Chapter 11 A mathematical model of microbial and chemical
advertisement

Chapter 11
I
A mathematical model of microbial and chemical
oxidation-reduction processes in the Scheldt estuary
by
G. BILLEN and J. SMITZ
Based on work by Y. ADAM 3, G. BILLEN 1,2, M. HOENIG 1 , Cl. JOIRIS 1 , J. LEFEVRE 2 ,
Y. RUNFOLA 3 , J. SMITZ3 •
1. Laboratoire de Chimie industrielle, U.L.B.
2. Laboratorium voor Ekologie en Systematiek, V.U.B.
3. Groupe de Mecanique des Fluides geophysiques, U. Lg.
Introduction
The deterioration of ehemieal and biologieal properties of natural
waters by domestie pOllution is not the direet eonsequenee of the presenee
of an organie load in the water, but is rather the result of heterotrophie baeterial aetivity which modifies oxidation-reduction characteristics of the water while degrading this charge. Because other oxidants
than oxygen can be used by heterotrophie mieroorganisms (anaerobie
respirations), a eomplete oxidation-reduction budget is necessary to
deseribe correctly thc evolution of the chemical composition of the water
under microbiological influence. The classical models of river pollution,
using oxygen as thc only chemieal state variable, are confronted with
serious dlffieulties as soon as anaerobie mlerobial metabolisms occur.
70
Fe ++r-----------------------------
-..
Mn ++
JULY 1974
J,lm
02
N0
pm
J,lm
3
250
20
200
200
150
i"\
I
,
, ,
,
I
, NO;
\.
I
10-
-
_100
I
\-
-!
100
\
\
\
50
\
o2
FEBRUARY 1974
20
/', -,,_ •
J
., J..'
200
....... "
\
\
\
200
\
\
\
\
\
\
r'-'--
,
, .......
J
100
, .... _ 1
10
,.,./
,
km
1 00
Dendermonde
100
.. _.." "
50
o
Sea
fi g. 1.
Longitudinal profiles of oxidation-reduction compounds in the Scheldt Estuary.
- 71 Fe++ .-_-:-
-.
~
Mn++
Na~
02
"0.0: ,<0#"\.
).Im
aerOBER 1974
\
).Im
pm
\
----/"'
20
.200
'.
'.,
\
,
\
\
\
...
200,
.'~.-.-',
\
150
'\
"
'\
"
"
\
"-,
\
10
_100
\
\
\
100.
50
~
......
~
Fe++
w
••
• __ •
• __
/
/S04-
APRIL-MAY 1974
;
20
/
10000
;'
/
. 200
!
I
i
i
200."
!
I
I
i
i
_ 100
10
100 _
\
.........
, / .... ,::;;,,,--.•,,,,.,.:::.--_...
--
---~
c"..- -- ....-
--'
'- .....-.--~ .....
··-·-·-·-·-·-·-·r·-·-·-·-·-·---·-·-·-·km
100
Dendermonde
50
o
Sea
fig. 1.
Longitudinal profiles of oxidation-reduction compounds in the Scheldt estuary.
- 72 ~ork
This
baete~ial
presents an oxidation-reduction model of the influence of
~ater
aetivity cn
ecmposition, applied to the ease of the
Seheldt est'\;.ary'. Basie!:',lly the same phenomena oeeurs in natural water
systems custaining an intenac heterotrophie aetivity, such as bottom
water of
stra~ified
basins [Riehards (1965)J or interstitial water of
sediments [Thorstenson (1970)J. With a few modirieations, the same model
eould be applied to these eases.
1.- Experimental results
Typieal longitudinal profiles of concentration of several oxidationreduetion
eompo~~~s
are shown in fig. 1 for winter and summer situations.
Note abrupt transitions between zones where a given substance is redueed
or oxidized. Aeeording to these profiles, it is possible to define an
upwards zone (whith ehlorinities less than
(° 2
, r-ln 4+,
Im; ,
2
e
Cl/i) where oxidants
Fe+++, S04-) are suceessively reduced, and a downwards
zone (with ehlorinities higher than
2 g Cl/i) where oxidants are rege-
nerated in the opposite order. The reductive stage proceeds lass far in
the winter than in the summer : for instanee, reduced iron does not
appear in the winter.
Dark 14C- biearbonate incorporation, an index of heterotriphic bacterial activity has been
de~er~ined
along several longitudinal profiles
of the Scheldt (rig. 2). Again, t'.w zones can be distinguished : intense
heterotrophie activity occurs upwards, approximately down to tbe point
where
2 g/i
ehlorinity is reached, und abruptly vanishes downwards.
m~ch
Bacterial activity is
less intense in the winter than in the summer;
an empirieal relation with water temperature
has been found : The
is proportional to
~ean
~
T
and river diseharge
d
upwards heterotrophie bicarbonate ineorporation
10 T/ d
•
2.- General principles of the model
The above mentioncd results are easily interpreted : The Seheldt
EstuarJ is heo.vily polluted upwards Antwerp by important amounts of
- 73 14C_bi carbonate
incorporation
(ILMC/9..h)
i;
January 1973
October 1973
November 1973
February 1974
May 1974
July 1974
Odober 1974
1I
I,
I,
,
f
I
\
I
I(
,,
}I
:, ,'\
I' \
1
2
, I' \
:!: 1
III 1/'
.' \ ,,' \
I \ ;1 :
\ '! 1
I
q :
I
11 I
I
lfi:
\
/
I
I
,
:
:,
:
I
I
I
I
/
/
,,-- ......
/
/
II
/"
~
I
I
I
I
.,
,
I
I
1
I
1
I
I
I
I
r
f
I
I
I
,
I
I
I
I
I
I
,
1
I
I
I
I
I
I
I
I
I
,
I
I
I
J
I
I
I
I
/
I
I
:
, '
:
/
I
l
\
: ,"
I
1I
I
I
I
\
", I
1
II
l
\
I
I
/\
I
I
I
I
,
I
.
I
I
I
J
,
I
I
I
0.1
o
km from the sea
120
100
t
50
o
R
fig. 2.
Dark 14 C-bicarbonate incorporation along a profile of the Scheldt, at different seasons.
- 74 -
organ~c
reatter. Owing to the intense bacterial activity) the redox
potential decreases from Dendermonde (km 120) to about Antwerp (km 80)
Oxygen is rapidly entirely depleted, and other oxidants are used by
Then~
anaerobic metabolisms.
near
km 70 , owing to increasing salinity,
floculation and precipitation of thc suspended organic matter occur
[Wollast (1973)J, bacterial activity falls down and a phase of recuperation begins, accelerated by mixing with unpolluted sea water. During this
stage, reaeration not only restores the
o~·gen
concentration but also
regenerates thc previously used oxidizing agents.
~ll
these phenomena will be simulated by a model in which measured
values of bacterial activity are introduced as a command parameter modifying the composition of thc watcr without disturbing the thermodynamic
internal equilibrium between all thc involved oxidation-reduction couples.
Thc variations in the concentration of thc considered chemical
compounds are the result of advection, turbulent diffusion or consumption
by oxidation reduction processes :
a
ät
xi + u(s,t)
adS
xi
= D(s,t)
2
a
-:2
xi - Ci(s)t)
dS
where
t
is the time,
concentration of the
water,
s
i
th
~s
the longitudinal coordinate)
species)
u
xi
~s
the
is the residual velocity of the
D is thc turbulent diffusion coefficient,
Ci
is the rate of
oxidation-reduction consumption.
The residual velocity of the water has been calculated as the
quotient of the residual outflow [the seasonal variation of which is
given by the measurements published by the An~erpse Zeediensten (1966)J
to the wet section area [reported by Wollast (1973) to fit a logarithmic
fonction of the distance to the mouthJ.
For the turbulent di ffusion coefficient, phenomenological values,
integrating the effects of the tides were calculated according to the
method described by Wollast (1973).
- 75 -
This one dimensional hydrodynamic treatment is admittedly very
simplistic. However, its ability to simulate the observed longitudinal
profile of chlorinity for each cruise has been shown to be satisfactory.
The oxidation reduction processes , represented by the term
Ci - ,
are controlled by heterotrophie bacterial activity in the water. For
oxygen, an additional aeration term occurs. It was taken as proportional
to the oxygen saturation deficit, witll a proportionality coefficient
1
about 6 x 10- 3 h- , as estimated by Wollast (1973).
K
The bacterial activity can be viewed as an electron flux imposed
to the water system, according to the reaction
bact.
~
By writing this equation, we assumed that, from an overall point of view,
fermentations play no important role, i.e. that no accumulation of uncompletely oxidized organie compounds (acids, cetones or alcohols) occurs.
For organic acids, this has been experimentally confirmed.
This electron flux,
H(s,t) , provocates one or several of the
following reactions to go on
+ 02 + 4 H+
a)
4e
b)
8 e - + NO; + 10 H+
"
""T
c)
2 e
+ Hn0 2 + 4 H+
""T
d)
2 H20
-.-
"-
.L_
e - + Fe(OH)3 + 3 H+
Fe++ +
d' )
e)
14 e
so that
H(s,t)
nco;
.<-r
NH+ + 3 H 0
4
2
Hn++ + 2 H20
Fe++ + 3 H20
"
~
FeC0 3 + H+
+ 2 S04"- + Fe++ + 16 H+
=
L
i
vi Ci(s,t)
"""T
FeS 2 + 8 H20
.
Some of these reactions are biologically mediated and the presence of the
responsible organisms has been demonstrated in the Scheldt (oxygen consumption, denitri fication , nitrification, sulfato-reduction), others occur
spontaneously although direct biological mediation can occur in certain
- 76 circumstances (iron oxidation and reduction, manganese oxidation and
reduction). However this distinction in the reaction nechanisms is not
essential for the overall
bal~,ce
we are doing.
To portion out the total electron flux
H(s,t)
imposed to the
system by heterotrophic activity between the different microbiological
or chemical electron consuming pathways, thc
internal
therm~dynamic
ass~~ption
is made that an
equilibrium is achieved in thc system for reactions
a) to c). This imposes that five simultaneous cquilibrium relations of
the general form
Ox·l .
Eh = E~ + RT log Red •
1
hold. This is equivalcnt to saying that the bacteria only use thermodynamicallY favourable half reactions in their energy yicldir.g metabolism.
Given a set of limit conditions (composition of thc watcr at
Rupelmonde and at thc seal, the knowledge of the total bacterial activity
H(s,t)
would allow to calculatc the consumption term
Ci(s,t)
for each
oxidant considered, and hence thc complete evolution of the water composition along a longitudinal profile of the estuary. Direct introduction
of bacterial activity as a command parameter would allow to take implicitcly into account a lot of phenomena that it would be difficult to
considcr explieitely, such as lateral import of organie matter, modification of the bacterial population with salinity, etc. According to
Romanenko (1964), total carbon mctabolisn rand hence electron flux
H(s,t)] can be evaluated from dark bicarbonate incorporation measurement
by simply multiplying it by a constant factor
H(s,t)
=4 x
(total
C metabolism)
= 4a
n:
x (bicarbonate incorporation) •
However, critical examination of the literature [Romanenko (1964),
Sorokin (1965), Overbeck (1974)J shows that this factor
n
can vary
greatly with the bacterial species, the growth phase, the quality of
the substrate, etc. Because of the lack of a precise value of
considering that
n
n , and
can vary because of changes in the baeterial commu-
nity, we treated it as an ajustable parameter which can have different
values from one seaSon to the other.
- 77 -
3.- Mathematical resolution
Ta summarize preceeding discussion, the problem posed is to calculate the concentrations
concentrations
Yi(s,t)
Xi(s,t)
of the considered oxidants and the
of corresponding reduced form, by solving
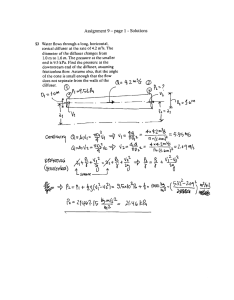
following system of equations :
i) 5 transp_ort-diffusion
If V'Ms,t)
equation_~
represents the operator
o
ät
0
02
+ u(s,t) ~ - D(s,t) os2
the equations are
(2)
V'LlX i ( X,t )
where the subscript
(3)
j
\lLlX.
J
= 2,5
= K(X 1 sat -
X1 ) - C1 (s,t)
refers to
°2
.
= Cj(s,t)
(i.e. NO; , Mn0 2 ' Fe(OH)3 , 80-4 -)
ii) oue-relation on the C 1
.'s( 4)
iii)
=
H(s,t)
2 logarithmic
and
5
E
i-1
v.1 C.
•
1
Eh
relations between a sixth variable
and the
X.'s
1 --
-
Y. 's
___ 1_
These
logarith~ic
relations enable to express the concentrations
of oxidants as a function of the redox potential
case, the function
Eh
.Q,i
= a·
1
Eh • In the general
has the form
X·
+ b.1 log
_1
Yi
(see fig. 3) where
Yi refers to the concentration of the reduced form
of the oxidant considered. (Note that if the oxidant or the reductor is
asolid species, the corresponding value of
Xi
the preceeding relation must be unity ar zero.)
For the reduced form
( 6)
Yj
,
we have
or
Yi
to be used in
- 78 By sumnang this equation with the corresponding equation (3) for the
xj 's
, we have :
vt.Z j ( s , t) = 0
if
ZJ':: X
,+ Y J, •
J
i.e.
Vt.(Im; + Im;}
m(Mn0
2
=0
+ Mn++}=0
m[Fe(OH}3 + Fe++
+ FeC0 3 + FeS 2 ] = 0
Vt.(SO;- + 2 FeS 2 } =
The maximum possible value of
mUID
0 •
Xi(x,t}
1S of course
Zi(s,t} • The ID1n1-
6
10- moles/~ , which is approximated to zero. This
considered is
divides the redox potential range into three domains for every oxidant
g1ven by the relation
X.1
:Eh
=
Eh > L B
,
The numerical method used to solve the system utilizes time and
space discretisation and, at each time step, the following sequential
procedure is used :
i) solution of the four equations (7) for
ii} computation of the limits
in the
Eh
LA(s,t}
and
domain.
iii} computation of an associated variable
( 8)
F(s,t}
=
5
E
i =1
v· X.
1
1
Zj(s,t) ;
Ls(s,t}
far every oxidant
log C
CH4
HS-
CO 2
NO-
-3
NW4
-----------T--------, 0
o
\ O2
V
2
\
Sreck}
2
\(Sato)
Fe (OH)3
FeS 2
FeC03
\
\
\
-5
Mn++ \
Mn02
\
\
-6
\
\
\
\
\
\
\
-7
-..;j
\()
\
\
I
\
\
\
\
\
\
-8
\
\
\
\
\
\
-9
\
\
\
\
\
0
.7
.6
.5
.4
.3
.,
.2
0
-.
,
fi 0. 3.
Eh -
C0
nc ,- nt
rat i end i
ii 9 r CI m
f 0" t""
W CI
t·:,. at Ru f"o 1 e, 0 n 1,., •
-.2
- .3
Eh (V)
- 80 for which the following equation holds :
Vb.F( s ,t)
= \) 1
K( x 1
sat
- X1) - H( s ,t )
As this equation contains oxygen concentration explicitely, the expression
(X 1
sat
step
-X 1 )
(if
~s calculated using the value of
X1
at the preceeding time
X1 varies slowly) or by an iterative scheme over points
3
and 4.
The discretization of transport-diffusion equations leads to tri-
di agonal form
- A.1 F.1 + 1+ B.1 F.1 - C.1, F.1- 1= D.1
which is then solved by recurrent algorithm [Adam and Runfola (1971),
Adam (1975)].
i v) Solution of an implicit relation
This relation allows to calculate the redox potential
Eh(s,t)
~~d
the
concentration of the oxidants Vla the logarithmic relations
4.- Results and discussion
The present discussion will be limited to the simulation of the
profile of February 1974, as an illustration of the capabilities and the
limitations of the model used. Most of the discussion will be devoted to
the validity of the assumption made that an internal equilibrium
1S
achieved in the system between all the redox species considered.
If it is assumed that perfeet internal equilibrium is achieved in
the system for reactions a) to e), the following relations must hold:
a)
Eh
= 1.26
- 0.059 pR + 0.015 log O2
- 81 -
b)
Eh
c)
Eh
Eh
= 0.882
= 1.229
= 1.057
- 0.074 pR + 0.007 log N03/NR~
- 0.118 pR - 0.029 log
- 0.177 pH - 0.059 log Fe
d' )
log Fe++ - log Reo; + pR
for
- 0.215 < Eh < 0.053
e)
Eh
= 0.354
l~++
=-
+ 0.008 log 80
++
0.286
4- -
0.067 pR + 0.004 log Fe++
These relations are diagramatically represented in figure 3 for the gross
chemical composition of the water at Rupelmonde.
Figure
4 shows the calculated profile obtained with this assumption.
Fe++ . - - - -
--,
Mn++
02
NO;
FEBRUARY 1974
_ 200
20
/-100
10 .
1
100
,
/'•.......
I
.- __ _...•.•__
_..
_-_
-
.•._..•...-
-........... ..._.._
/
.
., /
-
....
100
km
Denderl'1onde
.
o
5'0
Sea
fi g. 4.
Calculated profile for February 1974, obtained with a model assuming a
perfeet internal thermodynamic equilibrium.
Comparison with the experimental profile shows that the general trends of
the evolution of the water composition are reproduced by the simulation.
- 82 -
However~
oxygen concentration increases much later in the calculated pro-
file. Clearly this is the consequence of our assumption of perfeet internal equilibrium. With this assumption, oxygen can only begin to 1nNH~
crease after nil
and all
Mn ++ have been completely oxidized. To
explain the observed profiles, kinetical limitations must be invocated
the rate of the chemical and microbiological reactions involved is not
sufficient for an internal thermodynamic equilibrium between the
~fu++
/Mn02'
02/H20
NH:/NO;,
redox couples to be achieved.
~~E~!~~~_~i~~~~~_~2~~!_!2E_2~g~~
4.2.-
It is possible to obtain a quite good simulation (see fig. 5) of
the observed profile by expressing the oxygen consumption as a kinetical
Fe++ ,
Mn++
-,..
l
FEBRUARY 1974
.......................
.~
.~
20_~
.~
~N03
/~02
~
I
\
~
./
200
I
I
,
I
I
I
I
t
10 -
I
~--------..
!
I
_100
100 -
{
I
._
-
_ •••-
••;
';'.~'.
"" I _. :'::
•••
•
./.. ••••• >0 •••
~.~
o
km
100
Dendermonde
Sea
fig. 5.
Calculated profile for February 1974, obtained with a model using an empirical kinetic term far oxygen consumption.
term not immediately depending on
Eh , but proportional to the concentra-
tion of reduced forms of the other redox couples
- 83 -
In this treatment, only equations
assess~ng
Eh
b) to
and the relation on the
H(s,t)
5
= j E=1
Cj + k
e) are considered for
Ci 'C'"l
5
E
j =1
(Zj
becomes
- x.)
J
This treatment is not very satisfactory from the point of
v~ew
of the
microbiologist because it is only possible to justifY the particular
form given to the term representing oxygeu consumtion by assuming that
all heterotrophie aetivity is sustained by utilization of other oxidants
than oxygen, the lattcr only reaeting ehemieally to regenerate these
oxidunts. This is of course quite irrealistic, aerobic metabolisms
constituting always an important part of heterotrophie aetivity. However
the fact that this model does fit thc experimental profiles, while the
former does not, indicate that kinctical hindrances in the reactions of
02
with
NH~
and
Hn ++ must be somehow taken into ae count .
The first model, based on the assumption of thermodynamical equilibrium eould not aecount for two observations
i) Nitrate formation from amoonium is not eompleted before the beginn~ng
of oxygen eoneentration inerease.
This can be explaincd by the physiology of nitrir,ying bacteria. The
population of nitrifYing baeteria of thc Seheldt estuary are probably
made of fresh water
organ~sms
of terrestrial origin, carried down by the
river. As soon as thc convenient
Eh
value is reaehed, their aetivity
begins. Howcver, bccause of increasing salinity, they cannot divide rapidly
enough to counterbalance their dilution in sea water, so that nitrification cannot bc completcd [Billen (1975)J. A model ofnitrification in the
Scheldt cstuary, taking into account thc physiology of nitrir,ying baeteria is now in progress [Somville~ Billen and Vanderborght (in preparation)J.
- 84 ii) oxygen concentration increases before manganese oxidation begins
To
eA~lain
this discrepancYj slowness 01' bacterial action cannot
be invoked because manganese oxidation is a rapid spontaneous chemical
process. In this case, the kinetical hindrance with respect to thc
equilibrium model probably originates from the mechanisms 01'
Eh
control by oxygen. TIlis control has been discussed in detail by Sato
(1960) and Breck (1972), (1974). Both authors stressed that the chemical
reduction 01' oxygen is a multistep process in which oxygen peroxide is
an intermediate : summarizing
a' )
a")
The former 01' these processes is easy; the latter, involving rupture 01'
an
0-0 bond, is very slow. A definite non neglectable H202 concen-
tration can thus exist in natural oxygenated waters. A lot 01' chemical
spec1es (including oxidized forms 01' iron and manganese) readily react
wi th
~ 02
' decomposing i t back to oxygen and preventing further oxi-
dation 01' the solution by reaction alt). The stcady state so established
between
02
and
H2 0 2
is the effective way 01' control 01' the redox
potential. This implies that relation a) must be replaccd by thc corresponding Nernst equation for rcaction a'). The steady state concentration
01' H 0
has bcen estimated by Sato and Breck respecti vely to 10- 6
2 2
and
10 -11
. Nernst relat10ns
.
mo 1 es /n~ • The two result1ng
are represented
diagramatically in figure 3 (dotted lines).
Introduction 01' these relations in the model and comparison with
preceeding data (experimental and calculated) will allow to estimate
influence 01' oxygen peroxide process in thc control 01'
Eh
potential
in the Scheldt Estuary.
References
ADAN, Y., RUNF'OLA, Y.,
(1971). NwnencaZ ResoZution of diffusion equation,
Rapport N.9, Progr. Nat. Environnement Physique et Biologique,
Projet Her.
- 85 -
ADM!, Y. ~ (1915). A Hermitian finite differenee method for the solution
of paraboUe equations:; to be published.
AHTWERPSE ZEEDIENSTm1, (1966). Stormvloedcm op de Sehelde:; Ministerie
van openbare werken; Bestuur der waterwegen.
BILLEN, G., (1915). Nitrification in the Seheldt Estuary (Belgium and the
Netherlands), Estuarine and Coastal Harine Scienc:e" J:; 19-89.
BRECK, W.G.; (1912). Redox potentials by eQuilibration, J. MaI'.
121-139.
BRECK, W.G., (1914). Redox potentials in the
ed., yol. 5, I-liley, New York.
sea~ ~n
Res.~
30~
The Sea s Goldberg
OVERBECK, J., DALEY, R.J., (1913). Some preeautionary eomments on the
Romanenko tcehniQue for estimating heterotrophie bacterial produetion, BuU. Ec:ol. Res. Comm. (Stockholm) 17~ 342-344.
RICHARDS, F.A., (1965). Anoxie basins and Fjordß~ in Chemic:al oc:eanography" Vol. 1, 611-645~ Eds. Riley, S.P. and Skirrow, G., Aeademic
Press, New York.
ROHAHENKO, V.1., (1964). Heterotrophie C02 assimilation by baeterial flora
of water, Nikrobiol.~ 33" 119-683.
SATO, M., (1960). Oxidation of sulfide ore bodics, 1. Geochemical ennronments in terms of Eh and pHi Ec:on. ('reol. s 55 s 928-961.
SOROKIN, Y.I., (1965). On thc trophic rolc of chemosynthesis and baeterial
synthesis in water bodies, Nem. Ist. Ital. Idrobiol. s 18 SuppZ. 3
187-205.
THORSTENSON, D.C., (1970). EQuilibrium distribution of small oreanic
moleeules in natural waters:l Geoehim. Cosmoc:him. Ac:tas 34 s 145-110.
WOLLAST, R., (1973). Origine et mec:anicrrlec de Z'envacement de l 'estuaire
de l'Eseaut~ Rapport de synthece~ Recherche effectuee dans le cadre
dc l'Gtude de l'cnvasement de l'Eseaut dirigee par le Laboratoire
dc Recherehes HydrauliQues, Borgerhout, 11inistere des 'Travaux
Publies.
WOLLAST, R., (1913). Circulation s acc:umulation et biZan de masse dans
l 'estuaire de l 'Eseaut" in Modele mathematique de la pollution en
mer du Nord3 Rapport de synthese 1972 3 co~nässion interministerielle
de la politique scientifique (Beleium) .
.