normal subjects. Nephrology Dialysis Transplantation 10: 1376 –1380, 1995. 418. Hanley DA, Takatsuki K, Sultan JM, Schneider AB, Sherwood LM. Direct release of parathyroid hormone fragments from functioning bovine parathyroid glands in vitro. J Clin Invest 62: 1247–1254, 1978. 397. Green T, Dockray GJ. Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse and guinea-pig. Neuroscience 25: 181–193, 1988. 419. Hansen CP. The pharmacokinetics and pharmacodynamics of progastrin-derived peptides. Dan Med Bull 50: 310 –319, 2003. 398. Gregory DH, Van Uelft R. Calcium absorption following gastric resection. Am J Gastroenterol 57: 34 – 40, 1972. 420. Hansen CP, Goetze JP, Stadil F, Rehfeld JF. Excretion of progastrin products in human urine. Am J Physiol Gastrointest Liver Physiol 276: G985–G992, 1999. 399. Gregory RA, Tracy HJ. The constitution and properties of two gastrins extracted from hog antral mucosa. Gut 5: 103–114, 1964. 421. Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Ziegler TE, Penniston KL, Alvig AL, Shafer MM. Do proton pump inhibitors decrease calcium absorption? J Bone Miner Res 25: 2510 –2519, 2010. 400. Grisso JA, Kelsey JL, O’Brien LA, Miles CG, Sidney S, Maislin G, LaPann K, Moritz D, Peters B. Risk factors for hip fracture in men. Am J Epidemiol 145: 786 –793, 1997. 401. Gunness-Hey M, Hock JM. Increased trabecular bone mass in rats treated with human synthetic parathyroid hormone. Metab Bone Dis Relat Res 5: 177–181, 1984. 402. Guo YD, Strugnell S, Back DW, Jones G. Transfected human liver cytochrome P-450 hydroxylates vitamin D analogs at different side-chain positions. Proc Natl Acad Sci USA 90: 8668 – 8672, 1993. 403. Gupta RP, Patrick K, Bell NH. Mutational analysis of CYP27A1: assessment of 27hydroxylation of cholesterol and 25-hydroxylation of vitamin D. Metabolism 56: 1248 –1255, 2007. 404. Gyomorey K, Yeger H, Ackerley C, Garami E, Bear CE. Expression of the chloride channel ClC-2 in the murine small intestine epithelium. Am J Physiol Cell Physiol (could not determine journal name) 279: 1787–1794, 2000. 248 422. Hanzlik RP, Fowler SC, Fisher DH. Relative bioavailability of calcium from calcium formate, calcium citrate, and calcium carbonate. J Pharmacol Exp Ther 313: 1217– 1222, 2005. 423. Hardy P, Sechet A, Hottelart C, Oprisiu R, Abighanem O, Said S, Rasombololona M, Brazier M, Moriniere P, Achard JM, Pruna A, Fournier A. Inhibition of gastric secretion by omeprazole and efficiency of calcium carbonate on the control of hyperphosphatemia in patients on chronic hemodialysis. Artificial Organs 22: 569 –573, 1998. 424. Harmeyer J, Deluca HF. Calcium-binding protein and calcium absorption after vitamin D administration. Arch Biochem Biophys 133: 247–254, 1969. 425. Hartmann M, Theiss U, Huber R, Luhmann R, Bliesath H, Wurst W, Lucker PW. Twenty-four-hour intragastric pH profiles and pharmacokinetics following single and repeated oral administration of the proton pump inhibitor pantoprazole in comparison to omeprazole. Aliment Pharmacol Ther 10: 359 –366, 1996. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH 426. Hasebe K, Horie S, Komasaka M, Yano S, Watanabe K. Stimulatory effects of nitric oxide donors on gastric acid secretion in isolated mouse stomach. Eur J Pharmacol 420: 159 –164, 2001. 427. Hashizume Y, Waguri S, Watanabe T, Kominami E, Uchiyama Y. Cysteine proteinases in rat parathyroid cells with special reference to their correlation with parathyroid hormone (PTH) in storage granules. J Histochem Cytochem 41: 273–282, 1993. 448. Hess AF, Weinstock M. Antirachitic properties imparted to inert fluids by ultraviolet irradiation. Proc Soc Exp Biol Med 22: 6 –7, 1924. 449. Hess AF, Weinstock M. Antirachitic properties imparted to lettuce and to growing wheat by ultraviolet irradiation. Proc Soc Exp Biol Med 22: 5, 1924. 450. Hess AF, Weinstock M. A further report on imparting antirachitic properties to inert substances by ultra-violet irradiation. J Biol Chem 63: 297–307, 1925. 428. Hattersley G and Chambers TJ. Calcitonin receptors as markers for osteoclastic differentiation: correlation between generation of bone-resorptive cells and cells that express calcitonin receptors in mouse bone marrow cultures. Endocrinology 125: 1606 –1612, 1989. 451. Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol 103: 316 –321, 2007. 429. Haussler MR, Myrtle JF, Norman AW. The association of a metabolite of vitamin D3 with intestinal mucosa chromatin in vivo. J Biol Chem 243: 4055– 4064, 1968. 452. Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, Kilby MD, Moss PA, Chakraverty R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol 170: 5382–5390, 2003. 430. Haussler MR, Norman AW. Chromosomal receptor for a vitamin D metabolite. Proc Natl Acad Sci USA 62: 155–162, 1969. 431. He W, Liu W, Chew CS, Baker SS, Baker RD, Forte JG, Zhu L. Acid secretionassociated translocation of KCNJ15 in gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 301: G591–G600, 2011. 432. Heaney RP, Dowell MS, Bierman J, Hale CA, Bendich A. Absorbability and cost effectiveness in calcium supplementation. J Am Coll Nutr 20: 239 –246, 2001. 433. Heaney RP, Recker RR, Weaver CM. Absorbability of calcium sources: the limited role of solubility. Calcified Tissue Int 46: 300 –304, 1990. 434. Hebert SC, Brown EM, Harris HW. Role of the Ca2⫹-sensing receptor in divalent mineral ion homeostasis. J Exp Biol 200: 295–302, 1997. 435. Heersche JN, Marcus R, Aurbach GD. Calcitonin and the formation of 3=,5=-AMP in bone and kidney. Endocrinology 94: 241–247, 1974. 436. Heitzmann D, Koren V, Wagner M, Sterner C, Reichold M, Tegtmeier I, Volk T, Warth R. KCNE beta subunits determine pH sensitivity of KCNQ1 potassium channels. Cell Physiol Biochem 19: 21–32, 2007. 437. Helander KG, Bamberg K, Sachs G, Melle D, Helander HF. Localization of mRNA for the muscarinic M1 receptor in rat stomach. Biochim Biophys Acta 1312: 158 –162, 1996. 438. Heller HJ, Greer LG, Haynes SD, Poindexter JR, Pak CY. Pharmacokinetic and pharmacodynamic comparison of two calcium supplements in postmenopausal women. J Clin Pharmacol 40: 1237–1244, 2000. 439. Heller HJ, Stewart A, Haynes S, Pak CY. Pharmacokinetics of calcium absorption from two commercial calcium supplements. J Clin Pharmacol 39: 1151–1154, 1999. 440. Hendy GN, Kronenberg HM, Potts JT Jr, Rich A. Nucleotide sequence of cloned cDNAs encoding human preproparathyroid hormone. Proc Natl Acad Sci USA 78: 7365–7369, 1981. 441. Hennessy JF, Wells SA Jr, Ontjes DA, Cooper CW. A comparison of pentagastrin injection and calcium infusion as provocative agents for the detection of medullary carcinoma of the thyroid. J Clin Endocrinol Metab 39: 487– 495, 1974. 442. Henry HL. Parathyroid hormone modulation of 25-hydroxyvitamin D3 metabolism by cultured chick kidney cells is mimicked and enhanced by forskolin. Endocrinology 116: 503–510, 1985. 443. Henry HL. Regulation of the hydroxylation of 25-hydroxyvitamin D3 in vivo and in primary cultures of chick kidney cells. J Biol Chem 254: 2722–2729, 1979. 444. Herberth J, Fahrleitner-Pammer A, Obermayer-Pietsch B, Krisper P, Holzer H, Malluche HH, Dobnig H. Changes in total parathyroid hormone (PTH), PTH-(1– 84) and large C-PTH fragments in different stages of chronic kidney disease. Clin Nephrol 65: 328 –334, 2006. 445. Herlong HF, Recker RR, Maddrey WC. Bone disease in primary biliary cirrhosis: histologic features and response to 25-hydroxyvitamin D. Gastroenterology 83: 103– 108, 1982. 446. Hess AF. The prevention and cure of rickets by sunlight. Am J Public Health 12: 104 –107, 1922. 447. Hess AF, Unger LJ. The cure of infantile rickets by sunlight. J Am Med Assoc 77: 39, 1921. 453. Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol 215: 31–38, 2004. 454. Heynen G, Brassine A, Daubresse JC, Ligny G, Kanis JA, Gaspar S, Franchimont P. Lack of clinical and physiological relationship between gastrin and calcitonin in man. Eur J Clin Invest 11: 331–335, 1981. 455. Higham A, Noble P, Thompson DG, Dockray GJ. Increased sensitivity of gastrin cells to gastric distension following antral denervation in the rat. J Physiol 503: 169 –175, 1997. 456. Hildebrand P, Lehmann FS, Ketterer S, Christ AD, Stingelin T, Beltinger J, Gibbons AH, Coy DH, Calam J, Larsen F, Beglinger C. Regulation of gastric function by endogenous gastrin releasing peptide in humans: studies with a specific gastrin releasing peptide receptor antagonist. Gut 49: 23–28, 2001. 457. Hildmann B, Schmidt A, Murer H. Ca2⫹-transport across basal-lateral plasma membranes from rat small intestinal epithelial cells. J Membr Biol 65: 55– 62, 1982. 458. Hill LF, Van den Berg CJ, Mawer EB. Vitamin D metabolism in experimental uraemia: effects on intestinal transport 45Ca and on formation of 1,25-dihydroxycholecalciferol in rat. Nat New Biol 232: 189 –191, 1971. 459. Hills DM, Gerskowitch VP, Roberts SP, Welsh NJ, Shankley NP, Black JW. Pharmacological analysis of the CCKB/gastrin receptors mediating pentagastrin-stimulated gastric acid secretion in the isolated stomach of the immature rat. Br J Pharmacol 119: 1401–1410, 1996. 460. Hinata M, Yamamura H, Li L, Watanabe Y, Watano T, Imaizumi Y, Kimura J. Stoichiometry of Na⫹-Ca2⫹ exchange is 3:1 in guinea-pig ventricular myocytes. J Physiol 545: 453– 461, 2002. 461. Hirnet D, Olausson J, Fecher-Trost C, Bodding M, Nastainczyk W, Wissenbach U, Flockerzi V, Freichel M. The TRPV6 gene, cDNA and protein. Cell Calcium 33: 509 –518, 2003. 462. Hirsch PF, Baruch H. Is calcitonin an important physiological substance? Endocrine 21: 201–208, 2003. 463. Hirsch PF, Gauthier GF, Munson PL. Thyroid hypocalcemic principle and recurrent laryngeal nerve injury as factors affecting the response to parathyroidectomy in rats. Endocrinology 73: 244 –252, 1963. 464. Hirschfeld J. Immune-electrophoretic demonstration of qualitative differences in human sera and their relation to the haptoglobins. Acta Pathol Microbiol Scand 47: 160 –168, 1959. 465. Hirschfeld J. Individual precipitation patterns of normal rabbit sera. A preliminary report. Acta Pathol Microbiol Scand 46: 229 –238, 1959. 466. Hirschowitz BI, Fong J, Molina E. Effects of pirenzepine and atropine on vagal and cholinergic gastric secretion and gastrin release and on heart rate in the dog. J Pharmacol Exp Ther 225: 263–268, 1983. 467. Hjalm G, MacLeod RJ, Kifor O, Chattopadhyay N, Brown EM. Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase. J Biol Chem 276: 34880 –34887, 2001. 468. Hoag HM, Martel J, Gauthier C, Tenenhouse HS. Effects of Npt2 gene ablation and low-phosphate diet on renal Na⫹/phosphate cotransport and cotransporter gene expression. J Clin Invest 104: 679 – 686, 1999. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 249 SASCHA KOPIC AND JOHN P. GEIBEL 469. Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res 7: 65–72, 1992. droxyvitamin D(3) [1,24-(OH)(2)D(3)], 1,25-dihydroxyvitamin D(3) [1,25(OH)(2)D(3)] on selected vitamin D-regulated events in the rat. Biochem Pharmacol 60: 701–708, 2000. 470. Hocker M, Henihan RJ, Rosewicz S, Riecken EO, Zhang Z, Koh TJ, Wang TC. Gastrin and phorbol 12-myristate 13-acetate regulate the human histidine decarboxylase promoter through Raf-dependent activation of extracellular signal-regulated kinaserelated signaling pathways in gastric cancer cells. J Biol Chem 272: 27015–27024, 1997. 490. Horsting M, DeLuca HF. In vitro production of 25-hydroxycholecalciferol. Biochem Biophys Res Commun 36: 251–256, 1969. 491. Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 279: 909 –913, 2000. 471. Hocker M, John M, Anagnostopoulos J, Buhr HJ, Solimena M, Gasnier B, Henry JP, Wang TC, Wiedenmann B. Molecular dissection of regulated secretory pathways in human gastric enterochromaffin-like cells: an immunohistochemical analysis. Histochem Cell Biol 112: 205–214, 1999. 492. Howard A, Legon S, Walters JR. Human and rat intestinal plasma membrane calcium pump isoforms. Am J Physiol Gastrointest Liver Physiol 265: G917–G925, 1993. 472. Hocker M, Zhang Z, Fenstermacher DA, Tagerud S, Chulak M, Joseph D, Wang TC. Rat histidine decarboxylase promoter is regulated by gastrin through a protein kinase C pathway. Am J Physiol Gastrointest Liver Physiol 270: G619 –G633, 1996. 493. Huan J, Olgaard K, Nielsen LB, Lewin E. Parathyroid hormone 7– 84 induces hypocalcemia and inhibits the parathyroid hormone 1– 84 secretory response to hypocalcemia in rats with intact parathyroid glands. J Am Soc Nephrol 17: 1923–1930, 2006. 473. Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev 85: 373– 422, 2005. 474. Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ. Molecular identification of the apical Ca2⫹ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem 274: 8375– 8378, 1999. 475. Hoenderop JG, Vennekens R, Muller D, Prenen J, Droogmans G, Bindels RJ, Nilius B. Function and expression of the epithelial Ca2⫹ channel family: comparison of mammalian ECaC1 and 2. J Physiol 537: 747–761, 2001. 476. Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ. Homoand heterotetrameric architecture of the epithelial Ca2⫹ channels TRPV5 and TRPV6. EMBO J 22: 776 –785, 2003. 477. Hohne-Zell B, Galler A, Schepp W, Gratzl M, Prinz C. Functional importance of synaptobrevin and SNAP-25 during exocytosis of histamine by rat gastric enterochromaffin-like cells. Endocrinology 138: 5518 –5526, 1997. 478. Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res 22 Suppl 2: V28 –33, 2007. 479. Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT Jr, Anderson RR, Blank IH, Parrish JA, Elias P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science 210: 203–205, 1980. 480. Holick MF, Schnoes HK, DeLuca HF, Suda T, Cousins RJ. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry 10: 2799 –2804, 1971. 481. Hollande F, Gusdinar T, Bali JP, Magous R. Neurohormonal regulation of histamine release from isolated rabbit fundic mucosal cells. Agents Actions 38: 149 –157, 1993. 482. Hollande F, Imdahl A, Mantamadiotis T, Ciccotosto GD, Shulkes A, Baldwin GS. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology 113: 1576 –1588, 1997. 483. Hollander D, Muralidhara KS, Zimmerman A. Vitamin D-3 intestinal absorption in vivo: influence of fatty acids, bile salts, and perfusate pH on absorption. Gut 19: 267–272, 1978. 484. Hollander D, Truscott TC. Mechanism and site of small intestinal uptake of vitamin D3 in pharmacological concentrations. Am J Clin Nutr 29: 970 –975, 1976. 494. Huang C, Handlogten ME, Miller RT. Parallel activation of phosphatidylinositol 4-kinase and phospholipase C by the extracellular calcium-sensing receptor. J Biol Chem 277: 20293–20300, 2002. 495. Huang C, Hujer KM, Wu Z, Miller RT. The Ca2⫹-sensing receptor couples to G␣12/13 to activate phospholipase D in Madin-Darby canine kidney cells. Am J Physiol Cell Physiol 286: C22–C30, 2004. 496. Huang C, Wu Z, Hujer KM, Miller RT. Silencing of filamin A gene expression inhibits Ca2⫹-sensing receptor signaling. FEBS Lett 580: 1795–1800, 2006. 497. Huang JC, Sakata T, Pfleger LL, Bencsik M, Halloran BP, Bikle DD, Nissenson RA. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res 19: 235–244, 2004. 498. Huang Y, Niwa J, Sobue G, Breitwieser GE. Calcium-sensing receptor ubiquitination and degradation mediated by the E3 ubiquitin ligase dorfin. J Biol Chem 281: 11610 – 11617, 2006. 499. Huang Y, Zhou Y, Castiblanco A, Yang W, Brown EM, Yang JJ. Multiple Ca2⫹-binding sites in the extracellular domain of the Ca2⫹-sensing receptor corresponding to cooperative Ca2⫹ response. Biochemistry 48: 388 –398, 2009. 500. Huang Y, Zhou Y, Yang W, Butters R, Lee HW, Li S, Castiblanco A, Brown EM, Yang JJ. Identification and dissection of Ca2⫹-binding sites in the extracellular domain of Ca2⫹-sensing receptor. J Biol Chem 282: 19000 –19010, 2007. 501. Huda MS, Durham BH, Wong SP, Dovey TM, McCulloch P, Kerrigan D, Pinkney JH, Fraser WD, Wilding JP. Lack of an acute effect of ghrelin on markers of bone turnover in healthy controls and post-gastrectomy subjects. Bone 41: 406 – 413, 2007. 502. Hughes DA, Tang K, Strotmann R, Schoneberg T, Prenen J, Nilius B, Stoneking M. Parallel selection on TRPV6 in human populations. PLoS ONE 3: e1686, 2008. 503. Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol 18: 2660 –2671, 2004. 504. Huldschinsky K. Heilung von Rachitis durch künstliche Höhensonne. Deutsche Med Wochenschrift 45: 712, 1919. 485. Holst MC, Kelly JB, Powley TL. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 381: 81–100, 1997. 505. Hume EM, Smith HH. The effect of irradiation of the environment with ultra-violet light upon the growth and calcification of rats, fed on a diet deficient in fat-soluble vitamins: the part played by irradiated sawdust. Biochem J 18: 1334 –1345, 1924. 486. Holst Pedersen J, Skov Olsen P, Kirkegaard P. Effect of neurotensin and neurotensin fragments on gastric acid secretion in man. Regul Pept 15: 77– 86, 1986. 506. Humphries TJ, Merritt GJ. Review article: drug interactions with agents used to treat acid-related diseases. Aliment Pharmacol Ther 13 Suppl 3: 18 –26, 1999. 487. Holtrop ME, Raisz LG, Simmons HA. The effects of parathyroid hormone, colchicine, and calcitonin on the ultrastructure and the activity of osteoclasts in organ culture. J Cell Biol 60: 346 –355, 1974. 507. Hunyady B, Mezey E, Pacak K, Harta G, Palkovits M. Distribution of muscarinic receptor mRNAs in the stomachs of normal or immobilized rats. Inflammopharmacology 4: 399 – 413, 1996. 488. Hori K, Takahashi Y, Horikawa N, Furukawa T, Tsukada K, Takeguchi N, Sakai H. Is the ClC-2 chloride channel involved in the Cl⫺ secretory mechanism of gastric parietal cells? FEBS Lett 575: 105–108, 2004. 508. Ichikawa F, Sato K, Nanjo M, Nishii Y, Shinki T, Takahashi N, Suda T. Mouse primary osteoblasts express vitamin D3 25-hydroxylase mRNA and convert 1 alpha-hydroxyvitamin D3 into 1 alpha,25-dihydroxyvitamin D3. Bone 16: 129 –135, 1995. 489. Horst R, Prapong S, Reinhardt T, Koszewski N, Knutson J, Bishop C. Comparison of the relative effects of 1,24-dihydroxyvitamin D(2) [1, 24-(OH)(2)D(2)], 1,24-dihy- 509. Imai M. Calcium transport across the rabbit thick ascending limb of Henle’s loop perfused in vitro. Pflügers Arch 374: 255–263, 1978. 250 Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH 510. Imanishi Y, Kawata T, Kenko T, Wada M, Nagano N, Miki T, Arnold A, Inaba M. Cinacalcet HCl suppresses Cyclin D1 oncogene-derived parathyroid cell proliferation in a murine model for primary hyperparathyroidism. Calcif Tissue Int 89: 29 –35, 2011. 511. Imawari M, Kozawa K, Akanuma Y, Koizumi S, Itakura H, Kosaka K. Serum 25hydroxyvitamin D and vitamin D-binding protein levels and mineral metabolism after partial and total gastrectomy. Gastroenterology 79: 255–258, 1980. 512. IMSHealth. Top Products by U.S. Spending 2010 http://www.imshealth.com/ deployedfiles/ims/Global/Content/Corporate/Press%20Room/Top-line%20 Market%20Data/2010%20Top-line%20Market%20Data/2010_Top_Products_by_ Sales.pdf [retrieved November 2011]. 513. Inoue Y, Segawa H, Kaneko I, Yamanaka S, Kusano K, Kawakami E, Furutani J, Ito M, Kuwahata M, Saito H, Fukushima N, Kato S, Kanayama HO, Miyamoto K. Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem J 390: 325– 331, 2005. 514. Irving AD, Smith G, Catto GR. Does metabolic bone disease follow truncal vagotomy and gastrojejunostomy? Br Med J 287: 1516, 1983. 515. Isenberg JI, Walsh JH, Grossman MI. Zollinger-Ellison syndrome. Gastroenterology 65: 140 –165, 1973. 516. Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors– emphasis on rabeprazole. Aliment Pharmacol Ther 13 Suppl 3: 27– 36, 1999. 517. Ishizawa M, Matsunawa M, Adachi R, Uno S, Ikeda K, Masuno H, Shimizu M, Iwasaki K, Yamada S, Makishima M. Lithocholic acid derivatives act as selective vitamin D receptor modulators without inducing hypercalcemia. J Lipid Res 49: 763–772, 2008. 518. Ishizuya T, Yokose S, Hori M, Noda T, Suda T, Yoshiki S, Yamaguchi A. Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest 99: 2961–2970, 1997. 519. Ito R, Sato K, Helmer T, Jay G, Agarwal K. Structural analysis of the gene encoding human gastrin: the large intron contains an Alu sequence. Proc Natl Acad Sci USA 81: 4662– 4666, 1984. 520. Ivanovich P, Fellows H, Rich C. The absorption of calcium carbonate. Ann Intern Med 66: 917–923, 1967. 521. Jaffe BM, Kopen DF, Lazan DW. Endogenous serotonin in the control of gastric acid secretion. Surgery 82: 156 –163, 1977. 522. Jain RN, Al-Menhali AA, Keeley TM, Ren J, El-Zaatari M, Chen X, Merchant JL, Ross TS, Chew CS, Samuelson LC. Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice. J Clin Invest 118: 2459 –2470, 2008. 530. Jilka RL, O’Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC. Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone 44: 275–286, 2009. 531. Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 104: 439 – 446, 1999. 532. Jin HO, Lee KY, Chang TM, Chey WY, Dubois A. Secretin: a physiological regulator of gastric emptying and acid output in dogs. Am J Physiol Gastrointest Liver Physiol 267: G702–G708, 1994. 533. Johnson JA, Grande JP, Roche PC, Kumar R. Ontogeny of the 1,25-dihydroxyvitamin D3 receptor in fetal rat bone. J Bone Miner Res 11: 56 – 61, 1996. 534. Johnson LR, Lichtenberger LM, Copeland EM, Dudrick SJ, Castro GA. Action of gastrin on gastrointestinal structure and function. Gastroenterology 68: 1184 –1192, 1975. 535. Jones B, Jones EL, Bonney SA, Patel HN, Mensenkamp AR, Eichenbaum-Voline S, Rudling M, Myrdal U, Annesi G, Naik S, Meadows N, Quattrone A, Islam SA, Naoumova RP, Angelin B, Infante R, Levy E, Roy CC, Freemont PS, Scott J, Shoulders CC. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet 34: 29 –31, 2003. 536. Juppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF Jr, Hock J, Potts JT Jr, Kronenberg HM. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science 254: 1024 –1026, 1991. 537. Jurutka PW, Thompson PD, Whitfield GK, Eichhorst KR, Hall N, Dominguez CE, Hsieh JC, Haussler CA, Haussler MR. Molecular and functional comparison of 1,25dihydroxyvitamin D3 and the novel vitamin D receptor ligand, lithocholic acid, in activating transcription of cytochrome P450 3A4. J Cell Biochem 94: 917–943, 2005. 538. Juttmann JR, Visser TJ, Buurman C, de Kam E, Birkenhager JC. Seasonal fluctuations in serum concentrations of vitamin D metabolites in normal subjects. Br Med J 282: 1349 –1352, 1981. 539. Kahrilas PJ, Dent J, Lauritsen K, Malfertheiner P, Denison H, Franzen S, Hasselgren G. A randomized, comparative study of three doses of AZD0865 and esomeprazole for healing of reflux esophagitis. Clin Gastroenterol Hepatol 5: 1385–1391, 2007. 540. Kajikawa M, Ishida H, Fujimoto S, Mukai E, Nishimura M, Fujita J, Tsuura Y, Okamoto Y, Norman AW, Seino Y. An insulinotropic effect of vitamin D analog with increasing intracellular Ca2⫹ concentration in pancreatic beta-cells through nongenomic signal transduction. Endocrinology 140: 4706 – 4712, 1999. 541. Kajimura M, Reuben MA, Sachs G. The muscarinic receptor gene expressed in rabbit parietal cells is the m3 subtype. Gastroenterology 103: 870 – 875, 1992. 523. Jain RN, Samuelson LC. Transcriptional profiling of gastrin-regulated genes in mouse stomach. Physiol Genomics 29: 1–12, 2007. 542. Kameda T, Mano H, Yamada Y, Takai H, Amizuka N, Kobori M, Izumi N, Kawashima H, Ozawa H, Ikeda K, Kameda A, Hakeda Y, Kumegawa M. Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochem Biophys Res Commun 245: 419 – 422, 1998. 524. Jarrousse C, Audousset-Puech MP, Dubrasquet M, Niel H, Martinez J, Bataille D. Oxyntomodulin (glucagon-37) and its C-terminal octapeptide inhibit gastric acid secretion. FEBS Lett 188: 81– 84, 1985. 543. Kanai S, Hosoya H, Akimoto S, Ohta M, Matsui T, Takiguchi S, Funakoshi A, Miyasaka K. Gastric acid secretion in cholecystokinin-1 receptor, -2 receptor, and -1, -2 receptor gene knockout mice. J Physiol Sci 59: 23–29, 2009. 525. Jarrousse C, Niel H, Audousset-Puech MP, Martinez J, Bataille D. Oxyntomodulin and its C-terminal octapeptide inhibit liquid meal-stimulated acid secretion. Peptides 7 Suppl 1: 253–256, 1986. 544. Kanatani M, Sugimoto T, Kanzawa M, Yano S, Chihara K. High extracellular calcium inhibits osteoclast-like cell formation by directly acting on the calcium-sensing receptor existing in osteoclast precursor cells. Biochem Biophys Res Commun 261: 144 –148, 1999. 526. Jeffery DA, Roufogalis BD, Katz S. The effect of calmodulin on the phosphoprotein intermediate of Mg2⫹-dependent Ca2⫹-stimulated adenosine triphosphatase in human erythrocyte membranes. Biochem J 194: 481– 486, 1981. 527. Jeffery PL, McGuckin MA, Linden SK. Endocrine impact of Helicobacter pylori: focus on ghrelin and ghrelin o-acyltransferase. World J Gastroenterol 17: 1249 –1260, 2011. 528. Jeon TY, Lee S, Kim HH, Kim YJ, Son HC, Kim DH, Sim MS. Changes in plasma ghrelin concentration immediately after gastrectomy in patients with early gastric cancer. J Clin Endocrinol Metab 89: 5392–5396, 2004. 529. Jiang Y, Minet E, Zhang Z, Silver PA, Bai M. Modulation of interprotomer relationships is important for activation of dimeric calcium-sensing receptor. J Biol Chem 279: 14147–14156, 2004. 545. Karam SM, Straiton T, Hassan WM, Leblond CP. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells 21: 322–336, 2003. 546. Karaplis AC, Lim SK, Baba H, Arnold A, Kronenberg HM. Inefficient membrane targeting, translocation, and proteolytic processing by signal peptidase of a mutant preproparathyroid hormone protein. J Biol Chem 270: 1629 –1635, 1995. 547. Karp HJ, Ketola ME, Lamberg-Allardt CJ. Acute effects of calcium carbonate, calcium citrate and potassium citrate on markers of calcium and bone metabolism in young women. Br J Nutr 102: 1341–1347, 2009. 548. Karvar S, Yao X, Crothers JM Jr, Liu Y, Forte JG. Localization and function of soluble N-ethylmaleimide-sensitive factor attachment protein-25 and vesicle-associated Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 251 SASCHA KOPIC AND JOHN P. GEIBEL membrane protein-2 in functioning gastric parietal cells. J Biol Chem 277: 50030 – 50035, 2002. 549. Karvar S, Yao X, Duman JG, Hybiske K, Liu Y, Forte JG. Intracellular distribution and functional importance of vesicle-associated membrane protein 2 in gastric parietal cells. Gastroenterology 123: 281–290, 2002. 550. Karvar S, Zhu L, Crothers J Jr, Wong W, Turkoz M, Forte JG. Cellular localization and stimulation-associated distribution dynamics of syntaxin-1 and syntaxin-3 in gastric parietal cells. Traffic 6: 654 – 666, 2005. 551. Kato K, Hayashizaki Y, Takahashi Y, Himeno S, Matsubara K. Molecular cloning of the human gastrin gene. Nucleic Acids Res 11: 8197– 8203, 1983. 552. Kato K, Himeno S, Takahashi Y, Wakabayashi T, Tarui S, Matsubara K. Molecular cloning of human gastrin precursor cDNA. Gene 26: 53–57, 1983. 553. Kato S, Abe Y, Konishi M, Kuroda N, Takeuchi K. Mechanism of gastric hyperemic response during acid secretion in rats: relation to nitric oxide, prostaglandins, sensory neurons. J Clin Gastroenterol 25 Suppl 1: S48 –55, 1997. 554. Kato S, Aihara E, Yoshii K, Takeuchi K. Dual action of prostaglandin E2 on gastric acid secretion through different EP-receptor subtypes in the rat. Am J Physiol Gastrointest Liver Physiol 289: G64 –G69, 2005. 555. Kato S, Kitamura M, Korolkiewicz RP, Takeuchi K. Role of nitric oxide in regulation of gastric acid secretion in rats: effects of NO donors and NO synthase inhibitor. Br J Pharmacol 123: 839 – 846, 1998. 556. Kaufhold MA, Krabbenhoft A, Song P, Engelhardt R, Riederer B, Fahrmann M, Klocker N, Beil W, Manns M, Hagen SJ, Seidler U. Localization, trafficking, and significance for acid secretion of parietal cell Kir4.1 and KCNQ1 K⫹ channels. Gastroenterology 134: 1058 –1069, 2008. 557. Kawakami M, Blum CB, Ramakrishnan R, Dell RB, Goodman DS. Turnover of the plasma binding protein for vitamin D and its metabolites in normal human subjects. J Clin Endocrinol Metab 53: 1110 –1116, 1981. 558. Kawashima K, Ishihara S, Karim Rumi MA, Moriyama N, Kazumori H, Suetsugu H, Sato H, Fukuda R, Adachi K, Shibata M, Onodera S, Chiba T, Kinoshita Y. Localization of calcitonin gene-related peptide receptors in rat gastric mucosa. Peptides 23: 955– 966, 2002. 559. Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 28: 951–959, 2008. 560. Kazumori H, Ishihara S, Kawashima K, Fukuda R, Chiba T, Kinoshita Y. Analysis of gastrin receptor gene expression in proliferating cells in the neck zone of gastric fundic glands using laser capture microdissection. FEBS Lett 489: 208 –214, 2001. 561. Keeley TM, Samuelson LC. Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice. Am J Physiol Gastrointest Liver Physiol 299: G1241–G1251, 2010. 562. Keeton TP, Burk SE, Shull GE. Alternative splicing of exons encoding the calmodulinbinding domains and C termini of plasma membrane Ca2⫹-ATPase isoforms 1, 2, 3, and 4. J Biol Chem 268: 2740 –2748, 1993. 563. Kehayoglou AK, Holdsworth CD, Agnew JE, Whelton MJ, Sherlock S. Bone disease and calcium absorption in primary biliary cirrhosis with special reference to vitamin-D therapy. Lancet 1: 715–718, 1968. 564. Keinke O, Ehrlein HJ, Wulschke S. Mechanical factors regulating gastric emptying examined by the effects of exogenous cholecystokinin and secretin on canine gastroduodenal motility. Can J Physiol Pharmacol 65: 287–292, 1987. 565. Kemper B, Habener JF, Mulligan RC, Potts JT Jr, Rich A. Pre-proparathyroid hormone: a direct translation product of parathyroid messenger RNA. Proc Natl Acad Sci USA 71: 3731–3735, 1974. 568. Khanal RC, Peters TM, Smith NM, Nemere I. Membrane receptor-initiated signaling in 1,25(OH)2D3-stimulated calcium uptake in intestinal epithelial cells. J Cell Biochem 105: 1109 –1116, 2008. 569. Kidd M, Hauso O, Drozdov I, Gustafsson BI, Modlin IM. Delineation of the chemomechanosensory regulation of gastrin secretion using pure rodent G cells. Gastroenterology 137: 231–241, 2009. 570. Kidd M, Modlin IM, Black JW, Boyce M, Culler M. A comparison of the effects of gastrin, somatostatin and dopamine receptor ligands on rat gastric enterochromaffin-like cell secretion and proliferation. Regul Pept 143: 109 –117, 2007. 571. Kifor O, Diaz R, Butters R, Kifor I, Brown EM. The calcium-sensing receptor is localized in caveolin-rich plasma membrane domains of bovine parathyroid cells. J Biol Chem 273: 21708 –21713, 1998. 572. Kifor O, MacLeod RJ, Diaz R, Bai M, Yamaguchi T, Yao T, Kifor I, Brown EM. Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am J Physiol Renal Physiol 280: F291–F302, 2001. 573. Kikuchi K, Kikuchi T, Ghishan FK. Characterization of calcium transport by basolateral membrane vesicles of human small intestine. Am J Physiol Gastrointest Liver Physiol 255: G482–G489, 1988. 574. Kim HJ, Yang DK, So I. PDZ domain-containing protein as a physiological modulator of TRPV6. Biochem Biophys Res Commun 361: 433– 438, 2007. 575. Kim MS, Fujiki R, Murayama A, Kitagawa H, Yamaoka K, Yamamoto Y, Mihara M, Takeyama K, Kato S. 1Alpha,25(OH)2D3-induced transrepression by vitamin D receptor through E-box-type elements in the human parathyroid hormone gene promoter. Mol Endocrinol 21: 334 –342, 2007. 576. Kim SW, Her SJ, Park SJ, Kim D, Park KS, Lee HK, Han BH, Kim MS, Shin CS, Kim SY. Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells. Bone 37: 359 –369, 2005. 577. Kirchhoff P, Andersson K, Socrates T, Sidani S, Kosiek O, Geibel JP. Characteristics of the K⫹-competitive H⫹,K⫹-ATPase inhibitor AZD0865 in isolated rat gastric glands. Am J Physiol Gastrointest Liver Physiol 291: G838 –G843, 2006. 578. Kirton CM, Wang T, Dockray GJ. Regulation of parietal cell migration by gastrin in the mouse. Am J Physiol Gastrointest Liver Physiol 283: G787–G793, 2002. 579. Klenk C, Schulz S, Calebiro D, Lohse MJ. Agonist-regulated cleavage of the extracellular domain of parathyroid hormone receptor type 1. J Biol Chem 285: 8665– 8674, 2010. 580. Klenk C, Vetter T, Zurn A, Vilardaga JP, Friedman PA, Wang B, Lohse MJ. Formation of a ternary complex among NHERF1, beta-arrestin, parathyroid hormone receptor. J Biol Chem 285: 30355–30362, 2010. 581. Klotz U. Pharmacokinetic considerations in the eradication of Helicobacter pylori. Clin Pharmacokinet 38: 243–270, 2000. 582. Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol 95: 2– 8, 2004. 583. Ko SH, Choi KC, Oh GT, Jeung EB. Effect of dietary calcium and 1,25-(OH)2D3 on the expression of calcium transport genes in calbindin-D9k and -D28k double knockout mice. Biochem Biophys Res Commun 379: 227–232, 2009. 584. Kobayashi T, Tonai S, Ishihara Y, Koga R, Okabe S, Watanabe T. Abnormal functional and morphological regulation of the gastric mucosa in histamine H2 receptor-deficient mice. J Clin Invest 105: 1741–1749, 2000. 585. Kocian J, Brodan V. New observations on the absorption of 47Ca in patients with partial gastrectomy. Digestion 12: 193–200, 1975. 586. Kodicek E, Lawson DE, Wilson PW. Biological activity of a polar metabolite of vitamin D. Nature 228: 763–764, 1970. 566. Keusch I, Traebert M, Lotscher M, Kaissling B, Murer H, Biber J. Parathyroid hormone and dietary phosphate provoke a lysosomal routing of the proximal tubular Na/Pi-cotransporter type II. Kidney Int 54: 1224 –1232, 1998. 587. Koh TJ, Field JK, Varro A, Liloglou T, Fielding P, Cui G, Houghton J, Dockray GJ, Wang TC. Glycine-extended gastrin promotes the growth of lung cancer. Cancer Res 64: 196 –201, 2004. 567. Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev CD003244, 2007. 588. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656 – 660, 1999. 252 Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH 589. Komaba H, Nakanishi S, Fujimori A, Tanaka M, Shin J, Shibuya K, Nishioka M, Hasegawa H, Kurosawa T, Fukagawa M. Cinacalcet effectively reduces parathyroid hormone secretion and gland volume regardless of pretreatment gland size in patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol 5: 2305–2314, 2010. 590. Komasaka M, Horie S, Watanabe K, Murayama T. Antisecretory effect of somatostatin on gastric acid via inhibition of histamine release in isolated mouse stomach. Eur J Pharmacol 452: 235–243, 2002. 591. Kong XF, Zhu XH, Pei YL, Jackson DM, Holick MF. Molecular cloning, characterization, and promoter analysis of the human 25-hydroxyvitamin D3-1alpha-hydroxylase gene. Proc Natl Acad Sci USA 96: 6988 – 6993, 1999. 592. Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveirados-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397: 315–323, 1999. 593. Konturek JW, Stoll R, Konturek SJ, Domschke W. Cholecystokinin in the control of gastric acid secretion in man. Gut 34: 321–328, 1993. 594. Konturek SJ, Bilski J, Cieszkowski M. Role of cholecystokinin in the intestinal fat- and acid-induced inhibition of gastric secretion. Regul Pept 42: 97–109, 1992. 595. Konturek SJ, Kwiecien N, Obtulowicz W, Mikos E, Sito E, Oleksy J, Popiela T. Cephalic phase of gastric secretion in healthy subjects and duodenal ulcer patients: role of vagal innervation. Gut 20: 875– 881, 1979. 596. Kopin AS, Lee YM, McBride EW, Miller LJ, Lu M, Lin HY, Kolakowski LF Jr, Beinborn M. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc Natl Acad Sci USA 89: 3605–3609, 1992. 597. Kopp R, Pfeiffer A. Effects of phorbol ester treatment on dibutyryl cyclic adenosine-5= monophosphate- and carbachol-stimulated aminopyrine accumulation in isolated rat parietal cells. Scand J Gastroenterol 35: 686 – 693, 2000. 598. Kos CH, Karaplis AC, Peng JB, Hediger MA, Goltzman D, Mohammad KS, Guise TA, Pollak MR. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest 111: 1021–1028, 2003. 599. Koshihara Y, Hoshi K, Ishibashi H, Shiraki M. Vitamin K2 promotes 1alpha,25(OH)2 vitamin D3-induced mineralization in human periosteal osteoblasts. Calcif Tissue Int 59: 466 – 473, 1996. 600. Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, Conway SJ. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J 15: 1209 –1211, 2001. 601. Kozoni V, Tsioulias G, Shiff S, Rigas B. The effect of lithocholic acid on proliferation and apoptosis during the early stages of colon carcinogenesis: differential effect on apoptosis in the presence of a colon carcinogen. Carcinogenesis 21: 999 –1005, 2000. 602. Kretsinger RH, Mann JE, Simmonds JG. Model of facilitated diffusion of calcium by the intestinal calcium-binding protein. In: Proceedings of the Fifth Workshop on Vitamin D, edited by Norman AW, Schaefer K, Herrath DV, Grigoleit HG. Berlin: Debruyter, 1982, p. 232–248. 603. Kreutz M, Andreesen R, Krause SW, Szabo A, Ritz E, Reichel H. 1,25-Dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood 82: 1300 –1307, 1993. 604. Krisinger J, Strom M, Darwish HD, Perlman K, Smith C, DeLuca HF. Induction of calbindin-D 9k mRNA but not calcium transport in rat intestine by 1,25-dihydroxyvitamin D3 24-homologs. J Biol Chem 266: 1910 –1913, 1991. 605. Kubler N, Krause U, Wagner PK, Beyer J, Rothmund M. The secretion of parathyroid hormone and its fragments from dispersed cells of adenomatous parathyroid tissue at different calcium concentrations. Exp Clin Endocrinol 88: 101–108, 1986. 606. Kuestner RE, Elrod RD, Grant FJ, Hagen FS, Kuijper JL, Matthewes SL, O’Hara PJ, Sheppard PO, Stroop SD, Thompson DL. Cloning and characterization of an abundant subtype of the human calcitonin receptor. Mol Pharmacol 46: 246 –255, 1994. 607. Kuhn B, Schmid A, Harteneck C, Gudermann T, Schultz G. G proteins of the Gq family couple the H2 histamine receptor to phospholipase C. Mol Endocrinol 10: 1697–1707, 1996. 608. Kulaksiz H, Arnold R, Goke B, Maronde E, Meyer M, Fahrenholz F, Forssmann WG, Eissele R. Expression and cell-specific localization of the cholecystokinin B/gastrin receptor in the human stomach. Cell Tissue Res 299: 289 –298, 2000. 609. Kumar R, Cohen WR, Silva P, Epstein FH. Elevated 1,25-dihydroxyvitamin D plasma levels in normal human pregnancy and lactation. J Clin Invest 63: 342–344, 1979. 610. Kumar R, Schaefer J, Grande JP, Roche PC. Immunolocalization of calcitriol receptor, 24-hydroxylase cytochrome P-450, and calbindin D28k in human kidney. Am J Physiol Renal Fluid Electrolyte Physiol 266: F477–F485, 1994. 611. Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407: 971–977, 2000. 612. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997. 613. Kutuzova GD, Akhter S, Christakos S, Vanhooke J, Kimmel-Jehan C, DeLuca HF. Calbindin D9k knockout mice are indistinguishable from wild-type mice in phenotype and serum calcium level. Proc Natl Acad Sci USA 103: 12377–12381, 2006. 614. Kutuzova GD, Deluca HF. Gene expression profiles in rat intestine identify pathways for 1,25-dihydroxyvitamin D3 stimulated calcium absorption and clarify its immunomodulatory properties. Arch Biochem Biophys 432: 152–166, 2004. 615. Kutuzova GD, Sundersingh F, Vaughan J, Tadi BP, Ansay SE, Christakos S, Deluca HF. TRPV6 is not required for 1alpha,25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo. Proc Natl Acad Sci USA 105: 19655–19659, 2008. 616. Kwok CS, Yeong JK, Loke YK. Meta-analysis: risk of fractures with acid-suppressing medication. Bone 48: 768 –776, 2011. 617. Labuda M, Labuda D, Korab-Laskowska M, Cole DE, Zietkiewicz E, Weissenbach J, Popowska E, Pronicka E, Root AW, Glorieux FH. Linkage disequilibrium analysis in young populations: pseudo-vitamin D-deficiency rickets and the founder effect in French Canadians. Am J Hum Genet 59: 633– 643, 1996. 618. Labuda M, Morgan K, Glorieux FH. Mapping autosomal recessive vitamin D dependency type I to chromosome 12q14 by linkage analysis. Am J Hum Genet 47: 28 –36, 1990. 619. Lambers TT, Weidema AF, Nilius B, Hoenderop JG, Bindels RJ. Regulation of the mouse epithelial Ca2⫹ channel TRPV6 by the Ca2⫹-sensor calmodulin. J Biol Chem 279: 28855–28861, 2004. 620. Lamberts R, Stumps D, Plumpe L, Creutzfeldt W. Somatostatin cells in rat antral mucosa: qualitative and quantitative ultrastructural analyses in different states of gastric acid secretion. Histochemistry 95: 373–382, 1991. 621. Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest 102: 1627–1633, 1998. 622. Langhans N, Rindi G, Chiu M, Rehfeld JF, Ardman B, Beinborn M, Kopin AS. Abnormal gastric histology and decreased acid production in cholecystokinin-B/gastrin receptor-deficient mice. Gastroenterology 112: 280 –286, 1997. 623. Langub MC, Reinhardt TA, Horst RL, Malluche HH, Koszewski NJ. Characterization of vitamin D receptor immunoreactivity in human bone cells. Bone 27: 383–387, 2000. 624. Lapierre LA, Avant KM, Caldwell CM, Ham AJ, Hill S, Williams JA, Smolka AJ, Goldenring JR. Characterization of immunoisolated human gastric parietal cells tubulovesicles: identification of regulators of apical recycling. Am J Physiol Gastrointest Liver Physiol 292: G1249 –G1262, 2007. 625. Lark RK, Lester GE, Ontjes DA, Blackwood AD, Hollis BW, Hensler MM, Aris RM. Diminished and erratic absorption of ergocalciferol in adult cystic fibrosis patients. Am J Clin Nutr 73: 602– 606, 2001. 626. Larsen EH, Nedergaard S, Ussing HH. Role of lateral intercellular space and sodium recirculation for isotonic transport in leaky epithelia. Rev Physiol Biochem Pharmacol 141: 153–212, 2000. 627. Larsen FL, Katz S, Roufogalis BD. Calmodulin regulation of Ca2⫹ transport in human erythrocytes. Biochem J 200: 185–191, 1981. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 253 SASCHA KOPIC AND JOHN P. GEIBEL 628. Larsen FL, Vincenzi FF. Calcium transport across the plasma membrane: stimulation by calmodulin. Science 204: 306 –309, 1979. 649. Lepard KJ, Chi J, Mohammed JR, Gidener S, Stephens RL Jr. Gastric antisecretory effect of serotonin: quantitation of release and site of action. Am J Physiol Endocrinol Metab 271: E669 –E677, 1996. 629. Larsson B, Gritli-Linde A, Norlen P, Lindstrom E, Hakanson R, Linde A. Extracts of ECL-cell granules/vesicles and of isolated ECL cells from rat oxyntic mucosa evoke a Ca2⫹ second messenger response in osteoblastic cells. Regul Pept 97: 153–161, 2001. 650. LePard KJ, Stephens RL Jr. Serotonin inhibits gastric acid secretion through a 5-hydroxytryptamine1-like receptor in the rat. J Pharmacol Exp Ther 270: 1139 –1144, 1994. 630. Larsson B, Norlen P, Lindstrom E, Zhao D, Hakanson R, Linde A. Effects of ECL cell extracts and granule/vesicle-enriched fractions from rat oxyntic mucosa on cAMP and IP3 in rat osteoblast-like cells. Regul Pept 106: 13–18, 2002. 651. Leth RD, Abrahamsson H, Kilander A, Lundell LR. Malabsorption of fat after partial gastric resection. A study of pathophysiologic mechanisms. Eur J Surg 157: 205–208, 1991. 631. Larsson H, Carlsson E, Ryberg B, Fryklund J, Wallmark B. Rat parietal cell function after prolonged inhibition of gastric acid secretion. Am J Physiol Gastrointest Liver Physiol 254: G33–G39, 1988. 652. Levant JA, Walsh JH, Isenberg JI. Stimulation of gastric secretion and gastrin release by single oral doses of calcium carbonate in man. N Engl J Med 289: 555–558, 1973. 632. Larsson LI, Goltermann N, de Magistris L, Rehfeld JF, Schwartz TW. Somatostatin cell processes as pathways for paracrine secretion. Science 205: 1393–1395, 1979. 633. Lawson DE, Fraser DR, Kodicek E, Morris HR, Williams DH. Identification of 1,25dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature 230: 228 –230, 1971. 653. Levi R, Ben-Dov IZ, Lavi-Moshayoff V, Dinur M, Martin D, Naveh-Many T, Silver J. Increased parathyroid hormone gene expression in secondary hyperparathyroidism of experimental uremia is reversed by calcimimetics: correlation with posttranslational modification of the trans acting factor AUF1. J Am Soc Nephrol 17: 107–112, 2006. 634. Lawson DE, Wilson PW, Kodicek E. New vitamin D metabolite localized in intestinal cell nuclei. Nature 222: 171–172, 1969. 654. Levin F, Edholm T, Ehrstrom M, Wallin B, Schmidt PT, Kirchgessner AM, Hilsted LM, Hellstrom PM, Naslund E. Effect of peripherally administered ghrelin on gastric emptying and acid secretion in the rat. Regul Pept 131: 59 – 65, 2005. 635. Lawton DE, Simcock DC, Candy EJ, Simpson HV. Gastrin secretion by ovine antral mucosa in vitro. Comp Biochem Physiol A Mol Integr Physiol 126: 233–243, 2000. 655. Lewis JJ, Zdon MJ, Adrian TE, Modlin IM. Pancreastatin: a novel peptide inhibitor of parietal cell secretion. Surgery 104: 1031–1036, 1988. 636. Lawton GP, Tang LH, Miu K, Gilligan CJ, Absood A, Modlin IM. Adrenergic and cromolyn sodium modulation of ECL cell histamine secretion. J Surg Res 58: 96 –104, 1995. 656. Li P, Chang TM, Chey WY. Secretin inhibits gastric acid secretion via a vagal afferent pathway in rats. Am J Physiol Gastrointest Liver Physiol 275: G22–G28, 1998. 637. Le Moullec JM, Jullienne A, Chenais J, Lasmoles F, Guliana JM, Milhaud G, Moukhtar MS. The complete sequence of human preprocalcitonin. FEBS Lett 167: 93–97, 1984. 657. Li P, Chang TM, Coy D, Chey WY. Inhibition of gastric acid secretion in rat stomach by PACAP is mediated by secretin, somatostatin, and PGE2. Am J Physiol Gastrointest Liver Physiol 278: G121–G127, 2000. 638. Leclerc R, Pelletier G, Puviani R, Arimura A, Schally AV. Immunohistochemical localization of somatostatin in endocrine cells of the rat stomach. Mol Cell Endocrinol 4: 257–261, 1976. 639. Lee GS, Lee KY, Choi KC, Ryu YH, Paik SG, Oh GT, Jeung EB. Phenotype of a calbindin-D9k gene knockout is compensated for by the induction of other calcium transporter genes in a mouse model. J Bone Miner Res 22: 1968 –1978, 2007. 640. Lee JW, Fuda H, Javitt NB, Strott CA, Rodriguez IR. Expression and localization of sterol 27-hydroxylase (CYP27A1) in monkey retina. Exp Eye Res 83: 465– 469, 2006. 641. Lee MP, Ravenel JD, Hu RJ, Lustig LR, Tomaselli G, Berger RD, Brandenburg SA, Litzi TJ, Bunton TE, Limb C, Francis H, Gorelikow M, Gu H, Washington K, Argani P, Goldenring JR, Coffey RJ, Feinberg AP. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest 106: 1447–1455, 2000. 642. Lefkowitz ES, Garland CF. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int J Epidemiol 23: 1133–1136, 1994. 643. Leheste JR, Melsen F, Wellner M, Jansen P, Schlichting U, Renner-Muller I, Andreassen TT, Wolf E, Bachmann S, Nykjaer A, Willnow TE. Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J 17: 247–249, 2003. 644. Lehto-Axtelius D, Chen D, Surve VV, Hakanson R. Post-gastrectomy osteopenia in the rat: bone structure is preserved by retaining 10%-30% of the oxyntic gland area. Scand J Gastroenterol 37: 437– 443, 2002. 645. Leichtmann GA, Bengoa JM, Bolt MJ, Sitrin MD. Intestinal absorption of cholecalciferol and 25-hydroxycholecalciferol in patients with both Crohn’s disease and intestinal resection. Am J Clin Nutr 54: 548 –552, 1991. 646. Leitersdorf E, Reshef A, Meiner V, Levitzki R, Schwartz SP, Dann EJ, Berkman N, Cali JJ, Klapholz L, Berginer VM. Frameshift and splice-junction mutations in the sterol 27-hydroxylase gene cause cerebrotendinous xanthomatosis in Jews or Moroccan origin. J Clin Invest 91: 2488 –2496, 1993. 647. Lemay J, Demers C, Hendy GN, Gascon-Barré M. Oral calcium transiently increases calbindin(9k) gene expression in adult rat duodena. Calcified Tissue Int 60: 43– 47, 1997. 648. Lepage R, Roy L, Brossard JH, Rousseau L, Dorais C, Lazure C, D’Amour P. A non-(1– 84) circulating parathyroid hormone (PTH) fragment interferes significantly with intact PTH commercial assay measurements in uremic samples. Clin Chem 44: 805– 809, 1998. 254 658. Li XF, Kraev AS, Lytton J. Molecular cloning of a fourth member of the potassiumdependent sodium-calcium exchanger gene family, NCKX4. J Biol Chem 277: 48410 – 48417, 2002. 659. Li Y, Wu X, Yao H, Owyang C. Secretin activates vagal primary afferent neurons in the rat: evidence from electrophysiological and immunohistochemical studies. Am J Physiol Gastrointest Liver Physiol 289: G745–G752, 2005. 660. Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem 88: 327–331, 2003. 661. Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology 139: 4391– 4396, 1998. 662. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229 – 238, 2002. 663. Li YC, Pirro AE, Demay MB. Analysis of vitamin D-dependent calcium-binding protein messenger ribonucleic acid expression in mice lacking the vitamin D receptor. Endocrinology 139: 847– 851, 1998. 664. Li YY. Mechanisms for regulation of gastrin and somatostatin release from isolated rat stomach during gastric distention. World J Gastroenterol 9: 129 –133, 2003. 665. Lian J, Stewart C, Puchacz E, Mackowiak S, Shalhoub V, Collart D, Zambetti G, Stein G. Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc Natl Acad Sci USA 86: 1143–1147, 1989. 666. Lieben L, Benn BS, Ajibade D, Stockmans I, Moermans K, Hediger MA, Peng JB, Christakos S, Bouillon R, Carmeliet G. Trpv6 mediates intestinal calcium absorption during calcium restriction and contributes to bone homeostasis. Bone 47: 301–308, 2010. 667. Liedman B, Bosaeus I, Mellstrom D, Lundell L. Osteoporosis after total gastrectomy. Results of a prospective, clinical study. Scand J Gastroenterol 32: 1090 –1095, 1997. 668. Liedman B, Henningsson A, Mellstrom D, Lundell L. Changes in bone metabolism and body composition after total gastrectomy: results of a longitudinal study. Dig Dis Sci 45: 819 – 824, 2000. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH 669. Lin HY, Harris TL, Flannery MS, Aruffo A, Kaji EH, Gorn A, Kolakowski LF Jr, Lodish HF, Goldring SR. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science 254: 1022–1024, 1991. 670. Lindberg P, Nordberg P, Alminger T, Brandstrom A, Wallmark B. The mechanism of action of the gastric acid secretion inhibitor omeprazole. J Med Chem 29: 1327–1329, 1986. 671. Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 350: 550 –555, 1997. 672. Lindstrom E, Bjorkqvist M, Boketoft A, Chen D, Zhao CM, Kimura K, Hakanson R. Neurohormonal regulation of histamine and pancreastatin secretion from isolated rat stomach ECL cells. Regul Pept 71: 73– 86, 1997. 673. Lindstrom E, Eliasson L, Bjorkqvist M, Hakanson R. Gastrin and the neuropeptide PACAP evoke secretion from rat stomach histamine-containing (ECL) cells by stimulating influx of Ca2⫹ through different Ca2⫹ channels. J Physiol 535: 663– 677, 2001. 689. Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, Chandrasekhar S, Martin TJ, Onyia JE. Catabolic effects of continuous human PTH (1–38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and geneassociated bone formation. Endocrinology 142: 4047– 4054, 2001. 690. MacCallum WG, Voegtlin C. On the regulation of the parathyroid to calcium metabolism and the nature of tetany. Bull Johns Hopkins Hosp 19: 91, 1908. 691. Maccarinelli G, Sibilia V, Torsello A, Raimondo F, Pitto M, Giustina A, Netti C, Cocchi D. Ghrelin regulates proliferation and differentiation of osteoblastic cells. J Endocrinol 184: 249 –256, 2005. 692. Mace OJ, Morgan EL, Affleck JA, Lister N, Kellett GL. Calcium absorption by Cav1.3 induces terminal web myosin II phosphorylation and apical GLUT2 insertion in rat intestine. J Physiol 580: 605– 616, 2007. 693. MacGregor RR, Cohn DV, Hamilton JW. The content of carboxyl-terminal fragments of parathormone in extracts of fresh bovine parathyroids. Endocrinology 112: 1019 –1025, 1983. 674. Lindstrom E, Hakanson R. Neurohormonal regulation of secretion from isolated rat stomach ECL cells: a critical reappraisal. Regul Pept 97: 169 –180, 2001. 694. Madaus S, Bender H, Schusdziarra V, Kehe K, Munzert G, Weber G, Classen M. Vagally induced release of gastrin, somatostatin and bombesin-like immunoreactivity from perfused rat stomach. Effect of stimulation frequency and cholinergic mechanisms. Regul Pept 30: 179 –192, 1990. 675. Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol 82: C805–C816, 2002. 695. Madaus S, Schusdziarra V, Seufferlein T, Classen M. Effect of galanin on gastrin and somatostatin release from the rat stomach. Life Sci 42: 2381–2387, 1988. 676. Liu C, Chen J, Guo Y, Yang L, Zhao C, Bai L. The expression of PTHLH in human gastric mucosa enterochromaffin-like cells. Dig Dis Sci 56: 993–998, 2011. 696. Madhok TC, DeLuca HF. Characteristics of the rat liver microsomal enzyme system converting cholecalciferol into 25-hydroxycholecalciferol. Evidence for the participation of cytochrome p-450. Biochem J 184: 491– 499, 1979. 677. Lloyd KC, Amirmoazzami S, Friedik F, Heynio A, Solomon TE, Walsh JH. Candidate canine enterogastrones: acid inhibition before and after vagotomy. Am J Physiol Gastrointest Liver Physiol 272: G1236 –G1242, 1997. 678. Lo CW, Paris PW, Clemens TL, Nolan J, Holick MF. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr 42: 644 – 649, 1985. 679. Locklin RM, Khosla S, Turner RT, Riggs BL. Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. J Cell Biochem 89: 180 –190, 2003. 680. Loffing J, Loffing-Cueni D, Valderrabano V, Klausli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281: F1021–F1027, 2001. 681. Lorenz S, Frenzel R, Paschke R, Breitwieser GE, Miedlich SU. Functional desensitization of the extracellular calcium-sensing receptor is regulated via distinct mechanisms: role of G protein-coupled receptor kinases, protein kinase C and beta-arrestins. Endocrinology 148: 2398 –2404, 2007. 682. Lotscher M, Scarpetta Y, Levi M, Halaihel N, Wang H, Zajicek HK, Biber J, Murer H, Kaissling B. Rapid downregulation of rat renal Na/Pi cotransporter in response to parathyroid hormone involves microtubule rearrangement. J Clin Invest 104: 483– 494, 1999. 683. Lu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG. The -glucuronidase klotho exclusively activates the epithelial Ca2⫹ channels TRPV5 and TRPV6. Nephrol Dialysis Transplantation 23: 3397–3402, 2008. 684. Luiz de Freitas PH, Li M, Ninomiya T, Nakamura M, Ubaidus S, Oda K, Udagawa N, Maeda T, Takagi R, Amizuka N. Intermittent PTH administration stimulates preosteoblastic proliferation without leading to enhanced bone formation in osteoclastless c-fos(⫺/⫺) mice. J Bone Miner Res 24: 1586 –1597, 2009. 685. Lund B, Selnes A. Plasma 1,25-dihydroxyvitamin D levels in pregnancy and lactation. Acta Endocrinol 92: 330 –335, 1979. 686. Lund J, DeLuca HF. Biologically active metabolite of vitamin D3 from bone, liver, and blood serum. J Lipid Res 7: 739 –744, 1966. 687. Lytton J. Na⫹/Ca2⫹ exchangers: three mammalian gene families control Ca2⫹ transport. Biochem J 406: 365–382, 2007. 688. Lytton J, Li XF, Dong H, Kraev A. K⫹-dependent Na⫹/Ca2⫹ exchangers in the brain. Ann NY Acad Sci 976: 382–393, 2002. 697. Maeda M, Oshiman K, Tamura S, Futai M. Human gastric-ATPase gene. Similarity to (Na⫹ ⫹ K⫹)-ATPase genes in exon/intron organization but difference in control region. J Biol Chem 265: 9027–9032, 1990. 698. Mahon MJ, Donowitz M, Yun CC, Segre GV. Na⫹/H⫹ exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature 417: 858 – 861, 2002. 699. Mahoney AW, Holbrook RS, Hendricks DG. Effects of calcium solubility on adsorption by rats with induced achlorhydria. Nutr Metab 18: 310 –317, 1975. 700. Maier GW, Kreis ME, Zittel TT, Becker HD. Calcium regulation and bone mass loss after total gastrectomy in pigs. Ann Surg 225: 181–192, 1997. 701. Maislos M, Silver J, Fainaru M. Intestinal absorption of vitamin D sterols: differential absorption into lymph and portal blood in the rat. Gastroenterology 80: 1528 –1534, 1981. 702. Maiti A, Beckman MJ. Extracellular calcium is a direct effecter of VDR levels in proximal tubule epithelial cells that counter-balances effects of PTH on renal Vitamin D metabolism. J Steroid Biochem Mol Biol 103: 504 –508, 2007. 703. Makin G, Lohnes D, Byford V, Ray R, Jones G. Target cell metabolism of 1,25dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem J 262: 173–180, 1989. 704. Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science 296: 1313–1316, 2002. 705. Malinowska DH. Cl⫺ channel blockers inhibit acid secretion in rabbit parietal cells. Am J Physiol Cell Physiol 259: C536 –C543, 1990. 706. Malinowska DH, Kupert EY, Bahinski A, Sherry AM, Cuppoletti J. Cloning, functional expression, and characterization of a PKA-activated gastric Cl⫺ channel. Am J Physiol Cell Physiol 268: C191–C200, 1995. 707. Malinowska DH, Sherry AM, Tewari KP, Cuppoletti J. Gastric parietal cell secretory membrane contains PKA- and acid-activated Kir2.1 K⫹ channels. Am J Physiol Cell Physiol Cell Physiol 286: C495–C506, 2004. 708. Manela FD, Ren J, Gao J, McGuigan JE, Harty RF. Calcitonin gene-related peptide modulates acid-mediated regulation of somatostatin and gastrin release from rat antrum. Gastroenterology 109: 701–706, 1995. 709. Manolagas SC, Burton DW, Deftos LJ. 1,25-Dihydroxyvitamin D3 stimulates the alkaline phosphatase activity of osteoblast-like cells. J Biol Chem 256: 7115–7117, 1981. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 255 SASCHA KOPIC AND JOHN P. GEIBEL 710. Marcus CS, Lengemann FW. Absorption of Ca45 and Sr85 from solid and liquid food at various levels of the alimentary tract of the rat. J Nutr 77: 155–160, 1962. 711. Marie PJ. The calcium-sensing receptor in bone cells: a potential therapeutic target in osteoporosis. Bone 46: 571–576, 2010. 731. Melander T, Hokfelt T, Rokaeus A, Fahrenkrug J, Tatemoto K, Mutt V. Distribution of galanin-like immunoreactivity in the gastro-intestinal tract of several mammalian species. Cell Tissue Res 239: 253–270, 1985. 732. Melick RA, Benson JA Jr. Osteomalacia following partial gastrectomy. N Engl J Med 260: 976 –978, 1959. 712. Marks HD, Fleet JC, Peleg S. Transgenic expression of the human Vitamin D receptor (hVDR) in the duodenum of VDR-null mice attenuates the age-dependent decline in calcium absorption. J Steroid Biochem Mol Biol 103: 513–516, 2007. 733. Mellanby E. An experimental investigation on rickets. Lancet 193: 407– 412, 1919. 713. Martin DL, DeLuca HF. Influence of sodium on calcium transport by the rat small intestine. Am J Physiol 216: 1351–1359, 1969. 734. Mentaverri R, Yano S, Chattopadhyay N, Petit L, Kifor O, Kamel S, Terwilliger EF, Brazier M, Brown EM. The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. FASEB J 20: 2562–2564, 2006. 714. Martin SR, Linse S, Johansson C, Bayley PM, Forsen S. Protein surface charges and Ca2⫹ binding to individual sites in calbindin D9k: stopped-flow studies. Biochemistry 29: 4188 – 4193, 1990. 735. Meredith SC, Bolt MJ, Rosenberg IH. The supramolecular structure of vitamin D3 in water. J Colloid Interface Sci 99: 244 –255, 1984. 715. Martinez V, Curi AP, Torkian B, Schaeffer JM, Wilkinson HA, Walsh JH, Tache Y. High basal gastric acid secretion in somatostatin receptor subtype 2 knockout mice. Gastroenterology 114: 1125–1132, 1998. 736. Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol 20: 1447–1461, 2006. 716. Martz A, Forte LR, Langeluttig SG. Renal cAMP and 1,25(OH)2D3 synthesis in estrogen-treated chick embryos and hens. Am J Physiol Endocrinol Metab 249: E626 – E633, 1985. 717. Massheimer V, Boland R, de Boland AR. Rapid 1,25(OH)2-vitamin D3 stimulation of calcium uptake by rat intestinal cells involves a dihydropyridine-sensitive cAMPdependent pathway. Cell Signal 6: 299 –304, 1994. 718. Massry SG, Coburn JW, Chapman LW, Kleeman CR. Role of serum Ca, parathyroid hormone, NaCl infusion on renal Ca and Na clearances. Am J Physiol 214: 1403– 1409, 1968. 719. Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun 276: 905–908, 2000. 720. Mate L, Sakamoto T, Greeley GH Jr, Thompson JC. Regulation of gastric acid secretion by secretin and serotonin. Am J Surg 149: 40 – 45, 1985. 721. Matloff DS, Kaplan MM, Neer RM, Goldberg MJ, Bitman W, Wolfe HJ. Osteoporosis in primary biliary cirrhosis: effects of 25-hydroxyvitamin D3 treatment. Gastroenterology 83: 97–102, 1982. 722. Matsumoto M, Park J, Sugano K, Yamada T. Biological activity of progastrin posttranslational processing intermediates. Am J Physiol Gastrointest Liver Physiol 252: G315–G319, 1987. 723. Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab 64: 1165–1168, 1987. 724. Mawer EB, Taylor CM, Backhouse J, Lumb GA, Stanbury SW. Failure of formation of 1,25-dihydroxycholecalciferol in chronic renal insufficiency. Lancet 1: 626 – 628, 1973. 725. Mayer G, Arnold R, Feurle G, Fuchs K, Ketterer H, Track NS, Creutzfeldt W. Influence of feeding and sham feeding upon serum gastrin and gastric acid secretion in control subjects and duodenal ulcer patients. Scand J Gastroenterol 9: 703–710, 1974. 726. Mayer GP, Keaton JA, Hurst JG, Habener JF. Effects of plasma calcium concentration on the relative proportion of hormone and carboxyl fragments in parathyroid venous blood. Endocrinology 104: 1778 –1784, 1979. 727. McCollum EV, Simmonds N, Becker JE, Shipley PG. Studies on experimental rickets. XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J Biol Chem 53: 293–312, 1922. 728. McGehee DS, Aldersberg M, Liu KP, Hsuing S, Heath MJ, Tamir H. Mechanism of extracellular Ca2⫹ receptor-stimulated hormone release from sheep thyroid parafollicular cells. J Physiol 502: 31– 44, 1997. 729. McLaughlin JT, Ai W, Sinclair NF, Colucci R, Raychowdhury R, Koh TJ, Wang TC. PACAP and gastrin regulate the histidine decarboxylase promoter via distinct mechanisms. Am J Physiol Gastrointest Liver Physiol 286: G51–G59, 2004. 730. Mee AP, Hoyland JA, Braidman IP, Freemont AJ, Davies M, Mawer EB. Demonstration of vitamin D receptor transcripts in actively resorbing osteoclasts in bone sections. Bone 18: 295–299, 1996. 256 737. Miampamba M, Germano PM, Arli S, Wong HH, Scott D, Tache Y, Pisegna JR. Expression of pituitary adenylate cyclase-activating polypeptide and PACAP type 1 receptor in the rat gastric and colonic myenteric neurons. Regul Pept 105: 145–154, 2002. 738. Michelangeli F, Ruiz MC, Fernandez E, Ciarrocchi A. Role of Ca2⫹ in H⫹ transport by rabbit gastric glands studied with A23187 and BAPTA, an incorporated Ca2⫹ chelator. Biochim Biophys Acta 983: 82–90, 1989. 739. Millen AE, Dodd KW, Subar AF. Use of vitamin, mineral, nonvitamin, and nonmineral supplements in the United States: The 1987, 1992, and 2000 National Health Interview Survey results. J Am Diet Assoc 104: 942–950, 2004. 740. Miller A 3rd, Bronner F. Calcium uptake in isolated brush-border vesicles from rat small intestine. Biochem J 196: 391– 401, 1981. 741. Milne ML, Baran DT. End product inhibition of hepatic 25-hydroxyvitamin D production in the rat: specificity and kinetics. Arch Biochem Biophys 242: 488 – 492, 1985. 742. Mircheff AK, Wright EM. Analytical isolation of plasma membranes of intestinal epithelial cells: identification of Na, K-ATPase rich membranes and the distribution of enzyme activities. J Membr Biol 28: 309 –333, 1976. 743. Mitchell HH, Hamiltom TS, Steggerda FR, Bean HW. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J Biol Chem 158: 625– 637, 1945. 744. Mitsuma T, Rhue N, Kayama M, Mori Y, Adachi K, Yokoi Y, Ping J, Nogimori T, Hirooka Y. Distribution of calcium sensing receptor in rats: an immunohistochemical study. Endocr Regul 33: 55–59, 1999. 745. Miyake N, Hoshi K, Sano Y, Kikuchi K, Tadano K, Koshihara Y. 1,25-Dihydroxyvitamin D3 promotes vitamin K2 metabolism in human osteoblasts. Osteoporos Int 12: 680 – 687, 2001. 746. Mizobuchi M, Ogata H, Hatamura I, Saji F, Koiwa F, Kinugasa E, Koshikawa S, Akizawa T. Activation of calcium-sensing receptor accelerates apoptosis in hyperplastic parathyroid cells. Biochem Biophys Res Commun 362: 11–16, 2007. 747. Mogard MH, Maxwell V, Sytnik B, Walsh JH. Regulation of gastric acid secretion by neurotensin in man. Evidence against a hormonal role. J Clin Invest 80: 1064 –1067, 1987. 748. Monkawa T, Yoshida T, Wakino S, Shinki T, Anazawa H, Deluca HF, Suda T, Hayashi M, Saruta T. Molecular cloning of cDNA and genomic DNA for human 25-hydroxyvitamin D3 1 alpha-hydroxylase. Biochem Biophys Res Commun 239: 527–533, 1997. 749. Monstein HJ, Nylander AG, Salehi A, Chen D, Lundquist I, Hakanson R. Cholecystokinin-A and cholecystokinin-B/gastrin receptor mRNA expression in the gastrointestinal tract and pancreas of the rat and man. A polymerase chain reaction study. Scand J Gastroenterol 31: 383–390, 1996. 750. Morgan EL, Mace OJ, Affleck J, Kellett GL. Apical GLUT2 and Cav1.3: regulation of rat intestinal glucose and calcium absorption. J Physiol 580: 593– 604, 2007. 751. Morgan EL, Mace OJ, Helliwell PA, Affleck J, Kellett GL. A role for Ca(v)1.3 in rat intestinal calcium absorption. Biochem Biophys Res Commun 312: 487– 493, 2003. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH 752. Morii H, Lund J, Neville PF, DeLuca HF. Biological activity of a vitamin D metabolite. Arch Biochem Biophys 120: 508 –512, 1967. from ECL carcinoid tumor of Mastomys natalensis. Biochem Biophys Res Commun 187: 1151–1157, 1992. 753. Mosekilde L, Sogaard CH, Danielsen CC, Torring O. The anabolic effects of human parathyroid hormone (hPTH) on rat vertebral body mass are also reflected in the quality of bone, assessed by biomechanical testing: a comparison study between hPTH-(1–34) and hPTH-(1– 84). Endocrinology 129: 421– 428, 1991. 773. Nandi J, Bosche MC, Levine RA. Effects of a phorbol ester and isoquinoline sulfonamides on rabbit parietal cell function. J Pharmacol Exp Ther 279: 97–105, 1996. 774. Nandi J, Loo A, Kim SW, Levine RA. Expression and characterization of protein kinase C in isolated rabbit parietal cells. Int J Mol Med 3: 521–526, 1999. 754. Motoyama HI, Friedman PA. Calcium-sensing receptor regulation of PTH-dependent calcium absorption by mouse cortical ascending limbs. Am J Physiol Renal Physiol 283: F399 –F406, 2002. 775. Napoli N, Pedone C, Pozzilli P, Lauretani F, Bandinelli S, Ferrucci L, Incalzi RA. Effect of ghrelin on bone mass density: the InChianti study. Bone 49: 257–263, 2011. 755. Muallem S, Fimmel CJ, Pandol SJ, Sachs G. Regulation of free cytosolic Ca2⫹ in the peptic and parietal cells of the rabbit gastric gland. J Biol Chem 261: 2660 –2667, 1986. 776. Naro F, Perez M, Migliaccio S, Galson DL, Orcel P, Teti A, Goldring SR. Phospholipase D- and protein kinase C isoenzyme-dependent signal transduction pathways activated by the calcitonin receptor. Endocrinology 139: 3241–3248, 1998. 756. Muallem S, Karlish SJ. Studies on the mechanism of regulation of the red-cell Ca2⫹ pump by calmodulin and ATP. Biochim Biophys Acta 647: 73– 86, 1981. 777. Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinol 152: 845– 850, 2005. 757. Muhe L, Lulseged S, Mason KE, Simoes EA. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet 349: 1801–1804, 1997. 758. Mulderry PK, Ghatei MA, Spokes RA, Jones PM, Pierson AM, Hamid QA, Kanse S, Amara SG, Burrin JM, Legon S. Differential expression of alpha-CGRP and betaCGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience 25: 195–205, 1988. 759. Mun HC, Franks AH, Culverston EL, Krapcho K, Nemeth EF, Conigrave AD. The Venus Fly Trap domain of the extracellular Ca2⫹-sensing receptor is required for L-amino acid sensing. J Biol Chem 279: 51739 –51744, 2004. 760. Mungan Z, Hammer RA, Akarca US, Komaki G, Ertan A, Arimura A. Effect of PACAP on gastric acid secretion in rats. Peptides 16: 1051–1056, 1995. 761. Munson K, Garcia R, Sachs G. Inhibitor and ion binding sites on the gastric H⫹-K⫹ATPase. Biochemistry 44: 5267–5284, 2005. 762. Munson K, Law RJ, Sachs G. Analysis of the gastric H,K ATPase for ion pathways and inhibitor binding sites. Biochemistry 46: 5398 –5417, 2007. 763. Murayama A, Takeyama K, Kitanaka S, Kodera Y, Hosoya T, Kato S. The promoter of the human 25-hydroxyvitamin D3 1 alpha-hydroxylase gene confers positive and negative responsiveness to PTH, calcitonin, and 1 alpha,25(OH)2D3. Biochem Biophys Res Commun 249: 11–16, 1998. 764. Murayama A, Takeyama K, Kitanaka S, Kodera Y, Kawaguchi Y, Hosoya T, Kato S. Positive and negative regulations of the renal 25-hydroxyvitamin D3 1alpha-hydroxylase gene by parathyroid hormone, calcitonin, and 1alpha,25(OH)2D3 in intact animals. Endocrinology 140: 2224 –2231, 1999. 765. Murer H, Hernando N, Forster I, Biber J. Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol Rev 80: 1373–1409, 2000. 766. Murray CB, Morales MM, Flotte TR, McGrath-Morrow SA, Guggino WB, Zeitlin PL. CIC-2: a developmentally dependent chloride channel expressed in the fetal lung and downregulated after birth. Am J Respir Cell Mol Biol 12: 597– 604, 1995. 778. Naveh-Many T, Rahamimov R, Livni N, Silver J. Parathyroid cell proliferation in normal and chronic renal failure rats. The effects of calcium, phosphate, and vitamin D. J Clin Invest 96: 1786 –1793, 1995. 779. Nemere I. The 1,25D3-MARRS protein: contribution to steroid stimulated calcium uptake in chicks and rats. Steroids 70: 455– 457, 2005. 780. Nemere I, Norman AW. 1,25-Dihydroxyvitamin D3-mediated vesicular transport of calcium in intestine: time-course studies. Endocrinology 122: 2962–2969, 1988. 781. Nemere I, Norman AW. Parathyroid hormone stimulates calcium transport in perfused duodena from normal chicks: comparison with the rapid (transcaltachic) effect of 1,25-dihydroxyvitamin D3. Endocrinology 119: 1406 –1408, 1986. 782. Nemere I, Safford SE, Rohe B, DeSouza MM, Farach-Carson MC. Identification and characterization of 1,25D3-membrane-associated rapid response, steroid (1,25D3MARRS) binding protein. J Steroid Biochem Mol Biol 89 –90: 281–285, 2004. 783. Nemere I, Szego CM. Early actions of parathyroid hormone and 1,25-dihydroxycholecalciferol on isolated epithelial cells from rat intestine. I. Limited lysosomal enzyme release and calcium uptake. Endocrinology 108: 1450 –1462, 1981. 784. Nemere I, Szego CM. Early actions of parathyroid hormone and 1,25-dihydroxycholecalciferol on isolated epithelial cells from rat intestine. II. Analyses of additivity, contribution of calcium, and modulatory influence of indomethacin. Endocrinology 109: 2180 –2187, 1981. 785. Nemere I, Yoshimoto Y, Norman AW. Calcium transport in perfused duodena from normal chicks: enhancement within fourteen minutes of exposure to 1,25-dihydroxyvitamin D3. Endocrinology 115: 1476 –1483, 1984. 786. Nguyen-Yamamoto L, Rousseau L, Brossard JH, Lepage R, D’Amour P. Synthetic carboxyl-terminal fragments of parathyroid hormone (PTH) decrease ionized calcium concentration in rats by acting on a receptor different from the PTH/PTHrelated peptide receptor. Endocrinology 142: 1386 –1392, 2001. 767. Mutt V, Jorpes JE. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem 6: 156 –162, 1968. 787. Nguyen TM, Lieberherr M, Fritsch J, Guillozo H, Alvarez ML, Fitouri Z, Jehan F, Garabedian M. The rapid effects of 1,25-dihydroxyvitamin D3 require the vitamin D receptor and influence 24-hydroxylase activity: studies in human skin fibroblasts bearing vitamin D receptor mutations. J Biol Chem 279: 7591–7597, 2004. 768. Nakajima K, Nohtomi K, Sato M, Takano K, Sato K. PTH(7– 84) inhibits PTH(1–34)induced 1,25-(OH)2D3 production in murine renal tubules. Biochem Biophys Res Commun 381: 283–287, 2009. 788. Niall HD, Keutmann H, Sauer R, Hogan M, Dawson B, Aurbach G, Potts J Jr. The amino acid sequence of bovine parathyroid hormone I. Hoppe-Seylers Z Physiol Chem 351: 1586 –1588, 1970. 769. Nakajima T, Konda Y, Izumi Y, Kanai M, Hayashi N, Chiba T, Takeuchi T. Gastrin stimulates the growth of gastric pit cell precursors by inducing its own receptors. Am J Physiol Gastrointest Liver Physiol 282: G359 –G366, 2002. 789. Nicar MJ, Pak CY. Calcium bioavailability from calcium carbonate and calcium citrate. J Clin Endocrinol Metab 61: 391–393, 1985. 770. Nakamura E, Hasumura M, Gabriel AS, Uneyama H, Torii K. Functional role of calcium-sensing receptor on somatostatin release from rat gastric mucosa (Abstract). Gastroenterology 138: S404, 2010. 771. Nakamura K, Zhou CJ, Parente J, Chew CS. Parietal cell MAP kinases: multiple activation pathways. Am J Physiol Gastrointest Liver Physiol 271: G640 –G649, 1996. 772. Nakata H, Matsui T, Ito M, Taniguchi T, Naribayashi Y, Arima N, Nakamura A, Kinoshita Y, Chihara K, Hosoda S. Cloning and characterization of gastrin receptor 790. Nicholson GC, Moseley JM, Sexton PM, Mendelsohn FA, Martin TJ. Abundant calcitonin receptors in isolated rat osteoclasts. Biochemical and autoradiographic characterization. J Clin Invest 78: 355–360, 1986. 791. Niemeyer BA, Bergs C, Wissenbach U, Flockerzi V, Trost C. Competitive regulation of CaT-like-mediated Ca2⫹ entry by protein kinase C and calmodulin. Proc Natl Acad Sci USA 98: 3600 –3605, 2001. 792. Nijenhuis T, Hoenderop JG, van der Kemp AW, Bindels RJ. Localization and regulation of the epithelial Ca2⫹ channel TRPV6 in the kidney. J Am Soc Nephrol 14: 2731–2740, 2003. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 257 SASCHA KOPIC AND JOHN P. GEIBEL 793. Nilas L, Christiansen C. Vitamin D deficiency after highly selective vagotomy. Acta Med Scand 221: 303–306, 1987. 794. Nilas L, Christiansen C, Christiansen J. Regulation of vitamin D and calcium metabolism after gastrectomy. Gut 26: 252–257, 1985. 795. Nilsson BE, Westlin NE. The fracture incidence after gastrectomy. Acta Chir Scand 137: 533–534, 1971. 796. Nishimura M, Yaguti H, Yoshitsugu H, Naito S, Satoh T. Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity realtime reverse transcription PCR. Yakugaku Zasshi 123: 369 –375, 2003. 797. Nordin BE. Measurement and meaning of calcium absorption. Gastroenterology 54: 294 –301, 1968. 798. Norlen P, Bernsand M, Konagaya T, Hakanson R. ECL-cell histamine mobilization in conscious rats: effects of locally applied regulatory peptides, candidate neurotransmitters and inflammatory mediators. Br J Pharmacol 134: 1767–1777, 2001. 799. Norman AW, Lund J, Deluca HF. Biologically active forms of vitamin D3 in kidney and intestine. Arch Biochem Biophys 108: 12–21, 1964. 800. Norman AW, Myrtle JF, Midgett RJ, Nowicki HG, Williams V, Popjak G. 1,25Dihydroxycholecalciferol: identification of the proposed active form of vitamin D3 in the intestine. Science 173: 51–54, 1971. 801. Norman AW, Okamura WH, Hammond MW, Bishop JE, Dormanen MC, Bouillon R, van Baelen H, Ridall AL, Daane E, Khoury R, Farach-Carson MC. Comparison of 6-s-cis- and 6-s-trans-locked analogs of 1alpha,25-dihydroxyvitamin D3 indicates that the 6-s-cis conformation is preferred for rapid nongenomic biological responses and that neither 6-s-cis- nor 6-s-trans-locked analogs are preferred for genomic biological responses. Mol Endocrinol 11: 1518 –1531, 1997. 802. Norman AW, Olivera CJ, Barreto Silva FR, Bishop JE. A specific binding protein/ receptor for 1alpha,25-dihydroxyvitamin D(3) is present in an intestinal caveolae membrane fraction. Biochem Biophys Res Commun 298: 414 – 419, 2002. 813. Okamoto CT, Karam SM, Jeng YY, Forte JG, Goldenring JR. Identification of clathrin and clathrin adaptors on tubulovesicles of gastric acid secretory (oxyntic) cells. Am J Physiol Cell Physiol 274: C1017–C1029, 1998. 814. Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O’Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE. Targeted ablation of plasma membrane Ca2⫹-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem 279: 33742–33750, 2004. 815. Olesen C, Picard M, Winther AML, Gyrup C, Morth JP, Oxvig C, Moller JV, Nissen P. The structural basis of calcium transport by the calcium pump. Nature 450: 1036 –1042, 2007. 816. Oscarson J, Hakanson R, Liedberg G, Lundqvist G, Sundler F, Thorell J. Variated serum gastrin concentration: trophic effects on the gastrointestinal tract of the rat. Acta Physiol Scand Suppl 475: 2–27, 1979. 817. Owen TA, Aronow MS, Barone LM, Bettencourt B, Stein GS, Lian JB. Pleiotropic effects of vitamin D on osteoblast gene expression are related to the proliferative and differentiated state of the bone cell phenotype: dependency upon basal levels of gene expression, duration of exposure, and bone matrix competency in normal rat osteoblast cultures. Endocrinology 128: 1496 –1504, 1991. 818. Owyang C, Miller LJ, DiMagno EP, Brennan LA Jr, Go VL. Gastrointestinal hormone profile in renal insufficiency. Mayo Clin Proc 54: 769 –773, 1979. 819. Paillard F, Ardaillou R, Malendin H, Fillastre JP, Prier S. Renal effects of salmon calcitonin in man. J Lab Clin Med 80: 200 –216, 1972. 820. Pan Q, Ma J, Zhou Q, Li J, Tang Y, Liu Y, Yang Y, Xiao J, Peng L, Li P, Liang D, Zhang H, Chen YH. KCNQ1 loss-of-function mutation impairs gastric acid secretion in mice. Mol Biol Rep 37: 1329 –1333, 2010. 821. Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem 279: 16754 –16766, 2004. 803. Noto T, Nagasaki M, Endo T. Role of vagus nerves and gastrin in the gastric phase of acid secretion in male anesthetized rats. Am J Physiol Gastrointest Liver Physiol 272: G335–G339, 1997. 822. Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci USA 98: 7498 –7503, 2001. 804. Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res 13: 371–382, 1998. 823. Pannabecker TL, Chandler JS, Wasserman RH. Vitamin-D-dependent transcriptional regulation of the intestinal plasma membrane calcium pump. Biochem Biophys Res Commun 213: 499 –505, 1995. 805. Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96: 507–515, 1999. 824. Pansu D, Bellaton C, Bronner F. Effect of Ca intake on saturable and nonsaturable components of duodenal Ca transport. Am J Physiol Gastrointest Liver Physiol 240: G32–G37, 1981. 806. Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc Natl Acad Sci USA 98: 13895–13900, 2001. 825. Pansu D, Bellaton C, Roche C, Bronner F. Duodenal and ileal calcium absorption in the rat and effects of vitamin D. Am J Physiol Gastrointest Liver Physiol 244: G695– G700, 1983. 807. O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 118: 778 –781, 2005. 808. Offermanns S, Iida-Klein A, Segre GV, Simon MI. G alpha q family members couple parathyroid hormone (PTH)/PTH-related peptide and calcitonin receptors to phospholipase C in COS-7 cells. Mol Endocrinol 10: 566 –574, 1996. 809. Oginni LM, Worsfold M, Oyelami OA, Sharp CA, Powell DE, Davie MW. Etiology of rickets in Nigerian children. J Pediatr 128: 692– 694, 1996. 810. Oh DS, Lieu SN, Yamaguchi DJ, Tachiki K, Lambrecht N, Ohning GV, Sachs G, Germano PM, Pisegna JR. PACAP regulation of secretion and proliferation of pure populations of gastric ECL cells. J Mol Neurosci 26: 85–97, 2005. 811. Oh KW, Lee WY, Rhee EJ, Baek KH, Yoon KH, Kang MI, Yun EJ, Park CY, Ihm SH, Choi MG, Yoo HJ, Park SW. The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle-aged men. Clin Endocrinol 63: 131–138, 2005. 812. Ohyama Y, Ozono K, Uchida M, Yoshimura M, Shinki T, Suda T, Yamamoto O. Functional assessment of two vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase gene. J Biol Chem 271: 30381–30385, 1996. 258 826. Parhon C, Ureche CS. Untersuchungen uber den Einfluss den die Calcium und Sodiumsalze auf den Verlauf der experimentellen Tetanie ausuben. Neurol Centr 26: 1099, 1907. 827. Park J, Chiba T, Yamada T. Mechanisms for direct inhibition of canine gastric parietal cells by somatostatin. J Biol Chem 262: 14190 –14196, 1987. 828. Parkinson DB, Thakker RV. A donor splice site mutation in the parathyroid hormone gene is associated with autosomal recessive hypoparathyroidism. Nat Genet 1: 149 – 152, 1992. 829. Parthemore JG, Deftos LJ. Calcitonin secretion in normal human subjects. J Clin Endocrinol Metab 47: 184 –188, 1978. 830. Patterson EK, Hodsman AB, Hendy GN, Canaff L, Bringhurst FR, Fraher LJ. Functional analysis of a type 1 parathyroid hormone receptor intracellular tail mutant [KRK(484 – 6)AAA]: effects on second messenger generation and cellular targeting. Bone 46: 1180 –1187, 2010. 831. Patton MB. Further experiments on the utilization of calcium from salts by college women. J Nutr 55: 519 –526, 1955. 832. Patton MB, Sutton TS. The utilization of calcium from lactate, gluconate, sulfate and carbonate salts by young college women. J Nutr 48: 443– 452, 1952. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH 833. Pawlow JP, Schumowa-Simanowskaja EO. Beitrage zur Physiologie der Absonderungen. Die Innervation der Magendrusen beim Hunde. Arch Anat Physiol 54 – 69, 1895. 854. Pi M, Zhang L, Lei SF, Huang MZ, Zhu W, Zhang J, Shen H, Deng HW, Quarles LD. Impaired osteoblast function in GPRC6A null mice. J Bone Miner Res 25: 1092–1102, 2010. 834. Pearce SH, Bai M, Quinn SJ, Kifor O, Brown EM, Thakker RV. Functional characterization of calcium-sensing receptor mutations expressed in human embryonic kidney cells. J Clin Invest 98: 1860 –1866, 1996. 855. Picard N, Capuano P, Stange G, Mihailova M, Kaissling B, Murer H, Biber J, Wagner CA. Acute parathyroid hormone differentially regulates renal brush border membrane phosphate cotransporters. Pflügers Arch 460: 677– 687, 2010. 835. Pedersen JI, Holmberg I, Bjorkhem I. Reconstitution of vitamin D3 25-hydroxylase activity with a cytochrome P-450 preparation from rat liver mitochondria. FEBS Lett 98: 394 –398, 1979. 856. Pickard BW, Hodsman AB, Fraher LJ, Watson PH. Type 1 parathyroid hormone receptor (PTH1R) nuclear trafficking: association of PTH1R with importin alpha1 and beta. Endocrinology 147: 3326 –3332, 2006. 836. Peng JB, Brown EM, Hediger MA. Structural conservation of the genes encoding CaT1, CaT2, and related cation channels. Genomics 76: 99 –109, 2001. 837. Peng JB, Chen XZ, Berger UV, Vassilev PM, Brown EM, Hediger MA. A rat kidneyspecific calcium transporter in the distal nephron. J Biol Chem 275: 28186 –28194, 2000. 838. Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem 274: 22739 –22746, 1999. 839. Peng JB, Zhuang L, Berger UV, Adam RM, Williams BJ, Brown EM, Hediger MA, Freeman MR. CaT1 expression correlates with tumor grade in prostate cancer. Biochem Biophys Res Commun 282: 729 –734, 2001. 840. Perez JF, Ruiz MC, Michelangeli F. Simultaneous measurement and imaging of intracellular Ca2⫹ and H⫹ transport in isolated rabbit gastric glands. J Physiol 537: 735– 745, 2001. 841. Persson P, Gagnemo-Persson R, Chen D, Axelson J, Nylander AG, Johnell O, Hakanson R. Gastrectomy causes bone loss in the rat: is lack of gastric acid responsible? Scand J Gastroenterol 28: 301–306, 1993. 842. Persson P, Gagnemo-Persson R, Orberg J, Chen D, Hakanson R. Effects of gastrin on calcium homeostasis in chickens. Endocrinology 129: 1162–1166, 1991. 843. Persson P, Grunditz T, Axelson J, Sundler F, Hakanson R. Cholecystokinins but not gastrin-17 release calcitonin from thyroid C-cells in the rat. Regul Pept 21: 45–56, 1988. 844. Persson P, Hakanson R, Axelson J, Sundler F. Gastrin releases a blood calciumlowering peptide from the acid-producing part of the rat stomach. Proc Natl Acad Sci USA 86: 2834 –2838, 1989. 845. Petrovic S, Wang Z, Ma L, Seidler U, Forte JG, Shull GE, Soleimani M. Colocalization of the apical Cl⫺/HCO3⫺ exchanger PAT1 and gastric H⫹-K⫹-ATPase in stomach parietal cells. Am J Physiol Gastrointest Liver Physiol 283: G1207–G1216, 2002. 846. Pfeiffer A, Rochlitz H, Noelke B, Tacke R, Moser U, Mutschler E, Lambrecht G. Muscarinic receptors mediating acid secretion in isolated rat gastric parietal cells are of M3 type. Gastroenterology 98: 218 –222, 1990. 847. Pfister MF, Lederer E, Forgo J, Ziegler U, Lotscher M, Quabius ES, Biber J, Murer H. Parathyroid hormone-dependent degradation of type II Na⫹/Pi cotransporters. J Biol Chem 272: 20125–20130, 1997. 848. Phelps CB, Huang RJ, Lishko PV, Wang RR, Gaudet R. Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry 47: 2476 –2484, 2008. 849. Pi M, Chen L, Huang M, Luo Q, Quarles LD. Parathyroid-specific interaction of the calcium-sensing receptor and G alpha q. Kidney Int 74: 1548 –1556, 2008. 850. Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, Christenson L, Li B, Zhang J, Jackson PD, Faber P, Brunden KR, Harrington JJ, Quarles LD. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One 3: e3858, 2008. 851. Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, Quarles LD. Identification of a novel extracellular cationsensing G-protein-coupled receptor. J Biol Chem 280: 40201– 40209, 2005. 852. Pi M, Garner SC, Flannery P, Spurney RF, Quarles LD. Sensing of extracellular cations in CasR-deficient osteoblasts. Evidence for a novel cation-sensing mechanism. J Biol Chem 275: 3256 –3263, 2000. 853. Pi M, Oakley RH, Gesty-Palmer D, Cruickshank RD, Spurney RF, Luttrell LM, Quarles LD. Beta-arrestin- and G protein receptor kinase-mediated calcium-sensing receptor desensitization. Mol Endocrinol 19: 1078 –1087, 2005. 857. Pickard BW, Hodsman AB, Fraher LJ, Watson PH. Type 1 parathyroid hormone receptor (PTH1R) nuclear trafficking: regulation of PTH1R nuclear-cytoplasmic shuttling by importin-alpha/beta and chromosomal region maintenance 1/exportin 1. Endocrinology 148: 2282–2289, 2007. 858. Pidasheva S, Grant M, Canaff L, Ercan O, Kumar U, Hendy GN. Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CASR mutants retained intracellularly. Hum Mol Genet 15: 2200 – 2209, 2006. 859. Pines M, Fukayama S, Costas K, Meurer E, Goldsmith PK, Xu X, Muallem S, Behar V, Chorev M, Rosenblatt M, Tashjian AH Jr, Suva LJ. Inositol 1-,4-,5-trisphosphatedependent Ca2⫹ signaling by the recombinant human PTH/PTHrP receptor stably expressed in a human kidney cell line. Bone 18: 381–389, 1996. 860. Pioszak AA, Harikumar KG, Parker NR, Miller LJ, Xu HE. Dimeric arrangement of the parathyroid hormone receptor and a structural mechanism for ligand-induced dissociation. J Biol Chem 285: 12435–12444, 2010. 861. Pioszak AA, Xu HE. Molecular recognition of parathyroid hormone by its G proteincoupled receptor. Proc Natl Acad Sci USA 105: 5034 –5039, 2008. 862. Piqueras L, Tache Y, Martinez V. Peripheral PACAP inhibits gastric acid secretion through somatostatin release in mice. Br J Pharmacol 142: 67–78, 2004. 863. Polak JM, Sullivan SN, Bloom SR, Buchan AM, Facer P, Brown MR, Pearse AG. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nature 270: 183–184, 1977. 864. Ponchon G, DeLuca HF. The role of the liver in the metabolism of vitamin D. J Clin Invest 48: 1273–1279, 1969. 865. Ponchon G, Kennan AL, DeLuca HF. “Activation” of vitamin D by the liver. J Clin Invest 48: 2032–2037, 1969. 866. Popielski L. Beta-imidazolylathylamin und die Organextrakte. Erster Teil: beta-imidazolylathylamin als machtiger Erreger der Magendrusen. Pflügers Arch 178: 214 – 236, 1920. 867. Potts JT Jr, Tregear GW, Keutmann HT, Niall HD, Sauer R, Deftos LJ, Dawson BF, Hogan ML, Aurbach GD. Synthesis of a biologically active N-terminal tetratriacontapeptide of parathyroid hormone. Proc Natl Acad Sci USA 68: 63– 67, 1971. 868. Pouwels S, Lalmohamed A, Souverein P, Cooper C, Veldt BJ, Leufkens HG, De Boer A, Van Staa T, De Vries F. Use of proton pump inhibitors and risk of hip/femur fracture: A population-based case-control study. Osteoporosis Int 22: 903–910, 2011. 869. Prader A, Illig R, Heierli E. Eine besondere Form der primären Vitamin D resistenten Tachitis mit Hypocalcemie und autosomal dominantem Erbgang: die hereditäre Pseudomangelrachitis. Helv Paediatr Acta 16: 452– 468, 1961. 870. Prince CW, Butler WT. 1,25-Dihydroxyvitamin D3 regulates the biosynthesis of osteopontin, a bone-derived cell attachment protein, in clonal osteoblast-like osteosarcoma cells. Coll Relat Res 7: 305–313, 1987. 871. Prinz C, Kajimura M, Scott DR, Mercier F, Helander HF, Sachs G. Histamine secretion from rat enterochromaffinlike cells. Gastroenterology 105: 449 – 461, 1993. 872. Prinz C, Neumayer N, Mahr S, Classen M, Schepp W. Functional impairment of rat enterochromaffin-like cells by interleukin 1 beta. Gastroenterology 112: 364 –375, 1997. 873. Prinz C, Sachs G, Walsh JH, Coy DH, Wu SV. The somatostatin receptor subtype on rat enterochromaffinlike cells. Gastroenterology 107: 1067–1074, 1994. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 259 SASCHA KOPIC AND JOHN P. GEIBEL 874. Prosser DE, Guo Y, Jia Z, Jones G. Structural motif-based homology modeling of CYP27A1 and site-directed mutational analyses affecting vitamin D hydroxylation. Biophys J 90: 3389 –3409, 2006. 875. Prunier JH, Bearn AG, Cleve H. Site of formation of the group-specific component and certain other serum proteins. Proc Soc Exp Biol Med 115: 1005–1007, 1964. 876. Pyrah LN, Smith IB. Osteomalacia following gastrectomy. Lancet 270: 935–937, 1956. 897. Reeve J, Meunier PJ, Parsons JA, Bernat M, Bijvoet OL, Courpron P, Edouard C, Klenerman L, Neer RM, Renier JC, Slovik D, Vismans FJ, Potts JT Jr. Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. Br Med J 280: 1340 –1344, 1980. 898. Reiss AB, Martin KO, Rojer DE, Iyer S, Grossi EA, Galloway AC, Javitt NB. Sterol 27-hydroxylase: expression in human arterial endothelium. J Lipid Res 38: 1254 – 1260, 1997. 877. Quamme GA. Effect of calcitonin on calcium and magnesium transport in rat nephron. Am J Physiol Endocrinol Metab 238: E573–E578, 1980. 899. Remy C, Kirchhoff P, Hafner P, Busque SM, Mueller MK, Geibel JP, Wagner CA. Stimulatory pathways of the Calcium-sensing receptor on acid secretion in freshly isolated human gastric glands. Cell Physiol Biochem 19: 33– 42, 2007. 878. Quinn JM, Morfis M, Lam MH, Elliott J, Kartsogiannis V, Williams ED, Gillespie MT, Martin TJ, Sexton PM. Calcitonin receptor antibodies in the identification of osteoclasts. Bone 25: 1– 8, 1999. 900. Rengifo-Cam W, Umar S, Sarkar S, Singh P. Antiapoptotic effects of progastrin on pancreatic cancer cells are mediated by sustained activation of nuclear factor-B. Cancer Res 67: 7266 –7274, 2007. 879. Quinn SJ, Bai M, Brown EM. pH Sensing by the calcium-sensing receptor. J Biol Chem 279: 37241–37249, 2004. 901. Renkema KY, Velic A, Dijkman HB, Verkaart S, van der Kemp AW, Nowik M, Timmermans K, Doucet A, Wagner CA, Bindels RJ, Hoenderop JG. The calciumsensing receptor promotes urinary acidification to prevent nephrolithiasis. J Am Soc Nephrol 20: 1705–1713, 2009. 880. Quinn SJ, Ye CP, Diaz R, Kifor O, Bai M, Vassilev P, Brown E. The Ca2⫹-sensing receptor: a target for polyamines. Am J Physiol Cell Physiol 273: C1315–C1323, 1997. 881. Raczynski J. Recherches expérimentales sur le manque d’action du soleil comme cause du rachitisme. In: C R Assoc Internat Pediatrie. Paris: 1912, p. 308 –309. 882. Rakopoulos M, Ikegame M, Findlay DM, Martin TJ, Moseley JM. Short treatment of osteoclasts in bone marrow culture with calcitonin causes prolonged suppression of calcitonin receptor mRNA. Bone 17: 447– 453, 1995. 883. Rasmussen H, Anast C, Arnaud C. Thyrocalcitonin, EGTA, and urinary electrolyte excretion. J Clin Invest 46: 746 –752, 1967. 884. Rautureau M, Rambaud JC. Aqueous solubilisation of vitamin D3 in normal man. Gut 22: 393–397, 1981. 885. Ravazzola M, Benoit R, Ling N, Orci L. Prosomatostatin-derived antrin is present in gastric D cells and in portal blood. J Clin Invest 83: 362–366, 1989. 886. Ray JM, Squires PE, Curtis SB, Meloche MR, Buchan AM. Expression of the calciumsensing receptor on human antral gastrin cells in culture. J Clin Invest 99: 2328 –2333, 1997. 887. Ray K, Clapp P, Goldsmith PK, Spiegel AM. Identification of the sites of N-linked glycosylation on the human calcium receptor and assessment of their role in cell surface expression and signal transduction. J Biol Chem 273: 34558 –34567, 1998. 888. Ray K, Hauschild BC, Steinbach PJ, Goldsmith PK, Hauache O, Spiegel AM. Identification of the cysteine residues in the amino-terminal extracellular domain of the human Ca2⫹ receptor critical for dimerization. Implications for function of monomeric Ca2⫹ receptor. J Biol Chem 274: 27642–27650, 1999. 889. Raychowdhury R, Fleming JV, McLaughlin JT, Bulitta CJ, Wang TC. Identification and characterization of a third gastrin response element (GAS-RE3) in the human histidine decarboxylase gene promoter. Biochem Biophys Res Commun 297: 1089 –1095, 2002. 890. Raychowdhury R, Zhang Z, Hocker M, Wang TC. Activation of human histidine decarboxylase gene promoter activity by gastrin is mediated by two distinct nuclear factors. J Biol Chem 274: 20961–20969, 1999. 902. Repa JJ, Lund EG, Horton JD, Leitersdorf E, Russell DW, Dietschy JM, Turley SD. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia. Reversal by cholic acid feeding. J Biol Chem 275: 39685–39692, 2000. 903. Resnick RH, Adelardi CF, Gray SJ. Stimulation of gastric secretion in man by a serotonin antagonist. Gastroenterology 42: 22–25, 1962. 904. Reubi JC, Waser B, Horisberger U, Halter F, Soroka CJ, Kumar RR, Goldenring JR, Modlin IM. Identification of somatostatin and gastrin receptors on enterochromaffinlike cells from Mastomys gastric tumors. Endocrinology 131: 166 –172, 1992. 905. Reubi JC, Waser B, Laderach U, Stettler C, Friess H, Halter F, Schmassmann A. Localization of cholecystokinin A and cholecystokinin B-gastrin receptors in the human stomach. Gastroenterology 112: 1197–1205, 1997. 906. Riccardi D, Brown EM. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol 298: F485–F499, 2010. 907. Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2⫹/polyvalent cation-sensing protein in rat kidney. Am J Physiol Renal Physiol 274: F611–F622, 1998. 908. Riccardi D, Lee WS, Lee K, Segre GV, Brown EM, Hebert SC. Localization of the extracellular Ca2⫹-sensing receptor and PTH/PTHrP receptor in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F951–F956, 1996. 909. Riccardi D, Traebert M, Ward DT, Kaissling B, Biber J, Hebert SC, Murer H. Dietary phosphate and parathyroid hormone alter the expression of the calcium-sensing receptor (CaR) and the Na⫹-dependent Pi transporter (NaPi-2) in the rat proximal tubule. Pflügers Arch 441: 379 –387, 2000. 910. Richardson CT, Walsh JH, Cooper KA, Feldman M, Fordtran JS. Studies on the role of cephalic-vagal stimulation in the acid secretory response to eating in normal human subjects. J Clin Invest 60: 435– 441, 1977. 891. Recker RR. Calcium absorption and achlorhydria. N Engl J Med 313: 70 –73, 1985. 911. Ridefelt P, Hellman P, Stridsberg M, Akerstrom G, Rastad J. Different secretory actions of pancreastatin in bovine and human parathyroid cells. Biosci Rep 14: 221– 229, 1994. 892. Reddy GS, Norman AW, Willis DM, Goltzman D, Guyda H, Solomon S, Philips DR, Bishop JE, Mayer E. Regulation of vitamin D metabolism in normal human pregnancy. J Clin Endocrinol Metab 56: 363–370, 1983. 912. Robert A, Olafsson AS, Lancaster C, Zhang WR. Interleukin-1 is cytoprotective, antisecretory, stimulates PGE2 synthesis by the stomach, retards gastric emptying. Life Sci 48: 123–134, 1991. 893. Reddy GS, Tserng KY. Calcitroic acid, end product of renal metabolism of 1,25dihydroxyvitamin D3 through C-24 oxidation pathway. Biochemistry 28: 1763–1769, 1989. 913. Robinson CJ, Spanos E, James MF, Pike JW, Haussler MR, Makeen AM, Hillyard CJ, MacIntyre I. Role of prolactin in vitamin D metabolism and calcium absorption during lactation in the rat. J Endocrinol 94: 443– 453, 1982. 894. Reed JS, Meredith SC, Nemchausky BA, Rosenberg IH, Boyer JL. Bone disease in primary biliary cirrhosis: reversal of osteomalacia with oral 25-hydroxyvitamin D. Gastroenterology 78: 512–517, 1980. 914. Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell 5: 173–179, 2000. 895. Reenstra WW, Forte JG. Characterization of K⫹ and Cl⫺ conductances in apical membrane vesicles from stimulated rabbit oxyntic cells. Am J Physiol Gastrointest Liver Physiol 259: G850 –G858, 1990. 896. Reerink EH, Van Wijk W. The vitamin D problem. Biochem J 25: 1001–1009, 1931. 260 915. Rodriguez L, Tu C, Cheng Z, Chen TH, Bikle D, Shoback D, Chang W. Expression and functional assessment of an alternatively spliced extracellular Ca2⫹-sensing receptor in growth plate chondrocytes. Endocrinology 146: 5294 –5303, 2005. 916. Rodriguez ME, Almaden Y, Canadillas S, Canalejo A, Siendones E, Lopez I, AguileraTejero E, Martin D, Rodriguez M. The calcimimetic R-568 increases vitamin D Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH receptor expression in rat parathyroid glands. Am J Physiol Renal Physiol 292: F1390 – F1395, 2007. 936. Sachs G, Shin JM, Briving C, Wallmark B, Hersey S. The pharmacology of the gastric acid pump: the H⫹,K⫹ ATPase. Annu Rev Pharmacol Toxicol 35: 277–305, 1995. 917. Roepke TK, Anantharam A, Kirchhoff P, Busque SM, Young JB, Geibel JP, Lerner DJ, Abbott GW. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem 281: 23740 –23747, 2006. 937. Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, Liebhaber SA, Cooke NE. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest 103: 239 –251, 1999. 918. Rosen H, Reshef A, Maeda N, Lippoldt A, Shpizen S, Triger L, Eggertsen G, Bjorkhem I, Leitersdorf E. Markedly reduced bile acid synthesis but maintained levels of cholesterol and vitamin D metabolites in mice with disrupted sterol 27-hydroxylase gene. J Biol Chem 273: 14805–14812, 1998. 938. Sakurada T, Ro S, Onouchi T, Ohno S, Aoyama T, Chinen K, Takabayashi H, Kato S, Takayama K, Yakabi K. Comparison of the actions of acylated and desacylated ghrelin on acid secretion in the rat stomach. J Gastroenterol 45: 1111–1120, 2010. 939. Salvesen HA. The function of the parathyroids. J Biol Chem 56: 443– 456, 1923. 919. Rosenblatt M, Segre GV, Tyler GA, Shepard GL, Nussbaum SR, Potts JT Jr. Identification of a receptor-binding region in parathyroid hormone. Endocrinology 107: 545–550, 1980. 920. Ross TK, Darwish HM, Moss VE, DeLuca HF. Vitamin D-influenced gene expression via a ligand-independent, receptor-DNA complex intermediate. Proc Natl Acad Sci USA 90: 9257–9260, 1993. 921. Rost CR, Bikle DD, Kaplan RA. In vitro stimulation of 25-hydroxycholecalciferol 1 alpha-hydroxylation by parathyroid hormone in chick kidney slices: evidence for a role for adenosine 3=,5=-monophosphate. Endocrinology 108: 1002–1006, 1981. 922. Roth J, Thorens B, Hunziker W, Norman AW, Orci L. Vitamin D-dependent calcium binding protein: immunocytochemical localization in chick kidney. Science 214: 197– 200, 1981. 923. Roux C, Briot K, Gossec L, Kolta S, Blenk T, Felsenberg D, Reid DM, Eastell R, Glüer CC. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcified Tissue Int 84: 13–19, 2009. 924. Rovner AJ, Stallings VA, Schall JI, Leonard MB, Zemel BS. Vitamin D insufficiency in children, adolescents, and young adults with cystic fibrosis despite routine oral supplementation. Am J Clin Nutr 86: 1694 –1699, 2007. 925. Rowe DW, Kream BE. Regulation of collagen synthesis in fetal rat calvaria by 1,25dihydroxyvitamin D3. J Biol Chem 257: 8009 – 8015, 1982. 926. Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr 136: 2754 –2759, 2006. 927. Rumenapf G, Schwille PO, Erben RG, Schreiber M, Berge B, Fries W, Schmiedl A, Koroma S, Hohenberger W. Gastric fundectomy in the rat: effects on mineral and bone metabolism, with emphasis on the gastrin-calcitonin-parathyroid hormonevitamin D axis. Calcif Tissue Int 63: 433– 441, 1998. 928. Rumenapf G, Schwille PO, Wagner W, Tiecks FP, Fries W, Galewski D. Highly selective vagotomy in the rat: effects on bone and mineral metabolism. Scand J Gastroenterol 29: 232–237, 1994. 929. Rune SJ. Acid-base parameters of duodenal contents in man. Gastroenterology 62: 533–539, 1972. 930. Russell J, Bar A, Sherwood LM, Hurwitz S. Interaction between calcium and 1,25dihydroxyvitamin D3 in the regulation of preproparathyroid hormone and vitamin D receptor messenger ribonucleic acid in avian parathyroids. Endocrinology 132: 2639 – 2644, 1993. 931. Russell J, Lettieri D, Sherwood LM. Suppression by 1,25(OH)2D3 of transcription of the pre-proparathyroid hormone gene. Endocrinology 119: 2864 –2866, 1986. 932. Rutten MJ, Bacon KD, Marlink KL, Stoney M, Meichsner CL, Lee FP, Hobson SA, Rodland KD, Sheppard BC, Trunkey DD, Deveney KE, Deveney CW. Identification of a functional Ca2⫹-sensing receptor in normal human gastric mucous epithelial cells. Am J Physiol Gastrointest Liver Physiol 277: G662–G670, 1999. 933. Saarem K, Pedersen JI. Sex differences in the hydroxylation of cholecalciferol and of 5 beta-cholestane-3 alpha,7 alpha,12 alpha-triol in rat liver. Biochem J 247: 73–78, 1987. 940. Sandle GI, Fraser G, Long S, Warhurst G. A cAMP-activated chloride channel in the plasma membrane of cultured human gastric cells (HGT-1). Pflügers Arch 417: 259 – 263, 1990. 941. Sandor A, Kidd M, Lawton GP, Miu K, Tang LH, Modlin IM. Neurohormonal modulation of rat enterochromaffin-like cell histamine secretion. Gastroenterology 110: 1084 –1092, 1996. 942. Sands JM, Flores FX, Kato A, Baum MA, Brown EM, Ward DT, Hebert SC, Harris HW. Vasopressin-elicited water and urea permeabilities are altered in IMCD in hypercalcemic rats. Am J Physiol Renal Physiol 274: F978 –F985, 1998. 943. Sands JM, Naruse M, Baum M, Jo I, Hebert SC, Brown EM, Harris HW. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Invest 99: 1399 –1405, 1997. 944. Sandvik AK, Cui G, Bakke I, Munkvold B, Waldum HL. PACAP stimulates gastric acid secretion in the rat by inducing histamine release. Am J Physiol Gastrointest Liver Physiol 281: G997–G1003, 2001. 945. Sandvik AK, Dimaline R, Forster ER, Evans D, Dockray GJ. Differential control of somatostatin messenger RNA in rat gastric corpus and antrum. Role of acid, food, and capsaicin-sensitive afferent neurons. J Clin Invest 91: 244 –250, 1993. 946. Sandvik AK, Kleveland PM, Waldum HL. Muscarinic M2 stimulation releases histamine in the totally isolated, vascularly perfused rat stomach. Scand J Gastroenterol 23: 1049 –1056, 1988. 947. Saperas E, Cominelli F, Tache Y. Potent inhibition of gastric acid secretion by intravenous interleukin-1 beta and -1 alpha in rats. Peptides 13: 221–226, 1992. 948. Saperas E, Yang H, Tache Y. Interleukin-1 beta acts at hypothalamic sites to inhibit gastric acid secretion in rats. Am J Physiol Gastrointest Liver Physiol 263: G414 –G418, 1992. 949. Saperas ES, Yang H, Rivier C, Tache Y. Central action of recombinant interleukin-1 to inhibit acid secretion in rats. Gastroenterology 99: 1599 –1606, 1990. 950. Sawada N, Sakaki T, Ohta M, Inouye K. Metabolism of vitamin D3 by human CYP27A1. Biochem Biophys Res Commun 273: 977–984, 2000. 951. Schachter D. Toward a molecular description of active transport. In: Biological Membranes, edited by Dowben RM. Boston: Little, Brown, 1969, p. 157–176. 952. Schachter D, Dowdle EB, Schenker H. Active transport of calcium by the small intestine of the rat. Am J Physiol 198: 263–268, 1960. 953. Schachter D, Finkelstein JD, Kowarski S. Metabolism of vitamin D. I. Preparation of radioactive vitamin D and its intestinal absorption in the rat. J Clin Invest 43: 787–796, 1964. 954. Schachter D, Rosen SM. Active transport of Ca45 by the small intestine and its dependence on vitamin D. Am J Physiol 196: 357–362, 1959. 955. Schaffalitzky de Muckadell OB, Fahrenkrug J. Secretion pattern of secretin in man: regulation by gastric acid. Gut 19: 812– 818, 1978. 956. Schatzmann HJ. ATP-dependent Ca2⫹-extrusion from human red cells. Experientia 22: 364 –365, 1966. 934. Sabbieti MG, Agas D, Xiao L, Marchetti L, Coffin JD, Doetschman T, Hurley MM. Endogenous FGF-2 is critically important in PTH anabolic effects on bone. J Cell Physiol 219: 143–151, 2009. 957. Schayer RW. Formation and binding of histamine by rat tissues in vitro. Am J Physiol 187: 63– 65, 1956. 935. Saccomani G, Psarras CG, Smith PR, Kirk KL, Shoemaker RL. Histamine-induced chloride channels in apical membrane of isolated rabbit parietal cells. Am J Physiol Cell Physiol 260: C1000 –C1011, 1991. 958. Schepp W, Dehne K, Herrmuth H, Pfeffer K, Prinz C. Identification and functional importance of IL-1 receptors on rat parietal cells. Am J Physiol Gastrointest Liver Physiol 275: G1094 –G1105, 1998. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 261 SASCHA KOPIC AND JOHN P. GEIBEL 959. Schepp W, Dehne K, Riedel T, Schmidtler J, Schaffer K, Classen M. Oxyntomodulin: a cAMP-dependent stimulus of rat parietal cell function via the receptor for glucagon-like peptide-1 (7–36)NH2. Digestion 57: 398 – 405, 1996. 960. Schepp W, Prinz C, Hakanson R, Schusdziarra V, Classen M. Bombesin-like peptides stimulate gastrin release from isolated rat G-cells. Regul Pept 28: 241–253, 1990. 961. Schepp W, Prinz C, Tatge C, Hakanson R, Schusdziarra V, Classen M. Galanin inhibits gastrin release from isolated rat gastric G-cells. Am J Physiol Gastrointest Liver Physiol 258: G596 –G602, 1990. 962. Schiller LR, Walsh JH, Feldman M. Distention-induced gastrin release: effects of luminal acidification and intravenous atropine. Gastroenterology 78: 912–917, 1980. 963. Schinke T, Schilling AF, Baranowsky A, Seitz S, Marshall RP, Linn T, Blaeker M, Huebner AK, Schulz A, Simon R, Gebauer M, Priemel M, Kornak U, Perkovic S, Barvencik F, Beil FT, Del Fattore A, Frattini A, Streichert T, Pueschel K, Villa A, Debatin KM, Rueger JM, Teti A, Zustin J, Sauter G, Amling M. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nature Med 15: 674 – 681, 2009. 964. Schipani E, Karga H, Karaplis AC, Potts JT Jr, Kronenberg HM, Segre GV, AbouSamra AB, Juppner H. Identical complementary deoxyribonucleic acids encode a human renal and bone parathyroid hormone (PTH)/PTH-related peptide receptor. Endocrinology 132: 2157–2165, 1993. 965. Schjoldager BT, Baldissera FG, Mortensen PE, Holst JJ, Christiansen J. Oxyntomodulin: a potential hormone from the distal gut. Pharmacokinetics and effects on gastric acid and insulin secretion in man. Eur J Clin Invest 18: 499 –503, 1988. 966. Schmidt WE, Schenk S, Nustede R, Holst JJ, Folsch UR, Creutzfeldt W. Cholecystokinin is a negative regulator of gastric acid secretion and postprandial release of gastrin in humans. Gastroenterology 107: 1610 –1620, 1994. 967. Schmitz F, Goke MN, Otte JM, Schrader H, Reimann B, Kruse ML, Siegel EG, Peters J, Herzig KH, Folsch UR, Schmidt WE. Cellular expression of CCK-A and CCK-B/ gastrin receptors in human gastric mucosa. Regul Pept 102: 101–110, 2001. 968. Schmorl G. (Editor). Verhandlungen der Deutschen Pathologischen Gesellschaft. Jena: Gustav Fischer, 1909. 969. Schnoke M, Midura SB, Midura RJ. Parathyroid hormone suppresses osteoblast apoptosis by augmenting DNA repair. Bone 45: 590 – 602, 2009. 970. Schoeber JP, Topala CN, Wang X, Diepens RJ, Lambers TT, Hoenderop JG, Bindels RJ. RGS2 inhibits the epithelial Ca2⫹ channel TRPV6. J Biol Chem 281: 29669 –29674, 2006. 971. Schoeber JPH, Topala CN, Lee KP, Lambers TT, Ricard G, Van Der Kemp AWCM, Huynen MA, Hoenderop JGJ, Bindels RJM. Identification of Nipsnap1 as a novel auxiliary protein inhibiting TRPV6 activity. Pflügers Arch 457: 91–101, 2008. 972. Schoen MS, Lindenbaum J, Roginsky MS, Holt PR. Significance of serum level of 25-hydroxycholecalciferol in gastrointestinal disease. Am J Dig Dis 23: 137–142, 1978. 973. Schofield GC, Ito S, Bolender RP. Changes in membrane surface areas in mouse parietal cells in relation to high levels of acid secretion. J Anat 128: 669 – 692, 1979. 974. Scholz D, Schwille PO, Schley HW, Hanisch E, Bieger D, Zeuner E, Engelhardt W. Mineral metabolism and vitamin D status before and up to five years following highly selective vagotomy in duodenal ulcer patients. Hepatogastroenterology 30: 102–106, 1983. 975. Schubert ML, Edwards NF, Makhlouf GM. Regulation of gastric somatostatin secretion in the mouse by luminal acidity: a local feedback mechanism. Gastroenterology 94: 317–322, 1988. 976. Schubert ML, Jong MJ, Makhlouf GM. Bombesin/GRP-stimulated somatostatin secretion is mediated by gastrin in the antrum and intrinsic neurons in the fundus. Am J Physiol Gastrointest Liver Physiol 261: G885–G889, 1991. 977. Schubert ML, Makhlouf GM. Gastrin secretion induced by distention is mediated by gastric cholinergic and vasoactive intestinal peptide neurons in rats. Gastroenterology 104: 834 – 839, 1993. 978. Schubert ML, Saffouri B, Walsh JH, Makhlouf GM. Inhibition of neurally mediated gastrin secretion by bombesin antiserum. Am J Physiol Gastrointest Liver Physiol 248: G456 –G462, 1985. 262 979. Schuessler M, Astecker N, Herzig G, Vorisek G, Schuster I. Skin is an autonomous organ in synthesis, two-step activation and degradation of vitamin D(3): CYP27 in epidermis completes the set of essential vitamin D(3)-hydroxylases. Steroids 66: 399 – 408, 2001. 980. Schulak JA, Kaplan EL. Gastrin-induced hypocalcemia in thyroparathyroidectomized rats. Metabolism 23: 1103–1106, 1974. 981. Schulak JA, Kaplan EL. The importance of the stomach in gastrin-induced hypocalcemia in the rat. Endocrinology 96: 1217–1220, 1975. 982. Schusdziarra V, Harris V, Conlon JM, Arimura A, Unger R. Pancreatic and gastric somatostatin release in response to intragastric and intraduodenal nutrients and HCl in the dog. J Clin Invest 62: 509 –518, 1978. 983. Schwab M, Klotz U, Hofmann U, Schaeffeler E, Leodolter A, Malfertheiner P, Treiber G. Esomeprazole-induced healing of gastroesophageal reflux disease is unrelated to the genotype of CYP2C19: evidence from clinical and pharmacokinetic data. Clin Pharmacol Ther 78: 627– 634, 2005. 984. Schwartz GG, Wang MH, Zang M, Singh RK, Siegal GP. 1␣,25-Dihydroxyvitamin D (calcitriol) inhibits the invasiveness of human prostate cancer cells. Cancer Epidemiol Biomarkers Prev 6: 727–732, 1997. 985. Seal A, Liu E, Buchan A, Brown J. Immunoneutralization of somatostatin and neurotensin: effect on gastric acid secretion. Am J Physiol Gastrointest Liver Physiol 255: G40 –G45, 1988. 986. Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, Tatsumi S, Miyamoto K. Growth-related renal type II Na/Pi cotransporter. J Biol Chem 277: 19665– 19672, 2002. 987. Segawa H, Yamanaka S, Onitsuka A, Tomoe Y, Kuwahata M, Ito M, Taketani Y, Miyamoto K. Parathyroid hormone-dependent endocytosis of renal type IIc Na-Pi cotransporter. Am J Physiol Renal Physiol 292: F395–F403, 2007. 988. Segev H, Honigman A, Rosen H, Leitersdorf E. Transcriptional regulation of the human sterol 27-hydroxylase gene (CYP27) and promoter mapping. Atherosclerosis 156: 339 –347, 2001. 989. Segre GV, D’Amour P, Hultman A, Potts JT Jr. Effects of hepatectomy, nephrectomy, nephrectomy/uremia on the metabolism of parathyroid hormone in the rat. J Clin Invest 67: 439 – 448, 1981. 990. Selye H. Anesthetic effect of steroid hormones. Proc Soc Exp Biol Med 46: 116, 1941. 991. Serfaty-Lacrosniere C, Wood RJ, Voytko D, Saltzman JR, Pedrosa M, Sepe TE, Russell RR. Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. J Am Coll Nutr 14: 364 –368, 1995. 992. Seroussi E, Pan HQ, Kedra D, Roe BA, Dumanski JP. Characterization of the human NIPSNAP1 gene from 22q12: a member of a novel gene family. Gene 212: 13–20, 1998. 993. Setoguchi T, Salen G, Tint GS, Mosbach EH. A biochemical abnormality in cerebrotendinous xanthomatosis. Impairment of bile acid biosynthesis associated with incomplete degradation of the cholesterol side chain. J Clin Invest 53: 1393–1401, 1974. 994. Seva C, Dickinson CJ, Yamada T. Growth-promoting effects of glycine-extended progastrin. Science 265: 410 – 412, 1994. 995. Shareghi GR, Stoner LC. Calcium transport across segments of the rabbit distal nephron in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 235: F367–F375, 1978. 996. Sheikh MS, Ramirez A, Emmett M, Santa Ana C, Schiller LR, Fordtran JS. Role of vitamin D-dependent and vitamin D-independent mechanisms in absorption of food calcium. J Clin Invest 81: 126 –132, 1988. 997. Sheikh MS, Santa Ana CA, Nicar MJ, Schiller LR, Fordtran JS. Gastrointestinal absorption of calcium from milk and calcium salts. N Engl J Med 317: 532–536, 1987. 998. Sheinin Y, Kallay E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Immunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant human large intestinal mucosa. J Histochem Cytochem 48: 595– 602, 2000. 999. Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 73: 283–309, 2011. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH 1000. Shen LP, Pictet RL, Rutter WJ. Human somatostatin I: sequence of the cDNA. Proc Natl Acad Sci USA 79: 4575– 4579, 1982. 1001. Sherry AM, Malinowska DH, Morris RE, Ciraolo GM, Cuppoletti J. Localization of ClC-2 Cl⫺ channels in rabbit gastric mucosa. Am J Physiol Cell Physiol 280: C1599 – C1606, 2001. 1002. Shibata N, Matsui H, Yokota T, Matsuura B, Maeyama K, Onji M. Direct effects of nitric oxide on histamine release from rat enterochromaffin-like cells. Eur J Pharmacol 535: 25–33, 2006. 1003. Shimizu T, Yoshitomi K, Nakamura M, Imai M. Effects of PTH, calcitonin, cAMP on calcium transport in rabbit distal nephron segments. Am J Physiol Renal Fluid Electrolyte Physiol 259: F408 –F414, 1990. 1004. Shimoi K, Saka N, Nozawa R, Sato M, Amano I, Nakayama T, Kinae N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab Dispos 29: 1521–1524, 2001. 1005. Shin JM, Besancon M, Prinz C, Simon A, Sachs G. Continuing development of acid pump inhibitors: site of action of pantoprazole. Aliment Pharmacol Ther 8 Suppl 1: 11–23, 1994. 1006. Shin JM, Besancon M, Simon A, Sachs G. The site of action of pantoprazole in the gastric H⫹/K⫹-ATPase. Biochim Biophys Acta 1148: 223–233, 1993. ⫹ 1007. Shin JM, Cho YM, Sachs G. Chemistry of covalent inhibition of the gastric (H , K⫹)-ATPase by proton pump inhibitors. J Am Chem Soc 126: 7800 –7811, 2004. 1008. Shin JM, Homerin M, Domagala F, Ficheux H, Sachs G. Characterization of the inhibitory activity of tenatoprazole on the gastric H⫹,K⫹-ATPase in vitro and in vivo. Biochem Pharmacol 71: 837– 849, 2006. 1021. Slepchenko BM, Bronner F. Modeling of transcellular Ca transport in rat duodenum points to coexistence of two mechanisms of apical entry. Am J Physiol Cell Physiol 281: C270 –C281, 2001. 1022. Sneddon WB, Syme CA, Bisello A, Magyar CE, Rochdi MD, Parent JL, Weinman EJ, Abou-Samra AB, Friedman PA. Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50). J Biol Chem 278: 43787– 43796, 2003. 1023. Soejima M, Tachida H, Koda Y. Sequence analysis of human TRPV6 suggests positive selection outside Africa. Biochem Genet 47: 147–153, 2009. 1024. Soll AH. The interaction of histamine with gastrin and carbamylcholine on oxygen uptake by isolated mammalian parietal cells. J Clin Invest 61: 381–389, 1978. 1025. Soll AH. Potentiating interactions of gastric stimulants on [14C]aminopyrine accumulation by isolated canine parietal cells. Gastroenterology 83: 216 –223, 1982. 1026. Soll AH, Wollin A. Histamine and cyclic AMP in isolated canine parietal cells. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E444 –E450, 1979. 1027. Sone T, Kerner S, Pike JW. Vitamin D receptor interaction with specific DNA. Association as a 1,25-dihydroxyvitamin D3-modulated heterodimer. J Biol Chem 266: 23296 –23305, 1991. 1028. Song P, Groos S, Riederer B, Feng Z, Krabbenhoft A, Manns MP, Smolka A, Hagen SJ, Neusch C, Seidler U. Kir4.1 channel expression is essential for parietal cell control of acid secretion. J Biol Chem 286: 14120 –14128, 2011. 1029. Song P, Groos S, Riederer B, Feng Z, Krabbenhoft A, Smolka A, Seidler U. KCNQ1 is the luminal K⫹ recycling channel during stimulation of gastric acid secretion. J Physiol 587: 3955–3965, 2009. 1009. Shinki T, Ueno Y, DeLuca HF, Suda T. Calcitonin is a major regulator for the expression of renal 25-hydroxyvitamin D3-1alpha-hydroxylase gene in normocalcemic rats. Proc Natl Acad Sci USA 96: 8253– 8258, 1999. 1030. Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology 144: 3885–3894, 2003. 1010. Shoback DM, Membreno LA, McGhee JG. High calcium and other divalent cations increase inositol trisphosphate in bovine parathyroid cells. Endocrinology 123: 382– 389, 1988. 1031. Sopjani M, Kunert A, Czarkowski K, Klaus F, Laufer J, Föller M, Lang F. Regulation of the Ca2⫹ Channel TRPV6 by the Kinases SGK1, PKB/Akt, and PIKfyve. J Membr Biol 233: 35– 41, 2010. 1011. Shulkes A, Read M. Regulation of somatostatin secretion by gastrin- and acid-dependent mechanisms. Endocrinology 129: 2329 –2334, 1991. 1032. Sowa H, Kaji H, Iu MF, Tsukamoto T, Sugimoto T, Chihara K. Parathyroid hormoneSmaD3 axis exerts anti-apoptotic action and augments anabolic action of transforming growth factor beta in osteoblasts. J Biol Chem 278: 52240 –52252, 2003. 1012. Shyjan AW, Canfield VA, Levenson R. Evolution of the Na,K- and H⫹-K⫹-ATPase beta subunit gene family: structure of the murine Na⫹-K⫹-ATPase beta 2 subunit gene. Genomics 11: 435– 442, 1991. 1033. St-Arnaud R. The direct role of vitamin D on bone homeostasis. Arch Biochem Biophys 473: 225–230, 2008. 1013. Sidani SM, Kirchhoff P, Socrates T, Stelter L, Ferreira E, Caputo C, Roberts KE, Bell RL, Egan ME, Geibel JP. DeltaF508 mutation results in impaired gastric acid secretion. J Biol Chem 282: 6068 – 6074, 2007. 1034. St-Arnaud R, Messerlian S, Moir JM, Omdahl JL, Glorieux FH. The 25-hydroxyvitamin D 1-alpha-hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. J Bone Miner Res 12: 1552–1559, 1997. 1014. Silve C, Petrel C, Leroy C, Bruel H, Mallet E, Rognan D, Ruat M. Delineating a Ca2⫹ binding pocket within the venus flytrap module of the human calcium-sensing receptor. J Biol Chem 280: 37917–37923, 2005. 1035. Stamp TC, Round JM. Seasonal changes in human plasma levels of 25-hydroxyvitamin D. Nature 247: 563–565, 1974. 1015. Silver J, Fainaru M. Transport of vitamin D sterols in human plasma: effect of excess vitamin D, 25 hydroxyvitamin D and 1,25 dihydroxyvitamin D. Eur J Clin Invest 9: 433– 438, 1979. 1016. Singer FR, Foster GV, Jpolin GF, Nadarajah A, Parkinson DK, Thalassinos N, Woodhouse NJ, Clark MB, Fraser TR, MacIntyre I. Acute effects of administered calcitonin in man. Calcif Tissue Res Suppl: 20, 1968. 1017. Singh P, Velasco M, Given R, Varro A, Wang TC. Progastrin expression predisposes mice to colon carcinomas and adenomas in response to a chemical carcinogen. Gastroenterology 119: 162–171, 2000. 1018. Sitrin MD, Bengoa JM. Intestinal absorption of cholecalciferol and 25-hydroxycholecalciferol in chronic cholestatic liver disease. Am J Clin Nutr 46: 1011–1015, 1987. 1019. Sitrin MD, Pollack KL, Bolt MJ, Rosenberg IH. Comparison of vitamin D and 25hydroxyvitamin D absorption in the rat. Am J Physiol Gastrointest Liver Physiol 242: G326 –G332, 1982. 1020. Sizemore GW, Go VL, Kaplan EL, Sanzenbacher LJ, Holtermuller KH, Arnaud CD. Relations of calcitonin and gastrin in the Zollinger-Ellison syndrome and medullary carcinoma of the thyroid. N Engl J Med 288: 641– 644, 1973. 1036. Stedman CA, Barclay ML. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther 14: 963–978, 2000. 1037. Steenbock H, Black A. Fat-soluble vitamins. XVII. The induction of growth-promoting and calcifying properties in a ration by exposure to ultra-violet light. J Biol Chem 61: 405– 422, 1924. 1038. Steggerda FR, Mitchell HH. The effect of the citrate ion on the calcium metabolism of adult human subjects. J Nutr 31: 423– 438, 1946. 1039. Stepan V, Pausawasdi N, Ramamoorthy S, Todisco A. The Akt and MAPK signaltransduction pathways regulate growth factor actions in isolated gastric parietal cells. Gastroenterology 127: 1150 –1161, 2004. 1040. Stepan V, Sugano K, Yamada T, Park J, Dickinson CJ. Gastrin biosynthesis in canine G cells. Am J Physiol Gastrointest Liver Physiol 282: G766 –G775, 2002. 1041. Sternfeld L, Anderie I, Schmid A, Al-Shaldi H, Krause E, Magg T, Schreiner D, Hofer HW, Schulz I. Identification of tyrosines in the putative regulatory site of the Ca2⫹ channel TRPV6. Cell Calcium 42: 91–102, 2007. 1042. Sternfeld L, Krause E, Schmid A, Anderie I, Latas A, Al-Shaldi H, Kohl A, Evers K, Hofer HW, Schulz I. Tyrosine phosphatase PTP1B interacts with TRPV6 in vivo and Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 263 SASCHA KOPIC AND JOHN P. GEIBEL plays a role in TRPV6-mediated calcium influx in HEK293 cells. Cell Signal 17: 951– 960, 2005. 1043. Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev 81: 21–50, 2001. 1044. Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest 93: 347–354, 1994. 1045. Strushkevich N, Usanov SA, Plotnikov AN, Jones G, Park HW. Structural analysis of CYP2R1 in complex with vitamin D3. J Mol Biol 380: 95–106, 2008. 1046. Stumpf T, Zhang Q, Hirnet D, Lewandrowski U, Sickmann A, Wissenbach U, Dorr J, Lohr C, Deitmer JW, Fecher-Trost C. The human TRPV6 channel protein is associated with cyclophilin B in human placenta. J Biol Chem 283: 18086 –18098, 2008. 1047. Su HC, Bishop AE, Power RF, Hamada Y, Polak JM. Dual intrinsic and extrinsic origins of CGRP- and NPY-immunoreactive nerves of rat gut and pancreas. J Neurosci 7: 2674 –2687, 1987. 1048. Su P, Rennert H, Shayiq RM, Yamamoto R, Zheng YM, Addya S, Strauss JF 3rd, Avadhani NG. A cDNA encoding a rat mitochondrial cytochrome P450 catalyzing both the 26-hydroxylation of cholesterol and 25-hydroxylation of vitamin D3: gonadotropic regulation of the cognate mRNA in ovaries. DNA Cell Biol 9: 657– 667, 1990. 1049. Suda J, Zhu L, Okamoto CT, Karvar S. Rab27b localizes to the tubulovesicle membranes of gastric parietal cells and regulates acid secretion. Gastroenterology 140: 868 – 878, 2011. 1050. Sudo Y, Matsuo K, Tetsuo T, Tsutsumi S, Ohkura M, Nakai J, Uezono Y. Derived (mutated)-types of TRPV6 channels elicit greater Ca2⫹ influx into the cells than ancestral-types of TRPV6: evidence from Xenopus oocytes and mammalian cell expression system. J Pharmacol Sci 114: 281–291, 2010. 1051. Sugano K, Park J, Dobbins WO, Yamada T. Glycine-extended progastrin processing intermediates: accumulation and cosecretion with gastrin. Am J Physiol Gastrointest Liver Physiol 253: G502–G507, 1987. 1052. Suki WN, Eknoyan G, Rector FC Jr, Seldin DW. The renal diluting and concentrating mechanism in hypercalcemia. Nephron 6: 50 – 61, 1969. 1053. Sulimovici S, Roginsky MS, Duffy JL, Pfeifer RF. Calciferol 25-hydroxylase activity in smooth and rough endoplasmic reticulum of rat liver. Arch Biochem Biophys 195: 45–52, 1979. 1054. Sundler F, Ekblad E, Absood A, Hakanson R, Koves K, Arimura A. Pituitary adenylate cyclase activating peptide: a novel vasoactive intestinal peptide-like neuropeptide in the gut. Neuroscience 46: 439 – 454, 1992. 1055. Sunthornthepvarakul T, Churesigaew S, Ngowngarmratana S. A novel mutation of the signal peptide of the preproparathyroid hormone gene associated with autosomal recessive familial isolated hypoparathyroidism. J Clin Endocrinol Metab 84: 3792–3796, 1999. 1056. Suva LJ, Winslow GA, Wettenhall RE, Hammonds RG, Moseley JM, DiefenbachJagger H, Rodda CP, Kemp BE, Rodriguez H, Chen EY. A parathyroid hormonerelated protein implicated in malignant hypercalcemia: cloning and expression. Science 237: 893– 896, 1987. 1057. Suzuki H, Nakamura I, Takahashi N, Ikuhara T, Matsuzaki K, Isogai Y, Hori M, Suda T. Calcitonin-induced changes in the cytoskeleton are mediated by a signal pathway associated with protein kinase A in osteoclasts. Endocrinology 137: 4685– 4690, 1996. 1058. Suzuki M, Ishibashi K, Ooki G, Tsuruoka S, Imai M. Electrophysiologic characteristics of the Ca-permeable channels, ECaC and CaT, in the kidney. Biochem Biophys Res Commun 274: 344 –349, 2000. 1059. Tache Y, Saperas E. Potent inhibition of gastric acid secretion and ulcer formation by centrally and peripherally administered interleukin-1. Ann NY Acad Sci 664: 353–368, 1992. 1060. Takahashi N, Yamana H, Yoshiki S, Roodman GD, Mundy GR, Jones SJ, Boyde A, Suda T. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology 122: 1373–1382, 1988. 264 1061. Takahashi S, Goldring S, Katz M, Hilsenbeck S, Williams R, Roodman GD. Downregulation of calcitonin receptor mRNA expression by calcitonin during human osteoclast-like cell differentiation. J Clin Invest 95: 167–171, 1995. 1062. Takeuchi Y, Pausawasdi N, Todisco A. Carbachol activates ERK2 in isolated gastric parietal cells via multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol 276: G1484 –G1492, 1999. 1063. Takeuchi Y, Yamada J, Yamada T, Todisco A. Functional role of extracellular signalregulated protein kinases in gastric acid secretion. Am J Physiol Gastrointest Liver Physiol 273: G1263–G1272, 1997. 1064. Talmage RV, Neuenschwander J, Kraintz L. Evidence for the existence of thyrocalcitonin in the rat. Endocrinology 76: 103–107, 1965. 1065. Tam CS, Heersche JN, Murray TM, Parsons JA. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology 110: 506 –512, 1982. 1066. Tanaka S, Hamada K, Yamada N, Sugita Y, Tonai S, Hunyady B, Palkovits M, Falus A, Watanabe T, Okabe S, Ohtsu H, Ichikawa A, Nagy A. Gastric acid secretion in L-histidine decarboxylase-deficient mice. Gastroenterology 122: 145–155, 2002. 1067. Tanaka Y, Castillo L, Wineland MJ, DeLuca HF. Synergistic effect of progesterone, testosterone, estradiol in the stimulation of chick renal 25-hydroxyvitamin D3-1alpha-hydroxylase. Endocrinology 103: 2035–2039, 1978. 1068. Tanaka Y, Deluca HF. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys 154: 566 –574, 1973. 1069. Tanaka Y, DeLuca HF. Measurement of mammalian 25-hydroxyvitamin D3 24R-and 1 alpha-hydroxylase. Proc Natl Acad Sci USA 78: 196 –199, 1981. 1070. Tang LH, Stoch SA, Modlin IM, Goldenring JR. Identification of rab2 as a tubulovesicle-membrane-associated protein in rabbit gastric parietal cells. Biochem J 285: 715–719, 1992. 1071. Tanrattana C, Charoenphandhu N, Limlomwongse L, Krishnamra N. Prolactin directly stimulated the solvent drag-induced calcium transport in the duodenum of female rats. Biochim Biophys Acta 1665: 81–91, 2004. 1072. Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, Barchas JD. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature 324: 476 – 478, 1986. 1073. Taylor AN, Wasserman RH. Vitamin D3-induced calcium-binding protein: partial purification, electrophoretic visualization, and tissue distribution. Arch Biochem Biophys 119: 536 –540, 1967. 1074. Taylor IL, Byrne WJ, Christie DL, Ament ME, Walsh JH. Effect of individual L-amino acids on gastric acid secretion and serum gastrin and pancreatic polypeptide release in humans. Gastroenterology 83: 273–278, 1982. 1075. Taylor IL, Sells RA, McConnell RB, Dockray GJ. Serum gastrin in patients with chronic renal failure. Gut 21: 1062–1067, 1980. 1076. Teranishi H, Kasuya M, Aoshima K, Kato T, Migita S. Demonstration of vitamin D-binding protein (Gc-globulin) in the urine of Itai-itai disease patients. Toxicol Lett 15: 7–12, 1983. 1077. Thamsborg G, Jensen JE, Kollerup G, Hauge EM, Melsen F, Sorensen OH. Effect of nasal salmon calcitonin on bone remodeling and bone mass in postmenopausal osteoporosis. Bone 18: 207–212, 1996. 1078. Theodoropoulos C, Demers C, Petit JL, Gascon-Barre M. High sensitivity of rat hepatic vitamin D3-25 hydroxylase CYP27A to 1,25-dihydroxyvitamin D3 administration. Am J Physiol Endocrinol Metab 284: E138 –E147, 2003. 1079. Thomas WC Jr, Morgan HG, Connor TB, Haddock L, Bills CE, Howard JE. Studies of antiricketic activity in sera from patients with disorders of calcium metabolism and preliminary observations on the mode of transport of vitamin D in human serum. J Clin Invest 38: 1078 –1085, 1959. 1080. Thompson GR, Lewis B, Booth CC. Absorption of vitamin D3-3H in control subjects and patients with intestinal malabsorption. J Clin Invest 45: 94 –102, 1966. 1081. Thompson GR, Lewis B, Booth CC. Vitamin-D absorption after partial gastrectomy. Lancet 1: 457– 458, 1966. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH 1082. Thompson GR, Ockner RK, Isselbacher KJ. Effect of mixed micellar lipid on the absorption of cholesterol and vitamin D3 into lymph. J Clin Invest 48: 87–95, 1969. tive hormone and hypocalcemia-induced 1,25(OH)2D synthesis. Kidney Int 72: 1330 –1335, 2007. 1083. Thompson PD, Jurutka PW, Haussler CA, Whitfield GK, Haussler MR. Heterodimeric DNA binding by the vitamin D receptor and retinoid X receptors is enhanced by 1,25-dihydroxyvitamin D3 and inhibited by 9-cis-retinoic acid. Evidence for allosteric receptor interactions. J Biol Chem 273: 8483– 8491, 1998. 1103. Usui E, Noshiro M, Okuda K. Molecular cloning of cDNA for vitamin D3 25-hydroxylase from rat liver mitochondria. FEBS Lett 262: 135–138, 1990. 1084. Thunberg R. Localization of cells containing and forming histamine in the gastric mucosa of the rat. Exp Cell Res 47: 108 –115, 1967. 1085. Tian XQ, Chen TC, Matsuoka LY, Wortsman J, Holick MF. Kinetic and thermodynamic studies of the conversion of previtamin D3 to vitamin D3 in human skin. J Biol Chem 268: 14888 –14892, 1993. 1086. Tian XQ, Holick MF. A liposomal model that mimics the cutaneous production of vitamin D3. Studies of the mechanism of the membrane-enhanced thermal isomerization of previtamin D3 to vitamin D3. J Biol Chem 274: 4174 – 4179, 1999. 1087. Tinel N, Diochot S, Borsotto M, Lazdunski M, Barhanin J. KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. EMBO J 19: 6326 – 6330, 2000. 1088. Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology 149: 558 –564, 2008. 1089. Tondapu P, Provost D, Adams-Huet B, Sims T, Chang C, Sakhaee K. Comparison of the absorption of calcium carbonate and calcium citrate after Roux-en-Y gastric bypass. Obes Surg 19: 1256 –1261, 2009. 1090. Topala CN, Schoeber JP, Searchfield LE, Riccardi D, Hoenderop JG, Bindels RJ. Activation of the Ca2⫹-sensing receptor stimulates the activity of the epithelial Ca2⫹ channel TRPV5. Cell Calcium 45: 331–339, 2009. 1091. Tovey FI, Karamanolis DG, Godfrey J, Clark CG. Post-gastrectomy nutrition: methods of outpatient screening for early osteomalacia. Hum Nutr Clin Nutr 39: 439 – 446, 1985. 1092. Toyoshima C, Nomura H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418: 605– 611, 2002. 1093. Toyoshima C, Nomura H, Sugita Y. Crystal structures of Ca2⫹-ATPase in various physiological states. Ann NY Acad Sci 986: 1– 8, 2003. 1094. Tregear GW, Van Rietschoten J, Greene E, Keutmann HT, Niall HD, Reit B, Parsons JA, Potts JT Jr. Bovine parathyroid hormone: minimum chain length of synthetic peptide required for biological activity. Endocrinology 93: 1349 –1353, 1973. 1095. Tsunoda Y, Funasaka M, Modlin IM, Hidaka H, Fox LM, Goldenring JR. An inhibitor of Ca2⫹/calmodulin-dependent protein kinase II, KN-62, inhibits cholinergic-stimulated parietal cell secretion. Am J Physiol Gastrointest Liver Physiol 262: G118 –G122, 1992. 1104. Vagin O, Denevich S, Munson K, Sachs G. SCH28080, a K⫹-competitive inhibitor of the gastric H⫹-K⫹-ATPase, binds near the M5– 6 luminal loop, preventing K⫹ access to the ion binding domain. Biochemistry 41: 12755–12762, 2002. 1105. Vagin O, Denevich S, Sachs G. Plasma membrane delivery of the gastric H⫹-K⫹ATPase: the role of beta-subunit glycosylation. Am J Physiol Cell Physiol 285: C968 – C976, 2003. 1106. Vagin O, Munson K, Lambrecht N, Karlish SJ, Sachs G. Mutational analysis of the K⫹-competitive inhibitor site of gastric H⫹-K⫹-ATPase. Biochemistry 40: 7480 – 7490, 2001. 1107. Vagne M, Andre C. The effect of secretin on gastric emptying in man. Gastroenterology 60: 421– 424, 1971. 1108. Van Abel M, Hoenderop JG, van der Kemp AW, Friedlaender MM, van Leeuwen JP, Bindels RJ. Coordinated control of renal Ca2⫹ transport proteins by parathyroid hormone. Kidney Int 68: 1708 –1721, 2005. 1109. Van Abel M, Hoenderop JG, van der Kemp AW, van Leeuwen JP, Bindels RJ. Regulation of the epithelial Ca2⫹ channels in small intestine as studied by quantitative mRNA detection. Am J Physiol Gastrointest Liver Physiol 285: G78 –G85, 2003. 1110. Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA 98: 13324 –13329, 2001. 1111. Van de Graaf SF, Chang Q, Mensenkamp AR, Hoenderop JG, Bindels RJ. Direct interaction with Rab11a targets the epithelial Ca2⫹ channels TRPV5 and TRPV6 to the plasma membrane. Mol Cell Biol 26: 303–312, 2006. 1112. Van de Graaf SF, Hoenderop JG, Gkika D, Lamers D, Prenen J, Rescher U, Gerke V, Staub O, Nilius B, Bindels RJ. Functional expression of the epithelial Ca2⫹ channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex. EMBO J 22: 1478 –1487, 2003. 1113. Van de Graaf SF, Hoenderop JG, van der Kemp AW, Gisler SM, Bindels RJ. Interaction of the epithelial Ca2⫹ channels TRPV5 and TRPV6 with the intestine- and kidney-enriched PDZ protein NHERF4. Pflügers Arch 452: 407– 417, 2006. 1114. Van Leeuwen JP, van Driel M, van den Bemd GJ, Pols HA. Vitamin D control of osteoblast function and bone extracellular matrix mineralization. Crit Rev Eukaryot Gene Expr 11: 199 –226, 2001. 1115. Varrault A, Pena MS, Goldsmith PK, Mithal A, Brown EM, Spiegel AM. Expression of G protein alpha-subunits in bovine parathyroid. Endocrinology 136: 4390 – 4396, 1995. 1096. Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest 111: 1029 –1037, 2003. 1116. Varro A, Green T, Holmes S, Dockray GJ. Calcitonin gene-related peptide in visceral afferent nerve fibres: quantification by radioimmunoassay and determination of axonal transport rates. Neuroscience 26: 927–932, 1988. 1097. Tucker G 3rd, Gagnon RE, Haussler MR. Vitamin D3-25-hydroxylase: tissue occurrence and apparent lack of regulation. Arch Biochem Biophys 155: 47–57, 1973. 1117. Velluz L, Amirad G. Chimie organique-equilibre de reaction entre precalciferol et calciferol. Compt Rend Assoc Anat 288: 853– 855, 1949. 1098. Tudpor K, Teerapornpuntakit J, Jantarajit W, Krishnamra N, Charoenphandhu N. 1,25-Dihydroxyvitamin D(3) rapidly stimulates the solvent drag-induced paracellular calcium transport in the duodenum of female rats. J Physiol Sci 58: 297–307, 2008. 1118. Velluz L, Amirad G. Chimie organique-le precalciferol. Compt Rend Assoc Anat 228: 692– 694, 1949. 1099. Tzaneva MA. Ultrastructural immunohistochemical localization of gastrin, somatostatin and serotonin in endocrine cells of human antral gastric mucosa. Acta Histochem 105: 191–201, 2003. 1100. Uchida M, Teranishi H, Aoshima K, Katoh T, Kasuya M, Inadera H. Elevated urinary levels of vitamin D-binding protein in the inhabitants of a cadmium polluted area, Jinzu River basin, Japan. Tohoku J Exp Med 211: 269 –274, 2007. 1101. Uehara A, Okumura T, Sekiya C, Okamura K, Takasugi Y, Namiki M. Interleukin-1 inhibits the secretion of gastric acid in rats: possible involvement of prostaglandin. Biochem Biophys Res Commun 162: 1578 –1584, 1989. 1102. Usatii M, Rousseau L, Demers C, Petit JL, Brossard JH, Gascon-Barre M, Lavigne JR, Zahradnik RJ, Nemeth EF, D’Amour P. Parathyroid hormone fragments inhibit ac- 1119. Velluz L, Amirad G. Chimie organique-nouveau precursor de la vitamine D3. Compt Rend Assoc Anat 228: 1037–1038, 1949. 1120. Velluz L, Amirad G, Petit A. Le precalciferol: ses relations d’equilibre avec le calciferol. Bull Soc Chim France 16: 501–507, 1949. 1121. Verboven C, Rabijns A, De Maeyer M, Van Baelen H, Bouillon R, De Ranter C. A structural basis for the unique binding features of the human vitamin D-binding protein. Nat Struct Biol 9: 131–136, 2002. 1122. Vertino AM, Bula CM, Chen JR, Almeida M, Han L, Bellido T, Kousteni S, Norman AW, Manolagas SC. Nongenotropic, anti-apoptotic signaling of 1alpha,25(OH)2vitamin D3 and analogs through the ligand binding domain of the vitamin D receptor in osteoblasts and osteocytes. Mediation by Src, phosphatidylinositol 3-, and JNK kinases. J Biol Chem 280: 14130 –14137, 2005. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 265 SASCHA KOPIC AND JOHN P. GEIBEL 1123. Vilardaga JP, Krasel C, Chauvin S, Bambino T, Lohse MJ, Nissenson RA. Internalization determinants of the parathyroid hormone receptor differentially regulate betaarrestin/receptor association. J Biol Chem 277: 8121– 8129, 2002. 1143. Wang LD, Hoeltzel M, Gantz I, Hunter R, Del Valle J. Characterization of the histamine H2 receptor structural components involved in dual signaling. J Pharmacol Exp Ther 285: 573–578, 1998. 1124. Villa-Bellosta R, Ravera S, Sorribas V, Stange G, Levi M, Murer H, Biber J, Forster IC. The Na⫹-Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary Pi. Am J Physiol Renal Physiol 296: F691–F699, 2009. 1144. Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet 12: 17–23, 1996. 1125. Vinik AI, Gaginella TS, O’Dorisio TM, Shapiro B, Wagner L. The distribution and characterization of somatostatin-like immunoreactivity in epithelial cells, submucosa, muscle of the rat stomach and intestine. Endocrinology 109: 1921–1926, 1981. 1126. Voets T, Janssens A, Prenen J, Droogmans G, Nilius B. Mg2⫹-dependent gating and strong inward rectification of the cation channel TRPV6. J Gen Physiol 121: 245–260, 2003. 1127. Vogelsang H, Schofl R, Tillinger W, Ferenci P, Gangl A. 25-Hydroxyvitamin D absorption in patients with Crohn’s disease and with pancreatic insufficiency. Wien Klin Wochenschr 109: 678 – 682, 1997. 1128. Wada M, Furuya Y, Sakiyama J, Kobayashi N, Miyata S, Ishii H, Nagano N. The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. Control of parathyroid cell growth via a calcium receptor. J Clin Invest 100: 2977–2983, 1997. 1129. Wada S, Martin TJ, Findlay DM. Homologous regulation of the calcitonin receptor in mouse osteoclast-like cells and human breast cancer T47D cells. Endocrinology 136: 2611–2621, 1995. 1130. Wada S, Udagawa N, Nagata N, Martin TJ, Findlay DM. Calcitonin receptor downregulation relates to calcitonin resistance in mature mouse osteoclasts. Endocrinology 137: 1042–1048, 1996. 1131. Waldum HL, Sandvik AK, Brenna E, Petersen H. Gastrin-histamine sequence in the regulation of gastric acid secretion. Gut 32: 698 –701, 1991. 1132. Wallace JL, Cucala M, Mugridge K, Parente L. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. Am J Physiol Gastrointest Liver Physiol 261: G559 – G564, 1991. 1145. Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Dockray GJ. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest 98: 1918 –1929, 1996. 1146. Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Jarvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hypponen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genomewide association study. Lancet 376: 180 –188, 2010. 1147. Wang W, Lu M, Balazy M, Hebert SC. Phospholipase A2 is involved in mediating the effect of extracellular Ca2⫹ on apical K⫹ channels in rat TAL. Am J Physiol Renal Physiol 273: F421–F429, 1997. 1148. Wang WH, Lu M, Hebert SC. Cytochrome P-450 metabolites mediate extracellular Ca2⫹-induced inhibition of apical K⫹ channels in the TAL. Am J Physiol Cell Physiol 271: C103–C111, 1996. 1149. Wasserman RH, Corradino RA, Taylor AN. Vitamin D-dependent calcium-binding protein. Purification and some properties. J Biol Chem 243: 3978 –3986, 1968. 1150. Wasserman RH, Kallfelz FA, Comar CL. Active transport of calcium by rat duodenum in vivo. Science 133: 883– 884, 1961. 1133. Walling MW, Rothman SS. Apparent increase in carrier affinity for intestinal calcium transport following dietary calcium restriction. J Biol Chem 245: 5007–5011, 1970. 1151. Wasserman RH, Taylor AN. Vitamin D3-induced calcium-binding protein in chick intestinal mucosa. Science 152: 791–793, 1966. 1134. Walling MW, Rothman SS. Phosphate-independent, carrier-mediated active transport of calcium by rat intestine. Am J Physiol 217: 1144 –1148, 1969. 1152. Wasserman RH, Taylor AN. Vitamin D-dependent calcium-binding protein. Response to some physiological and nutritional variables. J Biol Chem 243: 3987–3993, 1968. 1135. Wallmark B, Brandstrom A, Larsson H. Evidence for acid-induced transformation of omeprazole into an active inhibitor of (H⫹ ⫹ K⫹)-ATPase within the parietal cell. Biochim Biophys Acta 778: 549 –558, 1984. 1136. Walters JR. Calbindin-D9k stimulates the calcium pump in rat enterocyte basolateral membranes. Am J Physiol Gastrointest Liver Physiol 256: G124 –G128, 1989. 1137. Walters JR, Howard A, Charpin MV, Gniecko KC, Brodin P, Thulin E, Forsen S. Stimulation of intestinal basolateral membrane calcium-pump activity by recombinant synthetic calbindin-D9k and specific mutants. Biochem Biophys Res Commun 170: 603– 608, 1990. 1138. Walters JR, Howard A, Lowery LJ, Mawer EB, Legon S. Expression of genes involved in calcium absorption in human duodenum. Eur J Clin Invest 29: 214 –219, 1999. 1139. Wang B, Bisello A, Yang Y, Romero GG, Friedman PA. NHERF1 regulates parathyroid hormone receptor membrane retention without affecting recycling. J Biol Chem 282: 36214 –36222, 2007. 1140. Wang B, Yang Y, Abou-Samra AB, Friedman PA. NHERF1 regulates parathyroid hormone receptor desensitization: interference with beta-arrestin binding. Mol Pharmacol 75: 1189 –1197, 2009. 1141. Wang DH, Hu YS, Du JJ, Hu YY, Zhong WD, Qin WJ. Ghrelin stimulates proliferation of human osteoblastic TE85 cells via NO/cGMP signaling pathway. Endocrine 35: 112–117, 2009. 1142. Wang L, Gantz I, DelValle J. Histamine H2 receptor activates adenylate cyclase and PLC via separate GTP-dependent pathways. Am J Physiol Gastrointest Liver Physiol 271: G613–G620, 1996. 266 1153. Watanabe S, Chey WY, Lee KY, Chang TM. Secretin is released by digestive products of fat in dogs. Gastroenterology 90: 1008 –1017, 1986. 1154. Watson F, Kiernan RS, Deavall DG, Varro A, Dimaline R. Transcriptional activation of the rat vesicular monoamine transporter 2 promoter in gastric epithelial cells: regulation by gastrin. J Biol Chem 276: 7661–7671, 2001. 1155. Watson PH, Fraher LJ, Hendy GN, Chung UI, Kisiel M, Natale BV, Hodsman AB. Nuclear localization of the type 1 PTH/PTHrP receptor in rat tissues. J Bone Miner Res 15: 1033–1044, 2000. 1156. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 67: 373–378, 1988. 1157. Weiss LA, Langenberg C, Barrett-Connor E. Ghrelin and bone: is there an association in older adults?: the Rancho Bernardo study. J Bone Miner Res 21: 752–757, 2006. 1158. Weitkamp LR, Rucknagel DL, Gershowitz H. Genetic linkage between structural loci for albumin and group specific component (Gc). Am J Hum Genet 18: 559 –571, 1966. 1159. Wettschureck N, Lee E, Libutti SK, Offermanns S, Robey PG, Spiegel AM. Parathyroid-specific double knockout of Gq and G11 alpha-subunits leads to a phenotype resembling germline knockout of the extracellular Ca2⫹-sensing receptor. Mol Endocrinol 21: 274 –280, 2007. 1160. Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med 17: 126 –139, 2011. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org GASTRIC ACID, CALCIUM ABSORPTION, AND BONE HEALTH 1161. Wiborg O, Berglund L, Boel E, Norris F, Norris K, Rehfeld JF, Marcker KA, Vuust J. Structure of a human gastrin gene. Proc Natl Acad Sci USA 81: 1067–1069, 1984. 1162. Wikvall K. Hydroxylations in biosynthesis of bile acids. Isolation of a cytochrome P-450 from rabbit liver mitochondria catalyzing 26-hydroxylation of C27-steroids. J Biol Chem 259: 3800 –3804, 1984. 1163. Wilkes JM, Kajimura M, Scott DR, Hersey SJ, Sachs G. Muscarinic responses of gastric parietal cells. J Membr Biol 122: 97–110, 1991. 1164. Windaus A. The chemistry of irradiated ergosterol. Proc R Soc Lond 108: 568 –575, 1931. 1165. Windaus A, Bock F. über das Provitamin aus dem Sterin der Schweineschwarte. Hoppe-Seyler’s Zeitschr physiol Chem 245: 168 –170, 1936. 1166. Windaus A, Hess A. Sterine und antirachitisches Vitamin. In: Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen aus dem Jahre 1926. Berlin: Weidmannsche Buchhandlung, 1927, p. 175–184. 1167. Windaus A, Lüttringhaus A, Deppe M. über das krystallisierte Vitamin D1. Just Lieb Ann Chem 489: 252–269, 1931. 1168. Wissenbach U, Niemeyer BA, Fixemer T, Schneidewind A, Trost C, Cavalie A, Reus K, Meese E, Bonkhoff H, Flockerzi V. Expression of CaT-like, a novel calciumselective channel, correlates with the malignancy of prostate cancer. J Biol Chem 276: 19461–19468, 2001. ⫹ ⫺ 1169. Wolosin JM, Forte JG. Stimulation of oxyntic cell triggers K and Cl conductances in apical H⫹-K⫹-ATPase membrane. Am J Physiol Cell Physiol 246: C537–C545, 1984. 1182. Yamada T, Soll AH, Park J, Elashoff J. Autonomic regulation of somatostatin release: studies with primary cultures of canine fundic mucosal cells. Am J Physiol Gastrointest Liver Physiol 247: G567–G573, 1984. 1183. Yamaguchi T, Chattopadhyay N, Kifor O, Brown EM. Extracellular calcium [Ca2⫹(o)]-sensing receptor in a murine bone marrow-derived stromal cell line (ST2): potential mediator of the actions of Ca2⫹(o) on the function of ST2 cells. Endocrinology 139: 3561–3568, 1998. 1184. Yamaguchi T, Chattopadhyay N, Kifor O, Butters RR Jr, Sugimoto T, Brown EM. Mouse osteoblastic cell line (MC3T3–E1) expresses extracellular calcium (Ca2⫹o)sensing receptor and its agonists stimulate chemotaxis and proliferation of MC3T3-E1 cells. J Bone Miner Res 13: 1530 –1538, 1998. 1185. Yamaguchi T, Chattopadhyay N, Kifor O, Ye C, Vassilev PM, Sanders JL, Brown EM. Expression of extracellular calcium-sensing receptor in human osteoblastic MG-63 cell line. Am J Physiol Cell Physiol 280: C382–C393, 2001. 1186. Yamauchi M, Yamaguchi T, Kaji H, Sugimoto T, Chihara K. Involvement of calciumsensing receptor in osteoblastic differentiation of mouse MC3T3–E1 cells. Am J Physiol Endocrinol Metab 288: E608 –E616, 2005. 1187. Yang F, Brune JL, Naylor SL, Cupples RL, Naberhaus KH, Bowman BH. Human group-specific component (Gc) is a member of the albumin family. Proc Natl Acad Sci USA 82: 7994 –7998, 1985. 1188. Yang F, Luna VJ, McAnelly RD, Naberhaus KH, Cupples RL, Bowman BH. Evolutionary and structural relationships among the group-specific component, albumin and alpha-fetoprotein. Nucleic Acids Res 13: 8007– 8017, 1985. 1170. Woodrow JP, Sharpe CJ, Fudge NJ, Hoff AO, Gagel RF, Kovacs CS. Calcitonin plays a critical role in regulating skeletal mineral metabolism during lactation. Endocrinology 147: 4010 – 4021, 2006. 1189. Yang W, Friedman PA, Kumar R, Omdahl JL, May BK, Siu-Caldera ML, Reddy GS, Christakos S. Expression of 25(OH)D3 24-hydroxylase in distal nephron: coordinate regulation by 1,25(OH)2D3 and cAMP or PTH. Am J Physiol Endocrinol Metab 276: E793–E805, 1999. 1171. Wortsman J, Pak CY, Bartter FC, Deftos L, Delea CS. Pathogenesis of osteomalacia in secondary hyperparathyroidism after gastrectomy. Am J Med 52: 556 –564, 1972. 1190. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 296: 2947–2953, 2006. 1172. Wrenn RW, Biddulph DM. Parathyroid hormone-induced calcium efflux from isolated renal cortical tubules: evidence for cyclic AMP mediation. Mol Cell Endocrinol 15: 29 – 40, 1979. 1191. Yao X, Karam SM, Ramilo M, Rong Q, Thibodeau A, Forte JG. Stimulation of gastric acid secretion by cAMP in a novel alpha-toxin-permeabilized gland model. Am J Physiol Cell Physiol 271: C61–C73, 1996. 1173. Wright MJ, Sullivan RR, Gaffney-Stomberg E, Caseria DM, O’Brien KO, Proctor DD, Simpson CA, Kerstetter JE, Insogna KL. Inhibiting gastric acid production does not affect intestinal calcium absorption in young, healthy individuals: a randomized, crossover, controlled clinical trial. J Bone Miner Res 25: 2205–2211, 2010. 1192. Ye CP, Yamaguchi T, Chattopadhyay N, Sanders JL, Vassilev PM, Brown EM. Extracellular calcium-sensing-receptor (CaR)-mediated opening of an outward K⫹ channel in murine MC3T3-E1 osteoblastic cells: evidence for expression of a functional CaR. Bone 27: 21–27, 2000. 1174. Wu G, Burzon DT, di Sant’Agnese PA, Schoen S, Deftos LJ, Gershagen S, Cockett AT. Calcitonin receptor mRNA expression in the human prostate. Urology 47: 376 – 381, 1996. 1193. Yergey AL, Abrams SA, Vieira NE, Aldroubi A, Marini J, Sidbury JB. Determination of fractional absorption of dietary calcium in humans. J Nutr 124: 674 – 682, 1994. 1175. Wu S, Grieff M, Brown AJ. Regulation of renal vitamin D-24-hydroxylase by phosphate: effects of hypophysectomy, growth hormone and insulin-like growth factor I. Biochem Biophys Res Commun 233: 813– 817, 1997. 1176. Wuster C, Raue F, Meyer C, Bergmann M, Ziegler R. Long-term excess of endogenous calcitonin in patients with medullary thyroid carcinoma does not affect bone mineral density. J Endocrinol 134: 141–147, 1992. 1177. Wyatt MA, Jarvie E, Feniuk W, Humphrey PP. Somatostatin sst2 receptor-mediated inhibition of parietal cell function in rat isolated gastric mucosa. Br J Pharmacol 119: 905–910, 1996. 1178. Xu H, Zhao H, Tian W, Yoshida K, Roullet JB, Cohen DM. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinasedependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J Biol Chem 278: 11520 –11527, 2003. 1194. Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16: 391–396, 1997. 1195. Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med 124: 519 –526, 2011. 1196. Yu PL, Fujimura M, Hayashi N, Nakamura T, Fujimiya M. Mechanisms in regulating the release of serotonin from the perfused rat stomach. Am J Physiol Gastrointest Liver Physiol 280: G1099 –G1105, 2001. 1197. Yue L, Peng JB, Hediger MA, Clapham DE. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature 410: 705–709, 2001. 1198. Zacharias DA, Kappen C. Developmental expression of the four plasma membrane calcium ATPase (Pmca) genes in the mouse. Biochim Biophys Acta 1428: 397– 405, 1999. 1179. Xu J, Henriksnas J, Barone S, Witte D, Shull GE, Forte JG, Holm L, Soleimani M. SLC26A9 is expressed in gastric surface epithelial cells, mediates Cl⫺/HCO3⫺ exchange, is inhibited by NH4⫹. Am J Physiol Cell Physiol 289: C493–C505, 2005. 1199. Zaidi M, Chambers TJ, Gaines Das RE, Morris HR, MacIntyre I. A direct action of human calcitonin gene-related peptide on isolated osteoclasts. J Endocrinol 115: 511–518, 1987. 1180. Xu J, Song P, Miller ML, Borgese F, Barone S, Riederer B, Wang Z, Alper SL, Forte JG, Shull GE, Ehrenfeld J, Seidler U, Soleimani M. Deletion of the chloride transporter Slc26a9 causes loss of tubulovesicles in parietal cells and impairs acid secretion in the stomach. Proc Natl Acad Sci USA 105: 17955–17960, 2008. 1200. Zanello LP, Norman AW. Rapid modulation of osteoblast ion channel responses by 1alpha,25(OH)2-vitamin D3 requires the presence of a functional vitamin D nuclear receptor. Proc Natl Acad Sci USA 101: 1589 –1594, 2004. 1181. Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology 136: 1317–1327, e1311–1312, 2009. 1201. Zanner R, Hapfelmeier G, Gratzl M, Prinz C. Intracellular signal transduction during gastrin-induced histamine secretion in rat gastric ECL cells. Am J Physiol Cell Physiol 282: C374 –C382, 2002. Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org 267 SASCHA KOPIC AND JOHN P. GEIBEL 1202. Zavros Y, Fleming WR, Hardy KJ, Shulkes A. Regulation of fundic and antral somatostatin secretion by CCK and gastrin. Am J Physiol Gastrointest Liver Physiol 274: G742–G750, 1998. 1214. Zhao CM, Chen D, Yamada H, Dornonville de la Cour C, Lindstrom E, Persson L, Hakanson R. Rat stomach ECL cells: mode of activation of histidine decarboxylase. Regul Pept 114: 21–27, 2003. 1203. Zavros Y, Shulkes A. Cholecystokinin (CCK) regulates somatostatin secretion through both the CCK-A and CCK-B/gastrin receptors in sheep. J Physiol 505: 811–821, 1997. 1215. Zhao CM, Jacobsson G, Chen D, Hakanson R, Meister B. Exocytotic proteins in enterochromaffin-like (ECL) cells of the rat stomach. Cell Tissue Res 290: 539 –551, 1997. 1204. Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M. Synthesis of 1,25-dihydroxyvitamin D3 by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol 13: 621– 629, 2002. 1205. Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 86: 888 – 894, 2001. 1206. Zehnder D, Evans KN, Kilby MD, Bulmer JN, Innes BA, Stewart PM, Hewison M. The ontogeny of 25-hydroxyvitamin D(3) 1alpha-hydroxylase expression in human placenta and decidua. Am J Pathol 161: 105–114, 2002. 1207. Zeng N, Athmann C, Kang T, Lyu RM, Walsh JH, Ohning GV, Sachs G, Pisegna JR. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Invest 104: 1383–1391, 1999. 1208. Zeng N, Kang T, Lyu RM, Wong H, Wen Y, Walsh JH, Sachs G, Pisegna JR. The pituitary adenylate cyclase activating polypeptide type 1 receptor (PAC1-R) is expressed on gastric ECL cells: evidence by immunocytochemistry and RT-PCR. Ann NY Acad Sci 865: 147–156, 1998. 1209. Zeng N, Kang T, Wen Y, Wong H, Walsh J, Sachs G. Galanin inhibition of enterochromaffin-like cell function. Gastroenterology 115: 330 –339, 1998. 1216. Zhao G, Simpson RU. Membrane localization, Caveolin-3 association and rapid actions of vitamin D receptor in cardiac myocytes. Steroids 75: 555–559, 2010. 1217. Zhong Y, Armbrecht HJ, Christakos S. Calcitonin, a regulator of the 25-hydroxyvitamin D3 1alpha-hydroxylase gene. J Biol Chem 284: 11059 –11069, 2009. 1218. Zhou AT, Assil I, Abou-Samra AB. Role of asparagine-linked oligosaccharides in the function of the rat PTH/PTHrP receptor. Biochemistry 39: 6514 – 6520, 2000. 1219. Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int 74: 170 –179, 2008. 1220. Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR. Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab Invest 82: 1755– 1764, 2002. 1221. Zhuang X, Adipietro KA, Datta S, Northup JK, Ray K. Rab1 small GTP-binding protein regulates cell surface trafficking of the human calcium-sensing receptor. Endocrinology 151: 5114 –5123, 2010. 1210. Zeng N, Walsh JH, Kang T, Wu SV, Sachs G. Peptide YY inhibition of rat gastric enterochromaffin-like cell function. Gastroenterology 112: 127–135, 1997. 1222. Zierold C, Mings JA, DeLuca HF. Parathyroid hormone regulates 25-hydroxyvitamin D(3)-24-hydroxylase mRNA by altering its stability. Proc Natl Acad Sci USA 98: 13572–13576, 2001. 1211. Zhang JX, Fasciotto BH, Darling DS, Cohn DV. Pancreastatin, a chromogranin Aderived peptide, inhibits transcription of the parathyroid hormone and chromogranin A genes and decreases the stability of the respective messenger ribonucleic acids in parathyroid cells in culture. Endocrinology 134: 1310 –1316, 1994. 1223. Zietkiewicz E, Labuda M, Sinnett D, Glorieux FH, Labuda D. Linkage mapping by simultaneous screening of multiple polymorphic loci using Alu oligonucleotide-directed PCR. Proc Natl Acad Sci USA 89: 8448 – 8451, 1992. 1212. Zhang W, Na T, Wu G, Jing H, Peng JB. Down-regulation of intestinal apical calcium entry channel TRPV6 by ubiquitin E3 ligase Nedd4–2. J Biol Chem 285: 36586–36596, 2010. 1224. Zornitzer AE, Bronner F. In situ studies of calcium absorption in rats. Am J Physiol 220: 1261–1266, 1971. 1213. Zhang Z, Sun S, Quinn SJ, Brown EM, Bai M. The extracellular calcium-sensing receptor dimerizes through multiple types of intermolecular interactions. J Biol Chem 276: 5316 –5322, 2001. 1225. Zuo Q, Claveau D, Hilal G, Leclerc M, Brunette MG. Effect of calcitonin on calcium transport by the luminal and basolateral membranes of the rabbit nephron. Kidney Int 51: 1991–1999, 1997. 268 Physiol Rev • VOL 93 • JANUARY 2013 • www.prv.org