Membrane Protein Structure & Func2on: Ac2ve Transport Importance of conforma-onal change in
advertisement

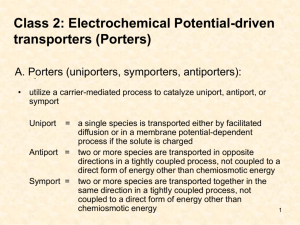
Membrane Protein Structure & Func2on: Ac2ve Transport Importance of conforma-onal change in transporter ac-vity Structure – Dynamics – Func-on Membrane proteins of known structure h=p://blanco.biomol.uci.edu/Membrane Proteins xtal.html h=p://www.drorlist.com/nmr/MPNMR.html Outline • Basics of membrane protein structure and differences from soluble proteins. • What is a transporter? Requirements for transport ac-vity. • Examples of mul-drug resistance transporter structure, dynamics and mechanism – Known mul-drug resistance transporter structures from each family, or close rela-ves – What can we observe/infer about how these proteins move in order to perform ac-ve transport • What’s leP to discover? – Common themes and differences between the known structures and mechanisms KcsA – First Crystal Structure Doyle et al. (1998) Science Vol. 280. no. 5360, pp. 69 ‐ 77 Diacyl Glycerol Kinase – Largest NMR Structure Van Horn et al. (2009) Science Vol. 324. no. 5935, pp. 1726 ‐ 1729 Rhodopsin: 7TM helices Palczewski, et al. (2000) Science: Vol. 289. no. 5480, pp. 739 – 745 Membrane Protein Structure •Dis-nct hydrophobic and hydrophilic domains required to interact with the lipid bilayer plus the aqueous environment on either side. •Even more important to sa-sfy hydrogen bonds in the hydrophobic membrane environment. Regular secondary structures. •Curved and kinked helices (& charged/polar residues) in TM domains play func-onal roles. Gly and Pro residues oPen found in conserved mo-fs. (GXXG, GXXXG, etc.) •Posi-ve inside rule: more Arg + Lys on cytoplasmic side of protein. Challenges with membrane protein structural biology: • Crystallography requires crystalliza-on…. – OPen low resolu-on – Crystalliza-on condi-ons? Detergents good for crystalliza-on may not be the best for func-on. – Lipid cubic phase tricky to work with – Use of an-bodies – FAB‐fragment assisted crystalliza-on • Solu-on NMR (and many biophysical methods) require a solubilized sample – Detergents are non‐ideal – Chemically different, highly curved – Ideally want li=le bilayer patches • Solid‐state NMR can work with oriented bilayers, bicelles, and vesicles but currently lacks resolu-on (problems with big, dynamic proteins). Landau & Rosenbusch PNAS 93:14533 (1996). Solubilizing Membrane Proteins Ac2ve Transporters: Crea2ng and Maintaining Gradients • Ionic and chemical gradients across membranes are cri-cal for life: ATP synthesis, nerve cell polariza-on and signal transmission, muscle contrac-on, maintenance of healthy intracellular condi-ons, etc. • Energy required: – ATP hydrolysis (primary ac-ve transporter) P‐type ATPases use 1/3 of our total energy. Examples: Na+/K+ ATPases, H+ ATPases, Ca+ ATPases – Coupling to an electrochemical gradient created by a primary ac-ve transporter (secondary ac-ve transporter) • Common mo-f: Dimer or pseudo‐dimer structure forming two halves of the molecule. Moving Molecules Across the Membrane Channel: open or closed Transporter: Only open to one side at a 2me Basic model of required features for channels and transporters, first proposed in the 1960s. Transporters & Channels Channel: open or closed •No energy input: Only flow down a concentra-on gradient •Free diffusion when pore is open – fast flow, 107 ions per second! •Ga-ng and selec-vity control what & when •Exact mechanism of opening/closing varies Transporter: Only open to one side at a 2me Transporters & Channels Channel: open or closed Transporter: Only open to one side at a 2me •Energy required: Can move molecule UP a concentra-on gradient •Only n molecules moved per transport cycle – rela-vely slow (102‐105 molecules per second) •2 sources of energy: 1) primary, ATP‐driven 2) secondary, use electrochemical gradient •Mechanism of opening/closing and coupling to energy source? Many transporters are alike… •4 phylogene-cally dis-nct families, no obvious sequence homology. •different func-ons: transport drugs, sugars, amino acids. Mul2drug Resistance Transporters • • • • • • Need to extrude drugs “uphill” against a concentra-on gradient Bacteria: mostly secondary ac-ve transporters in mul-drug resistance (proton gradient across inner membrane) Eukaryotes: mostly primary ac-ve transporters in mul-drug resistance Most recognize a wide range of hydrophobic, including drugs and lipids. Also implicated in transport of virulence factors and compounds important for quorum sensing. Creates an ideal environment inside the cell (low, non‐zero drug concentra-on) for evolu-on of other, stronger resistance mechanisms. Closely related transporters protect cells from toxic compounds and move lipids to the correct membrane. Drugs enter the cell: •Hydrophobic drugs may diffuse into the cell •Uptake through other channels or transporters? Transporters pump the drugs back out Mul2drug Resistance Transporters • ABC: ATP binding casse=e – couple ATP hydrolysis – Common eukaryo-c MDRs – Responsible for chemotherapeu-c resistance in human cancers • MFS: major facilitator superfamily – Couple proton gradient to drug export – 25% of all transport proteins, 12‐14 TM domains • SMR: small mul-drug resistance – Couple proton gradient to drug export – Very small, just over 100 amino acids, 4 TM domains • RND: resistance nodula-on division – Couple proton gradient across bacterial inner membrane to drug efflux across outer membrane • MATE: mul-an-microbial extrusion – Couple either proton or sodium gradient to substrate export Cartoon Models of Each Family Outer membrane RND Periplasm Membrane fusion protein MFS/SMR ABC Inner membrane ATP hydrolyzed RND or MFS Sobzcak & Lolkema Curr. Op. Microbiol. 8:161‐167 (2005) Borges‐Walmsley, et al, Biochem. J. 376:313 (2003) Primary ac2ve transport: ATP driven • ABC family of mul-drug resistance proteins 2 nucleo-de binding domains Ex. Sav1866 (bacterial), p‐glycoprotein, MDR1 More common in eukaryotes ‐ drug resistance in chemotherapy May be importers or exporters, roles in immune system, uptake of essen-al nutrients, ex: CFTR – Problem: only 1 structure of a mul-drug resistance protein, don’t know the structures of all relevant states of any family member – – – – • P‐type ATPases – Set up ionic gradients – use 1/3 of our total energy – 3 domains (N, A, P) interact with nucleo-de – Sarcoplasmic re-culum Ca2+‐ATPase: only primary ac-ve transporter where we have structures of mul-ple major intermediates • Both types have nucleo-de/motor domain dis-nct from TM transport pathway, believed to have similar coupling between the motor and TM domain conforma-on changes ABC transporters ATP binding Murakami, Curr. Opin. Struct. Biol. 18:459‐465 (2008) ATP hydrolysis Sav1866 Outward‐facing conforma-on open to the extracellular side of the membrane Consistent with solvent accessibility data, substrate access, mutagenesis and cross‐ linking studies in ATP‐ bound state (note mixed‐ monomers in TM “wings”, unlike lipid uptake ABC transporters) ADP bound But believed to be the ATP‐ bound conforma-on Dawson & Locher Nature 443:180‐185 (2006) Opening extends into bilayer – hydrophobic substrate may escape into outer bilayer leaflet. Sarcoplasmic Re2culum Ca2+‐ATPase conversion of ATP hydrolysis to TM domain conforma2onal change 1ATP hydrolyzed, 2 Ca2+ transported to SR lumen, 2H+ counter‐transported Dephosphoryla-on, open to cytoplasmic side, allowing Ca2+ binding TGES mo-f of A‐domain ATP binds, occluded conforma-on formed All structures be=er than 3Å resolu-on ATP hydrolysed, protein phosphorylated, occluded conforma-on forms ADP leaves, A‐domain swings, pulling TM domains open to lumen, releasing Ca2+ Olesen et al, Nature 450: 1036‐1042 (2007). ATP hydrolysis/protein phosphoryla2on N ADP released, A‐domain (yellow) swings in to replace N‐ domain (red) P A Ca2+‐ATPase: the movie in snapshots RND transporters Murakami, Curr. Opin. Struct. Biol. 18:459‐465 (2008) RND family: AcrB • Periplasmic loops confer substrate specificity • Structures solved by mul-ple groups in different crystal forms show same results • Func-onally rota-ng mechanism: three different states in each subunit of the trimer, the full mechanism revealed in a single crystal • Structure solved bound to different substrates – mul-ple drugs recogni-on revealed Func2onally Rota2ng Mechanism: AcrB The en-re complex Murakami, Curr. Opin. Struct. Biol. 18:459‐465 (2008) Func2onally Rota2ng Mechanism: AcrB 2.9 Å resolu-on access binding Seeger, et al, Science 313:1295 (2006) extrusion Func2onally Rota2ng Mechanism: AcrB 2D, 1K AcrB: Mul2drug Recogni2on minocycline doxyrubicin Mul-ple sites in a big hydrophobic binding pocket Murakami et al, (2006) Nature 443, 173‐179. MFS, SMR & MATE transporters Murakami, Curr. Opin. Struct. Biol. 18:459‐465 (2008) MFS, SMR & MATE families • Alterna-ng access model • Mostly TM, no big soluble domains – SMR: 4 TM helices, ≈110 amino acids – MFS: 12‐14 TM helices (2 bundles of 6 TM helices form core structure), ≈400 amino acids – MATE: 12 TM helices (2 bundles of 6 TM helices) similar to MFS family in size • Very common – not just mul-drug resistance, but MFS proteins transport sugars, amino acids, etc. – Only two MFS structures known: LacY (lactose permease), GlpT (glycerol‐3‐ phosphate transporter) • First MATE structure published in October 2010 – Export secondary metabolites important in host defense as well as xenobio-cs. – Widespread like MFS family. Think about the energeCcs involved in driving transport in this way Secondary transport: symport, an2port, and uniport Forrest & Rudnick (2009) Physiology, 24, 377‐386. Uniport A A A net transport A Symport Only doubly‐substrate‐bound and apo states undergo conforma-onal exchange B A B A B net transport A A A An2port Only single‐substrate‐bound states undergo conforma-onal exchange A A A net transport B B B MFS family: EmrD •Not really open to either side of the membrane. •True intermediate state? Or just trapped by crystal packing? Yin, Science 312:741 (2006). Alterna2ng access – an2port : GlpT Inward‐facing conforma-on, no substrate bound, 2 Arg face in toward ac-ve site, 3.3 Å resolu-on Lemieux, et al. Curr. Opin. Str. Biol. 14:405‐412 (2004) GlpT: What’s the other state? Two halves rocked rela-ve to each other to predict the open‐out conforma-on Lemieux, et al. Curr. Opin. Str. Biol. 14:405‐412 (2004) Alterna2ng access model: LacY Proton/lactose symport Abramson et al, Science 301:610‐615 (2003) Apo LacY at 3.0 Å resolu2on All structures are of C154G mutant, WT does not readily crystalize – C154G stabilizes inward‐facing conforma-on All known MFS crystal structures are of inward‐facing states or intermediate states. LacY: what’s the other state? Crystal structure with sugar bound (lactose analog TDG) at 3.6 Å, yellow residues show increased NEM labeling upon substrate binding Abramson et al, Science 301:610‐615 (2003) Model based on 60 degree rota-on (rocking) of curved helices, compa-ble with accessibility & cross‐linking data for other state LacY: what’s the other state? DEER (four‐pulse double electron‐electron resonance) measurements of distances in spin‐ labeled LacY Smirnova, et al, PNAS 104:16504‐16509 (2007) Distance changes upon sugar binding Blue/green – no sugar Red/purple‐ sugar LacY: what’s the other state model based on DEER measurements Open‐inward, no sugar bound Smirnova, et al, PNAS 104:16504‐16509 (2007) Open‐outward, sugar bound MATE: NorM from Vibrio cholerae 50Å 50Å It looks like an MFS transporter! cytoplasm cytoplasm He, et al. Nature 467: 991 (2010). NorM from Vibrio cholerae Note cleP open to the outer leaflet of the bilayer. He, et al. Nature 467: 991 (2010). NorM mechanis2c model Proposed transport mechanism. In the outward‐facing conforma-on, a ca-on (yellow) binds at a conserved site (blue oval; step 1). Ca-on binding induces structural changes to the inward‐ facing conforma-on (step 2), which is competent to bind substrate (organic ca-on in green) from the inner membrane leaflet or cytoplasm. Substrate binding causes structural changes back to the outward‐facing conforma-on (step 3), allowing export and ca-on binding. He, et al. Nature 467: 991 (2010). EmrE: SMR family • Small, minimal transport model • Single‐site alterna-ng access model – Glutamate residue cri-cal for ac-vity, E to D mutant alters pKa of transport – Aroma-c residues in ac-ve site key to aroma-c ca-on transport • Accessibility, cross‐linking, mutagenesis studies used to create mode • An-porter: 1 polyaroma-c ca-on per 2H+ EmrE: the structure? An-parallel homodimer? 3.5 Å resolu-on, crystallized from nonylglucoside Asymmetric, even in lipids Chen, Yen‐Ju et al. (2007) Proc. Natl. Acad. Sci. USA 104, 18999‐19004 7Å/16Å resolu-on, DMPC Ubarretxena‐Belandia, et al EMBO J 22:6175 (2003) FRET confirms an2parallel topology within the dimer Spectrum of EmrE Saturated with the Tight‐Binding Substrate TPP 45 °C pH 7.0 DMPC/DHPC≈0.33 Total lipid ≈ 400mM 15N (ppm) 0.5mM EmrE 1.5mM TPP+ 1H (ppm) 20m NaCl, 20mM potassium phosphate TPP+ Transport by EmrE Is peak doubling due to assymetric structure or slow interconversion? + + Characterizing Slow Conforma2onal Exchange by NMR ZZ‐exchange spectroscopy 1. Label ini-al chemical shiP 2. Mixing -me: wait tens of milliseconds Did the protein convert from state A to state B? 3. Label final chemical shiPs Determining the Exchange Timescale Starts and ends in same state Conforma-on changes kf = kr = 5 ± 0.5 s‐1 EmrE: the Movie What’s lei to study? • Biochemical data (cross‐linking, solvent & substrate accessibility) shows big conforma-onal changes during transport process. • Both structure and dynamics of transporters needed to really understand how they func-on: we need be=er movies, not just morphed structures. • Limited resolu-on of known structures. • Not all conforma-onal states crystallized for most cases. • Very li=le quan-ta-ve dynamics -ed to structural data – how fast is interconversion (apart from substrate on/off) – how is interconversion triggered (molecular mechanism) – Lack of structural data makes it difficult to design and analyze quan-ta-ve dynamics experiments in terms of structural change • We want a detailed mechanism – how can we target mul-drug efflux transporters to avoid an-bio-c resistance and chemotherapy resistance?