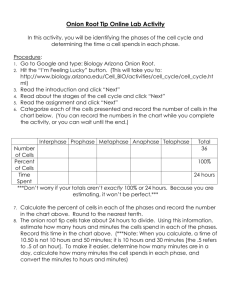
AN ABSTRACT OF THE THESIS OF
advertisement

AN ABSTRACT OF THE THESIS OF Danai Boonyakiat in for the degree of Horticulture Title: Master of Science presented on j July 16, 1980. Factors Affecting Storage of Yellow Globe Danvers Onions: Abscisic Acid During Maturation and Storage and Phenolic Substances Inhibitory to Botrytis allii and Botrytis cinerea Abstract approved: _ n f . Daryl G. Richardson ; Free abscisic acid (ABA) in Yellow Globe Danvers onion tissues was determined during maturation and storage. The amount of ABA in leaf tissue reached a maximum (1.81 ng/gm dry weight) at 40% top fall. Neck tissue ABA reached a maximum (0.33 ng/gm dry weight) at 60-70% top fall. ABA of bulb tissue slightly increased during maturation, then declined and remained steady (0.21 ng/gm dry weight) during storage with a slight increase again after 5 month storage. onion bulbs began to sprout. Bulb tissue ABA decreased when Some preliminary data indicates that bulb ABA concentration may be positively related to respiration rate. A study of phenolic constituents in bulb inner and dry outer scales, found that major phenolics are quercetin 0 and 26,750 yg/gm dry weight; chlorogenic acid 416.7 and 937.5 yg/gm dry weight; catechin 458.3 and 812.5 yg/gm dry weight; catechol 41.7 and 312.5 yg/gm dry weight; caffeic acid 141.7 and 62.5 yg/gm dry weight; and arbutin trace and absent in inner scale and outer scale, respectively. Mycelial growth and spore germination of ji. allii and B^. cinerea ::were inhibited by catechol concentration above 50 ug/1. The mechanism of inhibition appears to be different in the two species of Botrytis. Preliminary study on bulb weight loss at 3 different storage temperatures: 0°, 10° and 20oC showed that weight loss was 3, 5 and 10%, respectively, after 6 months storage. Onions with dry outer scales removed developed new dry outer scales in 4 weeks at room temperature, and room temperature with air circulation. Factors Affecting Storage of Yellow Globe Danvers Onion: Abscisic Acid During Maturation and Storage and Phenolic Substances Inhibitory to Botrytis allii and Botfytis cinerea by Danai Boonyakiat A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science Completed July 16, 1980 Commencement June 1981 APPROVED: Associate^rofessor of Horticulture in charge of major v v "jr v Head of Department of Horticulture Dean of Graduate School AJ- Date thesis is presented July 16, 1980 Typed by Larky Hansen for Danai Boonyakiat ACKNOWLEDGMENTS I would like to extend thanks to Dr. Daryl G. Richardson and Dr. Mary Powelson for their aid and guidance throughout my program and to Carol Standley for her very competent technical assistance. I would like to extend special thanks to the Anandhamahidol Foundation for support during my graduate program. TABLE OF CONTENTS I. II. III. IV. V. VI. VII. Introduction 1 Literature Review 2 Free Abscisic Acid Concentration in Onion Tissues During Maturation and Storage: Abscisic Acid and Respiration Rate 16 Materials and Methods 17 Results and Discussion 21 A Simple Method for Characterization and Quantitation of Onion Phenolic Compounds 26 Materials and Methods 28 Results and Discussion 30 Effect of Catechol on Mycelial Growth and Spore Germination of Botrytis allii and jB. cinerea 3A Literature Review 35 Materials and Methods 37 Results and Discussion 39 Effect of Storage Temperatures on Monthly and Cumulative Weight Loss of Onion 51 Dry Outer Scale Regeneration 57 Bibliography 59 Appendix 71 LIST OF FIGURES Figure 1 2 3 Percentage sprouting and hormonal changes of onion bulbs cultivar Rijnsburger.stored at 5-80C 12a ABA concentration in Yellow Globe Danvers onion tissues during maturation and storage 24a A. Regression line of respiration rate and ABA concentration of eight onionrvarieties 25a Regression line of respiration rate and ABA concentration of five onion varieties 25a Peak area weight relative to individual phenolic standards at various concentrations 33a Spore germination of Botrytis allii over time at 20 C relative to catechol concentration in the medium 47a Spore germination of B^. cinefea over time at 20 C relative to catechol concentration in the medium 48a Colony diameter of B^. allii (after 4 days) and j5. ciherea (after 2 days) incubated at 20oC relative to catechol concentration in the medium 49a Cumulative percent weight loss during 6 months storage of Yellow Globe Danvers onions 54a Percent weight loss per month during 6 months storage of Yellow Globe Danvers onions 55a B. 4 5 6 7 8 9 Page LIST OF TABLES Table 1 2 3 4 5 6 Page Comparison of respiration rate and ABA.concentration of eight onion varieties after 1 month storage at 0oC 23 Rf values of authentic standards and the major phenolic compounds in onion scales 29 The concentrations of extractable phenolic compounds in onion inner and outer scale tissues 32 The effect of catechol concentration on percent of spore germination of B^. allii after 15 hrs and IS. ciherea after 8 hrs incubation at 20oC 44 The effect of catechol concentration on colony diameter of B^. allii and 15. cinerea after 4 and 2 days, respectively, incubated at 20oC 45 Slope of regression line for different concentrations of catechol and B^. allii and B^. cinerea spore germination 46 LIST OF APPENDICES Appendix Page 1 Flow diagram for free ABA extraction from onion tissue 71 2 Free ABA concentration in onion tissues during maturation and storage 72 Comparison of respiration rate of eight onion varieties after 8 months storage at 0oC 73 ABA concentration and respiration rate of eight onion varities after 1 month storage at 0oC 74 IR spectrum of unknown phenolic with Rf = 0.86 in BAW and Rf = 0.74 in 2% acetic acid 75 3 4 5 6 7 A. Unknown B. Protocatechuic acid C. Catechol D. Catechol sample Chemical structures of major phenolics constituents of Yellow Globe Danvers onion 76 Comparison of respiration rate of eight onion varieties after 8 months storage at 0oC 78 Factors Affecting Storage of Yellow Globe Danvers Onion: Abscisic Acid During Maturation and Storage and Phenolic Substances Inhibitory to Botrytis allii and Botrytis cinerea INTRODUCTION The serious problems for storage onions are sprouting and storage diseases. Sprouting reduces onion storage life and diseases cause substantial storage losses for the growers. These studies examine the conditions that may help control onion sprouting and neck rot disease. Abscisic acid (ABA) may be involved in controlling sprouting and catechol, a plant phenolic compound, may inhibit growth of neck rot fungus. These studies characterize: ABA concentration in onion tissues during maturation and storage; 8 onion varieties with respect to ABA content, sprout elongation, and respiration rate; the effect of storage temperatures on weight loss; a preliminary experiment on dry outer scale regeneration; individual quantities of the major phenolic concentrations in onion inner and dry outer scales; and finally, the effect of catechol on radial growth and spore germination of Botrytis allii (onion neck rot fungus) and Botrytis cinerea (grey mold fungus). LITERATURE REVIEW Onion (Allium cepa L.) is an economically important crop throughout much of the world (47, 48). lily family (Liliaceae) (47) . The genus Allium is placed in the The onion has a complex life cycle. It is generally a biennial, although some forms, such as the tree onions are perennials (48). Growth in the first year is normally vegetative with a day-length induced bulb formation (6, 47). day and short-day types. There are both long- The Yellow Globe Danvers and Sweet Spanish varieties grown in Oregon are long-day types. In the second year, flower production is stimulated by environmental factors. When these are favorable, the shoot apex ceases to produce leaf primordia and initiates the inflorescence (6, 7, 47). The onset of bulbing is controlled by conditions of day-length and temperature and characterized by a rapid elongation of leaves due to an extension of the neck region of the sheath. When the leaf sheaths are swollen as a result of cell expansion rather than cell division, new.leaf blades cea.se to form. to swell to form storage tissue (6, 7). The scale leaves continue At the center of the bulb, the last 5-6 leaves are initials during the growing season and will renew growth as shoot sprouts after the resting period has ended in storage or as overwintering bulbs (47). As bulbing progresses, leaf blades cease to emerge and the older leaves begin to become senescent from the tip downwards. Maturation is characterized by a loss of turgidity in the upper part of the sheath causing the top to fall down while still green due to softening of the neck region. Older leaf blades and roots die off as maturity progresses (1, 6, 7, 47, 48, 88). A. Varieties. There are two general types of onions grown in the United States as dry bulbs, the pungent type and the mild type. inantly long day types. class: Both are predom- Four distinct colors are recognized in each red, white, yellow and brown (47, 48, 88). The pungent type can store longer than the mild type (45). B. Chemical constituents. Onion bulbs consist of a number of carbohydrates in the dry matter, protein constitutes 1.4% and fat 0.2%. In 100 gm of raw bulb tissues there were 50 international units of vitamin A, 0.03 gm of thiamine, 0.04 mg of riboflavin, 0.02 mg of niacin, and 9.0 mg of ascorbic acid (47). A characteristic of most members of Allium sp. is their onion or allinaceous odor which is attributable to organic sulphur compounds (47). Volatile compounds that impart the flavour and odor of Allium spp arise from odorless precursors (S-alkenyl-L-cysteine sulphoxides) which yield equimolar quantities of thiopropanol-S-oxide, pyruvate and ammonia. Thiopropanol-S-oxide is the lachrymatory (tear-inducing) factor of fresh onions. This breaks down rapidly in the cut or bruised tissue to yield a mixture of volatile disulphides and thiosulphonates that are responsible for odor and flavour. The concentration of flavour components in onions is increased by subjecting the growing plants to water stress and can be reduced by growth in sulphur deficient conditions (6, 7). It is interesting to note that these compounds which are responsible for allinaceous odors and flavours are formed only 4 after the onion has been cut or injured. Heating and freeze-drying, which destroy or suppress enzymatic changes, reduce the formation of onion odor (47). C. Curing. The objectives of curing onions are to encouraged the development of natural dormancy, seal the neck and outer scales to prevent diseases and to form intact dry outer scales to reduce the respiration rate of bulbs (47, 85, 91, 98). A properly cured onion has a dried shrunken neck and several intact dry outer scales. The weight loss during the curing period should not normally be more than 5%. The curing period normally takes about 6-14 days under natural conditions (66). the curing period the scale color is developed (42). During The scale color of onions after harvest is affected by temperature and humidity during the curing period. The optimum tempefature for artificial curing is about 240-320C for 2-3 weeks with relative humidity about 50-70% (42, 47). The curing period is very important for long storage and long distance shipping. Field curing can be accomplished when the production area is not subjected to rainfall in the harvest period (91). After onions have been lifted, onions lie in windrows 10 days or longer until the tops are quite dry (46, 47, 98). The dry outer scales contain natural sprout inhibitors that are necessary for extended storage periods (98). Onions that were not cured before storage had increased neck rot during the storage period (91). Onions cured with tops intact generally lose water from the neck and foliage more rapidly than those that are topped at lifting (61). D. Storage. The storability of onion bulbs is limited by weight loss, storage diseases and sprouting (1). Once curing is accomplished, optimum temperature for storage of onions is at or near 0 C and RH between 60-70%. If relative humidity rises above 80% root growth is promoted and decay may also increase. Storage temperatures about 10-15 C increase sprouting by accelerating the breaking of dormancy (1, 42, 46) Under storage conditions onions lose water and dry matter and serious losses due to storage rots, sprouting and rooting can occur (46, 47). There are many kinds of storage but the most common methods are bulk storage and pallet box storage (66) .. Ventilation is very important in storage of onions, since ventilation removes heat and moisture which are by-products of respiration and transpiration (85). Onions which were overmature or were excessively dried in storage caused larger numbers of green "peeler" onions, loose scales, and are variable in color. tion. This problem is prevented by proper management of air circulaIf air circulation is inadequate to remove moisture and to re- duce temperature from respiration the entire lot of onions may be lost due to sprouting and rots (44). Respiration rate declines immediately after harvest. Respiration rates of dormant onion bulbs are very low compared with those of other stored vegetables and fruit. Carbon dioxide evolution measured on the same apparatus at 10 C was 8 mg (XL kg and 34 mg CO kg hr hr for onion and 18, 31 for stored beet root, carrot and cauliflower heads respectively (87, 98). Respiration rates slowly increase as the bulbs remain in storage and begin to sprout (99). But even though the 6 respiration rate of onion bulbs is much lower than that of most vegetable crops, the loss of dry matter can be important, because onions are often stored for long periods (47, 99). relative weight loss due to CO (See calculations of and moisture loss p. 52 ). Factors affecting dormancy and storage life. 1. Genetic factors: Onions of the globe type are noted for greatest storability while the Bermuda type can be stored only for a period of 1 to 2 months. The Spanish types appear intermediate in storage potential (47, 62, 88). There is a positive correlation between the length of the growing season and length of storage life in the comparison of cultivars. Char- acteristics which vary among cultivars have been shown to influence storability include differences in dry matter content, growth inhibitor content, rate of respiration, rate of moisture loss, outer scale quality and rate of maturation (6, 86, 102). Cultivars containing higher dry matter can store longer than those with low dry matter. This may be related to metabolic substrate reserves (6, 7, 29, 73). A low rate of respiration reflects high storage potential (80, 81). Ward and Tucker (99, 100) have suggested that the low respiration rate of dormant bulbs and the very small rise in respiration observed with increasing temerpatures may indicate that respiration is controlled by the dormancy system. Cultivars that have good quality of dried outer scales can be stored longer. Quality of dried outer scales refers to the number. 7 thickness, color and completeness of the scales. Intact dried outer scales reduce respiratory gas exchange and also reduce moisture loss from the bulb. Intact scales also prevent infection by pathogens (6, 47). 2. Conditions during the growing season: Onion storage behavior is affected by the conditions under which the crop is grown. Onions that are grown on soils with high organic matter have poorer keeping quality than those grown on lower organic matter soils. This loss of keeping quality is thought to be due to the effects of organic soils in delaying bulb maturation through the retention of both nitrogen and soil moisture (31, 33). Drinkwater, et al. (26) found non-irrigated onions matured faster than onions receiving heavy and frequent irrigation. However weight loss during storage was greater in non-irrigated onions than in onions from irrigated treatments. The effect of various levels of N and K on keeping quality is not clear'. However applications of N without K tend to decrease the keeping quality (53). Increasing rates of P fertiliza- tion increased number and thickness of dried outer scales of onion, which should improve storage life. good scale quality (52). Copper is essential for color and Leaf diseases, premature topping, and prema- ture desiccation may increase decay, shorten dormancy and storage life. Bulbs grown under shaded conditions sprouted sooner than onions grown under full light (6, 7). Topping generally resulted in physiological shrinkage and generally more rot loss and total loss. Topping flush with the bulbs usually resulted in an increased shrivelling of the 8 bulbs but rotting was generally less than for bulbs receiving topping with longer residual stems (38)• 3. Storage temperature and humidity: The most influential storage factor is temperature. The ideal temperature of onion storage is about 0 C or near 0 C with 60-70% relative humidity (66). Both low temperatures (0-2.5 C) and high temperatures (25-30 C) have been found to extend dormancy and storage life (7, 64, 63). Bulbs from the lower storage temperatures had the lowest weight loss and generally a more desirable appearance (104). Dormancy disappears most rapidly at temperatures in the range 10-12 C (7, 64). Onion bulbs stored at 0 C or 30 C had rates of leaf initia- tion about half that observed for storage at 15 C. slowest at 0 storage at 10 Sprouts emerged and 30 C whereas most rapid emergence occurred after and 15 C (1). Because the rate of respiration rises with increasing temperature a lower storage temperature is generally more desirable due to slower consumption of stored metabolites and reduced loss of moisture (90). Storage temperatures also affect seed production for the following growing season. onions stored at 7.5 Jones (46) found that and 11-12 C were the earliest to produce seed stems and also the first to bloom when compared with other temperatures. than 7.5 stalks. Mother bulbs stored at either higher or lower1 temperatures and 11-12 C produced many vegetative bulbs and few seed Storage of onions at a temperature near the freezing point, but which does not freeze the bulbs, reduced the losses from growth and decay during storage (12). Temperatures in the range 0-20 C show onion (cultivar 'Rijnsburger') respiration rate to be linearly related to temperature, the mean increase of respiration rate is 0.55 mg CO kg Relative humidity should be held between 65-75%. humidity encourages root growth and decay. hr C (96, 97). Too high relative On the other hand with relative humidity lower than 50%, the bulbs become too dry and dry outer scales shed, leaving the edible scales exposed to environment (80, 101). Air circulation must be sufficient to prevent heat accumulation in the onions and to remove moisture from within piles, bins or sacks. Air circulation about 2 cfm of air per cubic foot of onion at 1 1/4 inches of static pressure is sufficient for curing. After curing, 1 cfm of air per cubic foot of onions is recommended for storage ventilation and circulation (66). The usefulness of CA storage for onions is still questionable. On balance 5% CO and 5% 0 seems capable of reducing losses from sprouting, root growth and other disorders. But increasing the CO to 10% induces a disorder that resembles the translucent scale defect (81). 4. Growth Regulators: In recent years many growth substances have been used to prevent or delay sprouting in stored onions. Pre-harvest application of maleic hydrazide, properly applied in the field is the most effective method of preventing sprouting in stored onions (43, 44, 45, 47). method of using maleic hydrazide is to apply 2-3 lbs/acre when the The 10 plant is nearly mature and tops begin to fall but are still green (47). Application of maleic hydrazide is usually 10-14 days before normal lifting. Overmature bulbs in the field or bulbs cured before applying maleic hydrazide did not respond to maleic hydrazide very well (41, 43, 44). Maleic hydrazide treated onions did not have normal mitotic cell division, the cells enlarged without cell division although many prominent nuclei were observed. When untreated onions were sprouting, treated onions were beginning to show cell enlargement at the apex (44). In maleic hydrazide treated bulbs, endogesnous growth promoters remained at low levels and inhibitors persisted at a high level longer than in untreated bulbs (44). Regrowth in untreated bulbs was characterized by formation of sheath and leaf initials and rapid cell division. All growth promoters were at high levels of activity and inhibitors were absent. But maleic hydrazide treated bulb tissues did not develop into organs. Growth promoting activities were at low levels, while inhibitor activity was still high (13, 87). Injection with 1 ml of 100 ppm maleic hydrazide during bulb formation prevented sprouting and the effect could not be reversed by injection of equal quantities of IAA, GA or kinetin (44, 87). The application of maleic hydrazide will reduce or prevent sprouting in most varieties, but it will not make a poor keeping variety equal to a good keeping variety (45). Onions go into a rest period during the fall and early winter. During the rest period, onions will not respond to rapid changes of environment. Onions treated with maleic hydrazide go into the normal 11 rest period but do not come out of it. When onions have completed the rest period, they enter the dormant period. This dormancy is the physiologic internal type; during this dormancy onion bulbs will respond to changes of environment, slowly in the early dormancy and rapidly as dormancy is lost. In early dormancy for most storage varieties, the shoot sprout will be found in the lower half of the bulb. And in late dormancy onion shoot sprouts extend into the upper two thirds of the bulbs, the basal plate is swollen and the scales around the base are cracked. When cut transversely the basal plate shows several conspicuous rings of tan to dark brown root primordia. In the case of onions properly treated with maleic hydrazide these characteristics are not apparent (7, 43, 44). Maleic hydrazide prolongs dormancy by effects on nucleic acid synthesis and cell division rather than by a direct effect on the level of natural growth inhibitors and growth promoters in the bulb (7). The effects of maleic hydrazide were not overcome by application of auxin, gibberellin or kinetin or their combination (2). Maleic hydrazide does not, however, inhibit all expansion as onions treated with 0, 0.33, 0.67, and 1.0 gallon maleic hydrazide per acre at 10%, 25%, 50% and 75% top fall stages all had the same yield and bulb size distribution (Richardson 1978 unpublished data). Dormancy and sprouting are under control of the balance between endogenous growth inhibitors and promoters. Growth inhibitors are pro- duced in the leaves and then translocated into the bulb during maturation. Early removal of thn leaves prior to harvest or early chemical (desiccation) of leaves can shorten the dormancy period (7, 15, 24). 12 In the resting stage, growth inhibitors were shown (Figure 1) to be present in the study by Thomas and Isenberg (87). In the dormancy stage inhibitors decreased gradually until the onset of sprouting. Auxin and gibberellins increased around the beginning of the sprouting period, but auxin activity preceded gibberellin. They suggested that inhibition was controlled by interactions between natural auxins and inhibitors but sprout extension may be controlled by natural gibberellin. Cytokinin activity also increased prior to sprouting. Cytokinin may be necessary to stimulate rapid cell division before shoot extension. It is possible that auxin affects cell permeability enabling the translocation of food reserves which are necessary for sprout growth (87). Long dormant period varieties contain higher amounts of inhibitor than short dormant period varieties. In a study of dormancy of shoot apices, it was found that kinetin and sucrose were very effective in promoting growth of shoot apices. of the excised shoot apices. 1618 but not ABA. AMO 1618 and ABA can reduce growth But sucrose overcomes the effect of AMO Kinetin has slightly counteracted the effect of ABA and AMO 1618 (63, 64). Aung and Peterson (7) have shown that both dormant and sprouting bulbs contain free gibberellin-like substances. The total gibberellin of dormant bulbs was greater than that of sprouting bulbs. This seeming contradiction was explained by the degrees of onion bulb dormancy lie in the modes of mobilization and/or utilization patterns of gibberellin by the bulb in response to external stimuli. The lesser amount of gibber- ellin in growth while the converse may be the case with dormant bulbs. 12a Figure 1. Hormcnal regulation of onion bulb donnancy 49 Hormone activity / I ,^ y •.. i^y^~y\ ' ' / ^ •' . \.' .\ y / / / /1 /'•. ' '■•.. A/ \S /••** ^^^^"^^ ^""V ^S. ^^ AUXIN 'i / \J \ / .••'' / \ \ N. • *.// .I /•../ \ :' \ •' /f^~~^\ ': \\ \ •' \ f X. '■••. ^s- INHIBITOR ^Ss,','"»^ * * f-^*^^ r50 [ /GIBBERELLIN ^' I 1 ^^^.^ / 1 / % Sprouting [ ■—T" Nov. "1 Dec. / ■ Jan. ^^^ r— • Feb. i Mar. i Apr. 1 May Storage period I-ig. i. Percentage sprouting and hormonal changes of onion bulbs cv. Rijnsburgcr stored at s-s-c FROM, Thomas, T. H. and F. M. Isenberg. Hormone physiology of. onion tulbs during dormancy. Experimental Horticulture No. 23. (85) 13 Perhaps the high level of bound gibberellin and low level of free fraction are associated with sprouting while the high level of free gibberellin and low level of bound are associated with dormancy. Greater amounts of growth inhibitors can be extracted from late compared to a medium maturing onion bulbs and this suggests that the earliness of sprouting in onion may be associated with a decrease in the concentration of growth inhibitors (50). Applications of ABA to maturing onions induced early senescence of the leaves and prolonged the rest period of bulbs. This effect was partially overcome with the application of gibberellins, auxins, and cytokinins, and totally overcome by applying a mixture of the three hormones (2). Pre-harvest spray applications of NAA and 2,4,5-T can stimulate early sprouting in stored bulbs. The injection of leaf blades during bulb formation with 1 ml of 100 ppm IAA, GA and kinetin either singly or together can reduce storage life due to sprouting (2, 7). In the case of rooting, exogenously applied ABA at 10 inhibits the growth of onion roots. Concentrations of 10 M or 10 M M inhibit 90% growth after 48 hours, and 10_ M inhibit 50% of growth after 48 hours (89). In the cortical area of onion roots between 3-4 mm from the root tip, roots treated by ABA have smaller cells and are 2.6 times shorter than untreated ABA roots. ABA treated cells show that the thickness of the longitudinal cell wall is greater than the untreated cell (78). Levy et al. (56) found that adding ethephon to the growth medium caused swelling of leaf bases but ethephon also caused retarded leaf 14 growth and a reduction in the final size of bulbs. Ethephon induced bulbing of short day, intermediate, and long day onion cultivars under non-inductive short day conditions (57, 60). E. Storage diseases. Important onion storage diseases are neck rot caused by Botrytis allii and bacterial soft rot caused by Erwinia carotovora (45). In- fected seeds have been shown to be a major source of B^. allii in the onion crop. Benomyl applied to seed samples containing 1, 10 and 20% infection reduced the average neck rot percentage in stored bulbs from 26.2 to 3.8% (67, 68). Seed dressing of benomyl (applied as a dry pow- der or as a slurry) virtually eliminated infection from the seeds and produced crops of healthy onions which remained free of neck rot during storage (68). 15. cinerea can cause brown stain symptoms on dry outer scales (22). Walker (93, 94, 92) indicated that Botrytis spp can cause serious diseases in storage for onion besides B^. allii such as B_. squamosa, 1$. cinerea and B^. byssoidea. Pseudomonas cepacia Burkh. was described by Burkholder as capable of causing decay of onion bulbs (94). tissue. Kawamoto (49) found that P^. cepacia infected only wounded onion Irwin (40) described the most prevalent symptom which appeared in infected plants as a wilting and chlorosis of two or more lower leaves which is followed closely by appearance of elongated lesions emerging from the neck and extending up the adaxial side of one or more young leaves. Soft rots of onion in Lake Labish area are caused by P^. cepacia, I\ eichorii and Erwinia spp (40). Bruising during lifting, curing and storing onions and failure to cure onions before placing 15 them in storage increased neck rot and other diseases in storage (91). Fusarium basal rot caused by Fusarium sp is soil borne and is thought to invade through basal wounds or through old root scars (94). Walker and Maude (96) found the occurrence of Gliocladium roseum on mycelia and sclerotia of ]$. allii and in culture G^. roseum produced substances inhibitory to the growth of j5. allii. Storage diseases are incited by prolonged periods of high relative humidity and presence of water on plant surfaces with high temperature (47). 16 FREE ABSCISIC ACID CONCENTRATION IN ONION TISSUES DURING MATURATION AND STORAGE: ABSCISIC ACID AND RESPIRATION RATE D. Boonyakiat and D. G. Richardson 2 Department of Horticulture, Oregon State University, Corvallis OR 97331. Additional index words, Abstract. dormancy, Allium cepa Free abscisic acid (ABA) in yellow Globe Danvers onion tis- sues was determined during maturation and storage. The amount of ABA in leaf tissue reached a maximum (1.81 ng/gm dry weight) at 40% top fall and then declined rapidly. Neck tissue ABA reached a maximum (0.33 ng/gm dry weight) at 60-70% top fall, then declined as the neck tissues dried. ABA of bulb tissue increased to 0.31 ng/gm dry weight at 60-70% top fall, then declined and remained steady (0.21 ng/gm dry weight) during storage with a slight increase again after 5 months storage. Bulb tissue ABA decreased slightly when onion bulbs began to sprout.Some preliminary data indicates that bulb ABA concentration may be positively related to respiration rate. The amount of abscisic acid (ABA) in onion tissues during top fall and storage has riot been fully characterized with respect to dormancy and respiration. Mahotiere (63) found that 1 he concentration of inhibitors of onion variety MSU 4535 which is a long dormant period is higher than "Downing's Yellow Globe" which is an intermediate dormant Received for publication , 1980. Oregon Agricultural Experiment Station Tech. Paper Nb._ . From the M.S. dissertation of the senior author. 2 Graduate Student and Associate Professor of Horticulture, respectively. 17 type. He indicated the inhibitor resembled abscisic acid (ABA) but was not ABA itself. Earlier research (2, 7, 15, 24, 87) referred to inhibitors (implying ABA) to correspond to dormancy of onion. Leopold (55) indicated that ABA was associated with changes in dormancy of buds and seeds of trees and that ABA could be increased by osmotic stress and mineral deficiencies. Thomas and Isenberg (87) found that a growth inhibitor was present in onions but this decreased gradually until just before the onset of sprouting. IAA content increased to a very high level before the onions showed visible signs of swelling of the leaf bases, then fell off rapidly after 5-7 days (24). of IAA increased again before sprouting in storage (86). The level It seems that dormancy is controlled by a balance of ABA levels and other growth regulators such as IAA, gibberellins and cytokinins (87) rather than by a single hormone. The inhibitor in the bulb was later identified as ABA by Stow (83). The objectives of these studies are: to determine the amount of ABA in onion tissues with respect to dormancy during maturation and storage, and to compare 8 onion varieties with respect to ABA and respiration. Materials and Methods Extractions of free abscisic acid from freshly harvested Yellow Globe Danvers leaves, neck and bulb tissues were begun in August 1979. The method was based on that of Powell et al. (75). Fresh onion tissue were frozen and lyophilized in a Virtis Uni-trap model 10-100 freeze dryer. Five grams of lyophilized onion tissue was added to 50 ml of 18 methanol and ground at high speed for 2 minutes by Sorval omni-mixer and the methanol solution was separated from the tissue residue by suction filtration through Whatman no. 1 filter paper. The residue was washed with small amounts of methanol until the rinse was clear. Radioactive 1-C 14 -cis, cis - ABA 100 pi containing 10 4 dpm dissolved in ethanol was added as an internal standard. Powell's method was modified by partitioning against 50 ml of ethyl acetate 3 times instead of methylene chloride in the final extraction of the acidified (pH 2.5) aqueous phase. This procedure was found to be more rapid and to produce higher recoveries of free ABA in Douglas-fir needles (W. K. Ferrell et_ al., manuscript in preparation) . The aqueous phase was discarded and the ethyl acetate was taken to dryness by rotary vacuum evaporator. The residue was taken up in 2 ml of ethyl acetate and transferred into a small test tube and dried by a stream of air. The residue was esterified by adding ether-diazo- methane generated from Diazald (Aldrich Chemical Co. Inc., Milwaukie, Wise), dried in an air stream and diluted with 400 yl ethyl acetate. The methyl esterified ABA was separated and quantitated by injecting 0.5 ul into a 5750 Hewlett-Packard gas chromatograph equipped with a 122 cm x .02 cm glass column charged with 2% Epon Resin 1001 liquid phase on chromosorb WAW-DMCS 80/100 support (Analabs Inc., 80 Republic Dr., New Haven, Conn.) with oven at 205 C, injector heater 215 C and electron capture detector 290 C. Nitrogen carrier gas flow was 25 ml/ min. Extraction losses were monitored by the recovery of internal standard 1-C 14 -cis, cis-ABA. The methyl esterified ABA and internal 19 standard were then injected (0.5 yl) into the GLC for analysis and the remainder of the sample was quantitatively transferred (toluene rinse) to a liquid scintillation vial and 12 ml of 4 gm of Omnifluor in 1000 ml of toluene was added for counting in order to calculate extraction efficiency. Percent recovery was calculated by converting cpm (counts per minute) read by Beckman LS 7500 (Irvine, Ca.) liquid scintillation counter to dpm (disintegrations per minute) by the formula: , dpm = Then dpm of 1-C with dpm of 1-C 14 14 cpm x 100 Hr TT-.—: counting efficiency -cis, cis-ABA in the sample extract was compared -cis, cis-ABA standard added initially. The respiration rate of onion hybrid XP-H667 was lowest (7.83 mg C0„ kg hr ) and significantly different (P_<.05) from the other 7 hybrids and varieties. There were no significant differences in respiration rate among XP-H742 Sapporo Yellow Globe, Autumn Beauty, XP-W207 and XP-H348. The onion variety Yellow Globe Danvers had the highest respiration rate but was not significantly different from XPW209, XP-H348, XP-H195 (Table 1). The concentration of ABA in variety XP-H195 was lowest but there was no significant difference (P<^05) between XP-H742, Sapporo Yellow Globe, Autumn Beauty, XP-H667, and Yellow Globe Danvers. Onion variety XP-H348 contained the greatest amount of ABA but this level of ABA was not significantly different from variety XP-H207 (Table 1). The correlation between the level of ABA in onion bulb and respiration rate was very low (r = -0.02) Fig. 3A. This data suggests no relationship between amount of ABA and respiration rates of onion. 20 However, it is very interesting that when the data for XP-H195, Yellow Globe Danvers and XP-H667 were excluded, the very high ( r = 0.99) correlation coefficiency for the remaining 5 onion varieties respira- tion rate and ABA data indicates (Fig. 3B) that ABA may in fact have a relationship to respiration rate of onion. The number of samples in this study may however, be too small to strongly support such a conclusion, but the possible involvement is intriguing. Further study is clearly needed to elucidate the strength of interaction between ABA and respiration rate. Eight varieties of onion were used; XP-H702 (Sweet Spanish type), SP-H667 (Danvers type. Sweet Spanish background), XP-H195 (Yellow Globe Danvers, Ontario Pink root resistance) obtained from Asgrow Seed Company, Autumn Beauty, XP-W207 and XP-H348 obtained from Crookham Seed Company, Sapporo Yellow Globe obtained from Takii Seed Company and Yellow Globe Danvers from Salem area. All of these onions were grown under the same cultural practices in the same field. The method for C09 determination in respiration studies followed the Claypool and Keefer method (25) except a Beckman model 865 Infrared gas analyzer was used to quantify C0_ rather than the methylene blue dye reduction solution. Onion bulbs were weighed to about 1 kg and put in 1 gallon glass jars fitted with flow through tubes, flow rate was adjusted to 50 ml/min and read daily by flowmeter. The res- piration rate of each variety consisted of 9 observations. Following the respiration studies, onion bulbs were removed from the jar to determine the ABA content as described previously. 21 Results and Discussion Based on 1-C 14 -cis, cis-ABA recoveries, the average extraction efficiency was found to be 75% with a range from 71% to 80%. The following ABA results are reported as adjusted values due to the 75% extraction efficiency. Adjusted free ABA was calculated by: adjusted ABA = J jL— 0.75 The concentration of ABA in leaf samples increased dramatically as onion top fall progressed and ABA reached the highest concentration (1.81 ng/g dry weight at 50% top fall) (August 30). ABA concentration in the leaves then decreased very rapidly after top fall when onion leaves were senescing but had not yet dried. Levels of ABA in leaves thereafter decreased gradually to 0.53 ng/gm dry weight on September 10, 0.22 ng/gm dry weight on September 20, and 0.01 ng/gm dry weight on October 1. ABA content in completely dried leaves was very low (Figure 2). The ABA concentration in the neck tissue reached its maximum (0.33 ng/gm dry weight) at 60-70% top fall, then ABA levels decreased gradually to 0.27 ng/gm dry weight on September 20 and 0.15 ng/gm dry weight on October 1 (Figure 2). In onion bulbs the amount of ABA was lowest at the onset of top fall (0.04 ng/gm dry weight) when compared with ABA concentrations in leaf and neck tissues. ABA in the bulbs, increased to 0.31 ng/gm dry weight at about 60-70% top fall and thereafter decreased to 0.21 ng/gm dry weight during the curing period and stayed at this level until November. Although no radio-tracer experiments were used to confirm 22 movement of ABA to the bulb following the decrease in leaf ABA, there is the suggestion that there may have been ABA translocation from the leaves to the bulb. ABA apparently has very weak effect on dormancy of onion, perhaps because onion bulbs contain other inhibitors and all of them may affect dormancy of onion by synergistic effects. Kato (50) indicated that onion bulbs also contain a growth inhibitor called allyl sulphide. Some plants have phenolic inhibitors such as p-couma- rin and chlorogenic acid (55). Bulb tissue ABA reached maximum amount (0.31 ng/gm dry weight) after leaf ABA was declining and could be explained as ABA translocated from leaves. The concentration of free ABA which declined during storage could be due to free ABA being bound as abscisyl-3-glucopyranoside. Bound ABA might then be released as free ABA which could correspond to the second peak in February which declined again as sprouting occurred. 23 Table 1. Comparison of respiration rate and ABA concentration of eight onion varieties after 1 month storage at 0 C. Respiration rate ABA7 Varieties mg of CO /kg/hr ng/g dry wt. XP-H667 7.83 a 0..33 b XP-H742 9.74 b 0,.24 a 'Sapporo Yellow Globe1 9.73 b 0..27 a 'Autumn Beauty' 9.98 b 0..32 b XP-W207 10.70 be 0..46 c XP-H348 10.80 be 0,.49 c XP-H195 11.49 c 0,.23 a 12.55 c 0..25 a 'Yellow Globe Danvers' Mean separation by Duncan's New Multiple Range Test (P=0.05)values sharing the same letter are not significantly different. x: n=9 for respiration rates y: n=3 for ABA determinations 24 Figure 2. ABA concentration in Yellow Globe Danvers onion tissues during maturation and storage. BULB • LEAF o NECK D o SPROUT 09 C H (0 8/20 8/30 9/10 9/20 10/1 ll/l DATE 25 Figure 3A. Regression line of respiration rates and ABA concentrations of eight onion varieties. 3B. Regression line of respiration rates and ABA concentrations of five onion varieties. 25a 13 I* * Figure 3A R«-.02 5cr a. CO UJ 01 UJ _j CD < 9 cr 8 21 1.6 VARIABLE 2 O 36 26 ABA Figure 3B 12-2 © R=.99 6 • £IIS ™" Q. CO UJ cr UJ _i CD < 114 5 106 *■ - - • > • • 18 .2 2 .2.7 VARIABLE .32 ABA .36 26 A SIMPLE METHOD FOR CHARACTERIZATION AND QUANTITATION OF ONION PHENOLIC COMPOUNDS D. Boonyakiat and D. G. Richardson Department of Horticulture, Oregon State University, Corvallis OR 97331. Additional index words; caffeic acid, catechin, catechol, chlorogenic acid, quercetin, fungal inhibitors. Abstract. A study of phenolic constituents in onion bulb inner and dry outer scales was performed by a method combining paper chromatography and Densicord recorder trace to densitometrically measure the amount of phenolics. Major phenolic constituents (yg/g dry weight) found in inner and outer dry scales respectively were quercetin 0 and 26,750, catechin 458 and 812, catechol 41 and 312, caffeic acid 141 and 62, and a minor phenolic (arbutin or quercetin glucoside) trace and absent. There has been interest for some time in the possible involvement of phenolic compounds in mechanisms associated with plant resistance to fungal pathogens. Walker's pioneering work (92, 95, 97) on phenolic compounds relative to onion smudge resistance has attracted some interest, but there have been few follow-up studies aimed at specific pehnolic compounds which are found in tissues relative to their effects on fungal spore germination or hyphal growth. studied the effect of phenolics: Clark and Lorbeer (22) Quercetin, protocatechuic acid and catechol on mycelial growth and spore germination of Botrytis cinerea Graduate Student and Associate Professor of Horticulture, respectively. Received for publication , 1980. Oregon Agriculture Experiment Station Tech. Paper No. . From the M.S. dissertation of the senior author. 27 and Colletotrichum circinans. Pappelis (72) found chlorogenic acid to be fungistatic for 13. allii. A preliminary study (C.E. Bubl, unpublished research notes) indicated qualitative differences in chlorogenic acid, catechol and quercetin with respect to their inhibitory activities on 15. allii mycelial growth. Although no concentrations were specified, quercetin was not inhibitory, whereas chlorogenic acid was progressively inhibitory comparing a low and a high concentration, and catechol had a very strong inhibition at the single concentration used. It was decided therefore to attempt to further characterize the concentrations of individual phenolic constituents which might possess anti-fungal properties. Although the method described by Anderson and Todd (4) has been used to quantify total phenols in tobacco tissue by binding to polyvinyl pyrrollidone (PVP) no information is available as to the quantitative nature of this technique relative to individual phenolic types, which are known to exhibit different binding properties with PVP. Since chlorogenic acid was used as the standard and quercetin is the major phenolic in onion dry outer scales, there is a reasonable question as to whether the PVP binding method gives an accurate estimate of individual phenolic concentrations. Attempts to elute individual phenolic compounds from paper for quantitation leads to losses, either from binding or to differential oxidations. While there are limitations, the method is fairly simple, rapid and gives reasonable accuracy provided suitable standards are available. 28 Materials and Methods Dried outer scales and dried inner scales of stored Yellow Globe Danvers onion bulb tissue were ground to pass a 40 mesh screen. One gram of ground tissues were extracted by enclosing the sample in a filter paper envelope held by a steel clip and placed in a 50 ml conical flask containing 20 ml of gently boiling absolute methanol for 10 minutes. The envelope containing the sample was transferred to a Soxhlet apparatus and extracted with additional methanol for 18 hours. The methanol extracts were combined, concentrated, and adjusted to 25 ml final volume (18, 19). Known standards; quercetin, chloro- genic acid, catechol, catechin, caffeic acid, protocatechuic acid and arbutin (Sigma Chemical Company, St. Louis, Mo.), and portions of extract were paper chromatographed on 7.5 x 45 cm Whatman No. 1 in n-butanol: acetic acid: water (BAW) (4:1:2.2 v/v/v) as one elution system or by 2% acetic acid as a second system. Spots on chromatograms were marked under ultraviolet light of 266 nm, before and after spraying with a visualization reagent according to Bhatia et^ al. (9). Sprayed chromatograms were placed in a 70 C hot air oven for 5 minutes and Rf's of unknown spots were compared to Rf's of standards (Table 2). An unknown was tentatively assigned as arbutin based on the identical chromatographic behavior in two solvent systems compared with authentic arbutin. There is the possibility that the compound in question could also be one or more of the glucosides of quercetin. Standard concentrations of catechol, catechin, chlorogenic acid and caffeic acid were spotted on 7.5 cm x 45 cm Whatman No. 1 paper at 29 Table 2. Rf values of authentic standards and the major phenolic compounds in onion scales. 2% acetic acid B A W 1. Catechin 0.41 0.72 2. Catechol 0.74 0.91 3. Chlorogenic ac:id 0.58 0.59 4. Caffeic acid 0.31 0.74 5. Quercetin 0.01 0.87 6. Arbutin 0.86 0.74 30 5, 10, 25, 50, 100 yg. Four hundred yl extract (equal to 16 mg dry weight) of inner and dry outer scales of onion were spotted between the standards. Phenolic compounds were separated by descending chroma- tography in 2% acetic acid (18, 19). Paper chromatograms were sprayed with Bhatia's visualization reagent (9), then were cut into 23 x 3 cm strips. The amount of phenolic compound in each spot was densitome- trically measured by Densicord recorder trace (Photovolt Corp., New York City). The area under the recorder trace peak for each spot was cut out and weighed as this was proportional to the concentration used. Standard curves for each phenolic compound were developed from the relation between weight of area under peak and the concentration applied (Fig. 4). The amounts of the individual phenolics in onion samples were then estimated by comparison to the standard curve for that particular phenolic substance. Quercetin and onion phenolic extracts were spotted in the same amount as above but quercetin was separated by descending chromatography in (BAW) n-butanol, acetic acid, and water (40:10:22 v:v:v). The con- centration of quercetin in the onion tissues was estimated by the same method as above. Results arid Discussion The approximate amounts of major phenolics found per gram of onion dry outer scales are: quercetin 26,750 yg, chlorogenic acid 937.5 yg, catechin 812.5 yg, catechol 312.5 yg and caffeic acid 62.5 yg. The inner scales of onion contain the approximate amounts of the following phenolic compounds per gram dry weight: catechin 458.3 yg, chlorogenic 31 acid 416.7 yg, caffeic acid 141.7 yg and catechol 81.7 yg. No querce- tin was detected in the inner scales (Table 3). Jones (47) indicated that the major phenolic in dry outer scales of a brown onion was quercetin at about 4% on a dry weight basis. The Yellow Globe Danvers variety used here had 2.6% quercetin on a dry weight basis. K. P. Link et al. (58, 59) found 0.01% or less of onion outer scale dry weight was catechol, whereas 0.03% were found for Yellow Globe Danvers in this study. Pappelis (72) found chlorogenic acid synthesis was induced in exposed bulb scales of onions in the ambient atmosphere at 25 C for 48 hours. both the exposed and unexposed tissues. Caffeic acid was present in He reported 18 mg of chloro- genic acid per gram fresh weight and this amount was fungistatic for 13. allii. About 8 mg of caffeic acid was contained per gram fresh tissue, but this level of caffeic acid stimulated the germination of B. allii spores. Clark et al. (22) studied the role of phenolics in Botrytis brown stain of onion. They found only catechol inhibited growth of 15. cinerea and Colletotrichum circihans. Quercetin had no effect on these fungi. The inhibitory effects of catechol concentrations on spore germination and growth of Botrytis allii, and B^. cinerea are described in Boonyakiat Thesis (10). The amounts found in this study should be more than adequate for protection of onions from.neck rot disease.in storage. The dried outer scales of onions contain a higher concentra- tion than inner scales of all phenolic compounds except caffeic acid. This may explain why the dry outer scales protect the bulb from the invasion of Botrytis spp. and possibly other fungal pathogens. 32 Table 3. The concentrations of extractable phenolic compounds in onion inner and outer scale tissues. outer scale inner scale (yg/gm dry wt.) (yg/gm dry wt.) 1. Catechin 812.5 458.3 2. Catechol 312.5 41.7 3. Chlorogenic acid 937.5 416.7 4. Caffeic acid 62.5 141.7 5. Quercetin 6. Arbutin (on the glycosides of quercetin) 26,750.0 absent 0 trace 33 Figure 4. Peak area weight relative to individual phenolic standards at various concentrations. o CATECHIN - CAFFEIC ACID r1 CATECHOL • CHLOROGENIC A QUERCETIN ACID 6" S.4 < u < a Q. .1 0 IO 20 30 40 50 CONCENTRATION 60 70 80 ( jjg ) u> 34 EFFECT OF CATECHOL ON MYCELIAL GROWTH AND SPORE GERMINATION OF BOTRYTIS ALLII AND BOTRYTIS CINEREA1 2 3 4 D. Boonyakiat , M. L. Powelson and D. G. Richardson Department of Horticulture and Department of Botany and Plant Pathology, Oregon State University, Corvallis OR 97331. Additional index words: Allium cepa, neck rot, onion phenolics, onion disease resistance. Abstract. Catechol, a naturally occurring phenolic constituent of onions exhibits inhibition of the neck rot organism, Botrytis allii and may be inhibitory to other pathogens. Mycelial growth of ]5. allii and B^. ciherea were progressively inhibited by catechol concentrations above 50 and 100 Ug/1 respectively. Spore germination of B^. allii was in- hibited by catechol concentrations above 150 Pg/l and 50 yg/1 in the case of B^. ciherea. The mechanism of response to catechol inhibition appears to be different in the two species of Botrytis. Catechol has a greater effect on the length of time for maximum germination of B.* allii than for B^. cinerea. High concentrations (400 yg catechol/1) provided a clue in that visual oxidation of phenolics occurred in the presence of B. cinerea but not in the presence of B. allii. Received for .publication Experiment Station Tech. Paper No. tion of the senior author. 2 Graduate Student in Department of 3 Assistant Professor in Department 4 Associate Professor in Department ; 1980. Oregon Agricultural . From the M.S. dissertaHorticulture. of Botany and Plant Pathology. of Horticulture. 35 Literature Review Neck rot caused by Botrytis allii Munn. has been identified in many countries as one of the major bulb-destroying diseases of stored onions (93, 94, 97). after harvest. The disease is found most commonly on the bulbs Initial bulb symptoms are characterized as having a soft and watery texture. As the disease progresses this area enlarges, turns tan to brown and finally dark brown. At advanced stages of disease the tissue becomes spongy or corky and the bulb is light in weight. Mycelia often develop on the surface of the bulbs or between decayed bulb scales and black sclerotia can be observed on the surface of the rotted tissues (93, 94). Onion bulbs have been reported to contain the phenolic compounds 4,-glucocide, 3,4,-diglucoside and y^'-diglucosides of quercetin (36). Onion bulbs also contain protocatechuic acid approximately 1000 yg/gm of the dry scales and catechol, 1000-2000 yg/gm dry weight of scales (58, 59). Pappelis et al., 1976 found that onions exposed to 25 C for 48 hrs in ambient atmosphere resulted in the accumulation of about 18 mg of chlorogenic acid per gram fresh weight. to be fungistatic to ]J. allii (22). This amount, was found About 8 mg of caf feic acid was also found per gram fresh weight but at this level caffeic acid stimulated the germination of B^. allii spores (72) . Other phenolic com- pounds in onion scales were phloroglucinol, a methyl ester of protocatechuic acid, and ferulic acid from fleshy scales (47). Onion varieties resistant to the smudge disease caused by Colletotrichum circinans accumulated anthocyanins and simple phenolics such as protocatechuic acid and catechol in the dried outer scales of red 36 pigmented varieties. Protocatechuic acid and catechol inhibit germina- tion and penetration of fungi (28). Protocatechuic acid and catechol, two water soluble phenolic compounds present in dry outer scales of colored onions are not present in the scales of the white varieties. These two phenolics inhibit the infection by C. circinans and are toxic to fungi at dilutions up to about 1-3000 (5, 37, 47, 58, 59). Clark and Lorbeer (1974) indicated, that catechol inhibited growth of both B^. cinerea and C^. circinans. Mycelial growth of these two fungi was not inhibited by protocatechuic acid or quercetin at concentrations up to 600 yg/ml. In vivo, crushed, pigmented yellow onion scales inhibited germination of conidia of jB. cinerea and C^. circinans (22) . Onion, leaves, inoculated with spores of 15. allii, exhibited a sharp increase in flavonoids and chlorogenic acids, especially in the epidermal cells (84). Owen et_ al., 1949 (71) found a relationship between pungency (which refers to strong, odor of onion) and disease resistance in the onion. An abundance of moisture in the air greatly increased the occurrence of neck rot. The diseases were more severe on mild varie- ties than pungent ones. 1$. cinerea is the causal organism for brown stain symptoms on onion bulbs (21) . Ellerbrook e^t al. (27) indicated that both _B. cinerea and 15. allii caused flower blighting and seed yield losses of 98 and 47% respectively. Wukasch ^t al. (103) found that ozone injured leaves of onions are more susceptible to B^. cinerea infection. 15. cinerea also caused lesions on onion leaves and produced a cavity within each lesion as a result of collapse and separation of mesophyll and epidermal 36a cells (23). Phenolic compounds have been implicated in resistance to other diseases. Byrde (16) indicated that there is strong evidence to suggest that the resistance to brown rot of certain high tannin varieties of apples increases as a result of reduced activity of fungal macerating enzymes by oxidized polyphenols produced by the fruit which are most effective in inactivating the pectolytic enzymes of Sclerotihia fructigena. The oxidation products may be expected to have the ability to precipitate pectolytic enzyme of the fungus which are necessary for its pathogenicity. The young roots of potatoes resistant to Verticillium alboatrum contain higher levels of chlorogenic acid than the susceptible varieties (74). Chlorogenic acid content in the roots of resistant varieties of potatoes is approximately five times higher than susceptible ones. Roots of both resistant and susceptible varieties lose their ability to synthesize chlorogenic acid when they age (71). Glomerella perennans, (syn. G^. cingulata), which causes bitter rot of stored apples, is a major source of wastage in some years (39). Chlorogenic acid had been shown to inhabit spore germination of this fungus in culture. Apples containing higher chlorogenic acid level in the peel were more resistant to G. perennans. Anthocyanins, a group of compounds normally present in the peel of the apple variety Cox's Orange Pippin, also inhibited the germination of spores of (J. perennans (39). A study of the role of oxidized polyphenols by Byrde et al. in 1969 (16) suggested that polyphenols in apple fruits form a defense mechanism against the brown rot disease caused by Sclerotihia 37 fructigena. Polyphenols were oxidized by most enzymes of the fungus, which are necessary for its pathogenicity (16). In some cases phenolic compounds are localized in non-living "protective layers" of plant organs and play a fungistatic role in preventing further infection from fungi. An example is the wax covering of apple leaves which contains soluble acidic constituents, including phenolics. These compounds reportedly play a role in resistance to powdery mildew (28). Phenolic compounds also play a role in the hypersensitive reaction. The appearance of a hypersensitive reaction is a consequence of the disturbance of the balance between oxidative and reductive processes of polyphenol compounds. The result is an excessive oxidation of poly- phenol compounds and a breakdown of cellular structure related to necrosis (30). In the case of viruses, phenolics influence resistance of plants to virus mostly by the hypersensitive reaction. In some cases, tannic acid in raspberry probably inactivates most viruses by clumping the viruses particles into complexes which are largely uninfective (17). Materials and Methods Cultures; A culture of 3. allii isolated from diseased Yellow Globe Danvers onion (Allium cepa L.) and B^. ciherea isolated from bean pods (Phaseolus vulgaris) were maintained on potato dextrose agar. Spores and mycelia were obtained by incubating the cultures in the dark for 10 days at 20OC. Basal medium: The basal medium consisted of 20 g agar; 5 g 38 dextrose; 5 g peptone; 1.0 g KH distilled water (22). PO ; 0.5 g Mg SO -IE 0 and 1000 ml of Stock solutions of catechol and the other pheno- lic compounds (Sigma Chem. Co. and Eastman Kodak Company, Rochester, N.Y.) were added to molten media (50 C) to achieve final concentrations in the medium of 0, 50, 100, 150, 200, 250, 300, 350 or 400 pg/liter. Radial growth and spore germination of Botrytis spp were evaluated relative to catechol concentrations. From the margins of five day old cultures of B^. allii and IJ. cinerea, 4 mm diameter plugs were cut with a cork borer. Plugs were placed upside down onto the phenolic test agars and the 9 cm diam. petri dishes were incubated in darkness at 20 C. Two measurements of radial growth per plate were made each day thereafter until the colony on the control plate covered the entire plate. This was 5 days for 13. allii and 3 days for B^. cinerea. Each concentration treatment consisted of 23 plates as replicates. Spores from ten day old B^. allii and B^. cinerea cultures were harvested in water and the final spore concentration was adjusted to 10 conidia per ml. 3 This suspension was sprayed onto agar media which con- tained the different concentrations of catechol. Plates were incubated in the dark at 20 C according to the optimum conditions described (20). (Microscopic examination at 100X was used to assess germination of spores). A spore was considered germinated if the germination tube was longer than the width of the spore. Counts were made at 4, 6, 8, 9, 12, 15, 18 and 24 hrs. for B. allii and 4, 6, 8, 9, 12, 15 for B. cinerea. Each treatment consisted of 4 replications. Statistic analysis: Radial growth and spore germination test data were analyzed by using a one-way analysis of variance as described by 39 Steel and Torrie (82). The Student Newman-Keul's test was used to determine significant differences between treatments. Results arid Discussion Extent of mycelial growth after A days incubation of _B. allii steadily decreased as the concentration of catechol increased (Figure 7). Every concentration of catechol significantly reduced radial growth of 15. allii when compared with the control (Table 5). Each treatment also differed significantly from the others except for the 200 yg/1 and 250 yg/1 treatments. Mycelial growth of _B. cinerea in all treatments except the 50 yg/1 treatment differed significantly (P<0.5) from the control (Table 5). One hundred yg/1 of catechol had statistically the same effect on mycelial growth of 15. cinerea as 150 yg/1 of catechol. Similarly, no significant difference was found between the 200 and 250 yg/1 treatments. At 400 yg/1 of catechol, no mycelial growth was observed for B^. cinerea in 2 of the 23 observations and in 5 observations where some mycelial growth had occurred a deep brown to black color was observed in the media. Slopes of the regression lines for colony size for both fungi and different concentrations of catechol were significantly different (p=0.005) (Figure 7). Mycelial growth of both species of Botrytis was 2 negatively correlated (R =.90) with increasing concentrations of catechol. Mycelial growth of 15. cinerea was more vigorous than that of B^. allii as IJ. cinerea grew to the edge of the 9 cm plates in 3 days 40 compared to 5 days for 15. allii. In an earlier study, Clark and Lorbeer (22) reported that catechol concentrations of 50 yg/1 to 500 yg/1 steadily reduced mycelial growth of 15. cihefea. In our experiment, however, 50 yg/1 of catechol inhibited growth of j3. allii but not 13. cinerea. At 100 yg/1 catechol 15. ciherea mycelial growth was slightly inhibited after 2 days. Clark and Lorbeer (22) studied the effect of different concentrations of catechol, protocatechuic acid and quercetin on growth of 13. cinerea. Only catechol inhibited growth whereas con- centrations of protocatechuic acid and quercetin up to 500 yg/1 did not inhibit mycelial growth of this fungus. The reason that some observations on mycelial growth of jB. cinerea showed deep brown color may be due to the reaction of oxidative enzymes on phenolics present in the medium. Whether oxidations can be conclusively ascribed to the action of fungal enzymes on the phenolics is yet to be determined. Approximately 8% of the conidia of 13. allii began to germinate during the first 2-4 hours of incubation for control cultures and after 15 hrs of incubation 90% of the spores had germinated. Percent germi- nation of B. allii spores after 15 hrs incubation on media containing 50 and 100 yg/1 of catechol was not significantly different from the control (Table 4). However, with all other treatments the percent spore germination was significantly reduced after 15 hrs incubation when compared with the control. With the highest experimental concen- tration of catechol (400 yg/1) only 20 percent of the spores had germinated after 15 hrs of incubation. About 77% of the conidia of 13. cinerea began to germinate after 2-4 hours incubation on control media. After 8 hrs of incubation. 41 spore germination of 1J. cinerea was 90 percent in the control (Figure 5) . All catechol treatments of B^. cinerea spore germination were sig- nificantly different (P=.05) from the control. However there were no significant differences in germination between treatments of 50 u.g/1 " and 100 yg/1 of catechol; between 150 yg/1 and 200 yg/1; 250 yg/1, 300 yg/1, and 350 yg/1 concentrations. The effect of increasing catechol concentration on inhibiting spore germination of both Botrytis allii and Botrytis cinerea is readily evident in Table 4. Although there are some spore germinations which are not significantly different than spore germination of adjacent catechol treatments, nevertheless, the trend of inhibition is clear. For all experimental concentrations of catechol at 20 C, rates of spore germination of B^. cinerea were faster than that of B^. allii. The rate of spore germination of 15. allii reached over 90% at 15 hrs incubation on PDA without catechol, whereas it took only 8 hrs for that of 15. cinerea. On 400 yg/1 of catechol after 15 hrs incubation, spore germination of 15. allii was only 19 percent compared to 49 percent for B. cinerea. This may suggest that catechol affects the length of time for maximum germination of B^. allii more than it directly affects B^. cinerea spores. In order to try to determine whether the nature of inhibition mechanisms might be the same or different, regression lines of percent spore germination over time for 15. allii at different concentrations of catechol were compared using analysis of covariance. The slope of each regression line was compared with all others using the t-test following the method of Neter (70). The slope of the regression line 42 for 0 yg/1 of catechol was not found to be significantly different (p=.05) from that of 50 yg/1. However slopes of the regression lines for 100, 150, 200, and 250 yg/1 catechol differed from the control but not from each other. The slopes of the regression line of 400 yg/1 catechol differed significantly (P=.05) from all other treatments (Table 6, Figure 5). In the case of 15. cinerea, there were no significant differences among the slopes of the regression lines for spore germination and different concentrations of catechol even though there were significant differences in percent of spore germination at different concentrations of catechol after 8 hrs incubation (Table 6, Figure 6). The effect of varying catechol concentrations on 15. allii and jB. cinerea mycelial growth as determined by regression analysis suggests that they probably differ in the mechanisms by which they react to catechol in their environments. The calculation of slope data was used in order to ask the question as to whether both species of Botrytis were reacting to catechol via the same mechanism and it appears from the data that they are not. The differences (if, indeed they are real) in mechanisms are beyond the scope of this study, although the high concentration (400 yg catechol/1) may have provided a clue in that substantial oxidation of phenolics occurred in the case of Botrytis cinerea but not in the case of Botrytis allii. Concentrations of catechol greater than 150 yg/1 inhibited spore germination of both 15. allii and B^. cinerea after incubation of 15 and 8 hrs respectively. This raises an interesting hypothesis with respect to the nature of resistance to neck rot in those resistant 43 onion varieites which contain catechol greater than some critical concentration in the dry outer scales or neck tissues as they are cured. Since there are many more hosts that 1$. cinerea attacks, this may indicate that 13. cinerea may.be better adapted to react to diverse environments including media containing catechol than is Botrytis allii which has a narrower host range. The early study by Clark et^ al. (22) incidated that the number of B^. cinerea colonies was reduced with concentration ranging from 350450 yg/1 of catechol and spore germination was completely inhibited at 500 yg/1. Crushed pigmented yellow onion scales contain compounds which inhibit spore germination of 1$. cinerea. Protocatechuic acid and quercetin up to 500 yg/1 reportedly had no effect on inhibiting spore germination of ]$. cinerea. It' is likely that onion scales have several phenolic compounds which may act additively or synergistically to inhibit various pathogens. Onion cultivars that contain high levels of catechol may be expected to resist infection by .B. allii and 15. cinerea. The amounts of catechol in the inner and outer scales (Table 3) are 41.7 and 312.5 yg/1 dry wt. respectively in the onion variety Yellow Globe Danvers. The outer scale amounts appear to be quite sufficient to inhibit infection by IS. allii, but the inner scale concentrations may be only marginally effective. 44 Table 4. The effect of catechol concentration on percent of spore germination of .B. allii after 15 hrs and B^. cinefea after 8 hrs incubation at 20oC.* percent spore germination catechol concentration (yg/1) B. allii B^. cinefea 0 92.60 a 91.50 a 50 90.40 a 68.18 b 100 88.53 a 67.49 b 150 74.93 b 61.00 c 200 66.90 c 58.60 c 250 64.90 c 33.57 d 300 46.45 d 33.57 d 350 44.65 d 29.72 d 400 19.65 e 4.81 e * values followed by the same letter are not significantly different according to Student-Newman-Kuel's test (P<0.05). 45 Table 5. The effect of catechol concentration on colony diameter of Botrytis allii and B^ ciherea after 4 and 2 days respectively, incubated at 20 C. Mycelial growth (mm) B. allii B. cinerea 0 57.15 a 81.,4 a 50 45.98 b 79.,9 a 100 38.44 c 68..6 b 150 34.96 d 60..7 be 200 31.04 e 60..7 c 250 31.04 e 51..3 e 300 26.89 f 38,.6 d 350 24.28 g 33..8 e 400 20.63 h 25,.7 f Catechol concentration (yg/1) *Values followed by the same letter are not significantly different according to Student-Newman-Kuel's test (P<0.05). 46 Table 6. Slope of regression line for different concentrations of catechol and 1$. allii and B^. cirierea spore germination.* Catechol concentration (ug/1) B^. allii (slope) 15. cinerea (slope) 0 8.6 a 1.53 a 50 8.2 a 1.38 a 100 6.0 b 1.41 a 150 5.8 b 1.49 a 200 5.2 b 1.42 a 250 5.0 b 1.10 a 300 4.1 c 1.10 a 350 4.5 c 0.85 a 400 3.6 d 0.88 a * Values followed by the same letter are not significantly different according to Student-Newman-Kuel's test (P^0.05). 47 Figure 5. Spore germination of Botrytis allii over time at 20 C relative to catechol concentration in the medium. 47a Figure 5. I I _i 2 ^~ ~_ ^oi **•_ "^CT _ a> _ C oi o> c» - - oo c In omo om —~wcvjfO D» * • c D 0 ! o« Oi - o in o ro v D ! I I OOOOOOOOOOO ocT>oor^c£>»o^-tocvi N0llVNIWa3£) % — 48 Figure 6. Spore germination of I[. cinerea over time at 20oC relative to catechol concentration in the medium. 48a Figure 6, _J 2 C ~ ~ "^ ^ ■"* -^ ~ 2 OoOOO O 0 O OOc inOiOQ in um — — CJCVIIO ro D# * • c DO D © t I ! I ooooooooooo oa>cDh-c0ir5^rrooj N0!1VNMU39 % — 49 Figure 7. Colony diameter of 1$. allii (after 4 days) and 15. cirierea (after 2 days) incubated at 20oC relative to catechol concentration in the medium. COLONY DIAMETER (cm) ' L aanSxa e6<7 50 Preliminary Experiment 'Weight Loss Over Time at 3 Storage Temperatures' 51 EFFECT OF STORAGE TEMPERATURES ON WEIGHT LOSS Field cured Yellow Glove Danvers onions from the Lake Labish area near Salem, Oregon were placed in cold storage rooms at 0 , 10 , and 20 C. Fifteen onion bulbs were individually weighed on October 15, 1979 and stored at each temperature. The same onion bulbs were removed to be weighed every month until April 15, 1980. Split plot analysis of variance was performed to determine the difference of weight loss between treatments by the method described in Steel and Torrie (82). Effect of storage temperature on weight loss. Onion bulbs stored for six months at 20 C, 10 C and 0 C had weight losses of 10%, 5%, and 3% respectively (Figure 8). Onion bulbs stored at 20 C had 5 layers of paper-like dry outer scales compared with 3 layers of dry outer scales on onions stored at 10 or 0 C. Cumulative weight losses at 20 C, 10 C and 0 C were significantly different at 0.05 level both by temperature and by sampling date (Figure 9). Weight loss (shrinkage) in storage of onions is due to respiration losses of carbon as CO and transpiration losses of water and other volatiles (46, 47, 66). Brewster (6, 7) indicated that within the o o range 0 -20 C onion bulb respiration rate was linearly related to temperature. Ward and Tucker (100) reported a mean increase of 0.55 mg C0„ Kg C h and the data of Robinson et al. (79) show an increase of about 0.3 CO /Kg/h/ C. Onion bulbs stored at low temperature (-1 to +2 C) and high temperatures (25 to 30 C) have longer storage lives than those stored at 12 -16 C, but onions stored at high temperatures 52 were subjected to more storage diseases (2, 7). Onions stored from 30 -35 C yield satisfactory onions for the fresh market, but their external color may be less attractive than bulbs from cold storage. Most cultivars of onions can not be stored effectively between 5 C and 20 C because development of sprouting and decay are most rapid in this range (80, 81). At 0 C and 25 to 30 C temperatures are expected to delay and prevent progress of inflorescence initiation. Tempera- tures of 9 -13 C appeared most favorable for flower initiation. Storage at 25 C to 30 C after floral initiation can promote reversion from a floral to a vegetative condition (8). The results of this study indicate that onions are best stored at low temperature (0 C). There was no incidence of rotting for the onion bulbs stored at 10 and 20 C. Likely explanations are that relative humidity was not suitable for pathogens or onion bulbs were free from pathogens from the field. Previous work cited above, showed that at 0 C onions can be stored longer with less weight loss and with lower incidence of diseases. The dramatic increase in weight loss in the 10 C storage at the 5th month was likely due to excessive sprouting evident at that temperature which did not occur in the 20 C or 0 C stored onions (Figure 9). Relative weight losses for this study at 20 C Assume 8-10 mg CO kg hr is the respiration rate. So after six months, respiration rate = 6 months x 30 day/month x 24 hrs/day x 8-10 mg C02./kg/hr = 43,200 mg/kg 53 This is about 4.32% at 20OC 4.32%. as. CO 10% weight loss = 43% loss in respiration lost in transpiration = 57% 54 Figure 8. Cumulative percent weight loss during 6 month storage of Yellow Globe Danvers onions. 54a Figure 8. O CSJ O CD <0 ^- oJ SSOl 1H9I3M% "3AllVlffWnO 55 Figure 9. Percent weight loss per month during 6 month storage of Yellow Globe Danvers onions. 55a Figure 9. - to - w - <3Z O IO - oo H1N0IN y3d *- SS0-I1H9I3M% o CsJ 56 Preliminary Experiment "Dry Outer Scales Regeneration" 57 DRY OUTER SCALES REGENERATION A preliminary experiment attempted to determine the development of dried outer scales as affected by different temperatures and air velocity conditions. Dried outer scales of onion were removed until fleshy scales were exposed. Peeled onions were placed in portable food dehydrators set at 40OC and 50 FPM of air, 350C and 50 FPM of air, 30OC and 50 FPM of air and 20OC and 50 FPM of air. Other onions were dipped in chloroform for 1 minute or in 100 ppm ethephon for 5 minutes and placed in room temperature on the lab bench. Another treatment merely left the peeled onions at room temperature. Evalua- tion of scale regeneration was made after peeled onions had been exposed to the above conditions for 4 weeks. Each treatment consisted of 5 observations. Dry outer scale regeneration: A preliminary study. Room temperature and room temperature with air circulation promoted the best scale development in 4 weeks. At 40 C, 35 C, and 30 C onion bulbs appeared cooked, becoming soft and shrunken within 2 weeks. Dipping onion bulbs in chloroform in an effort to accelerate moisture loss from the outer scales by altering cuticular wax structures caused the basal plate to dry and split, and fungal attack occurred by the end of 4 weeks. Ethrel at 100 ppm had no effect on scale development. Further study for dry outer scale regeneration should be done, because the occasional seasonal variation in curing results in losses from "bald" or "skinned" onions which do not store well and are unacceptable in the market. 58 Leaving onions cure in tjie field too long can result in loss of outer wrapper-scales and cause bald onions in storage (98). Similarly increased risk of rain can.lead to storage rot problems (91). Irrigations which are continued after the onions have matured may allow rot organisms in the soil to separate the dry wrapper-scales from the root plate and these scales fall off during harvest and result in many bald onions (98). Intact dry outer scales of onion reduce respiration rate, see appendix 2, prevent moisture loss from the bulb, and prevent the entry of pathogens (66). Quality of the outer dried leaf scales of onion refers to the number, thickness, color and completeness of the scales. hr kg Rate of onion respiration is increased about 10 mg CO by removal of dry outer scales from the bulbs. Onions with cracked or missing scales are more subject to weight loss (6). Again earlier work has indicated that dry outer scales of onion provide benefits for storage life of onions. Determining the best methods for development of dry outer scales on "bald" or "peeler" onions would represent substantial reductions in losses to onion growers and packers. 59 BIBLIOGRAPHY 1. Abdalla, A. A. and L. K. Mann. 1963. Bulb development in the onion (Allium cepa L.) and the effect of storage temperature on bulb rest. 2. Hilgardia 35(5):55-112. Abdel - Rahman, M. and Isenberg, F. M. R. 1974. The role of exogenous plant regulators in the dormancy of onion bulbs. J. Agric. Sci. 82(1):113-116. 3. Agrios, G. N. 1978. Plant Pathology - 2nd ed. Academic Press, New York, 703 pp. 4. Anderson, R. A. and J. R. Todd. 1968. Estimation of total phe- nols by their binding to polyvinylpyrrolidone. Tobacco Sci. 112:107-111. 5. Angell, H. R., J. C. Walker and K. P. Link. 1930. The relation of protocatechuic acid to disease resistance in the onion. Phytopathology 20:431-438. 6. Apeland, J. 1971. processes in onion. 7. Effects of scale quality on physiological Acta Hort. 20:72-79. Aung, L. H. and C. E. Peterson. 1974. Gibberellin-like substan- ces of dormant and non-dormant bulbs of Allium cepa L. J. Amer. Soc. Hort. Sci. 99:279-281. 8. Aura, K. 1963. Studies on the vegetatively propagated onions cultivated in Finland, with special reference to flowering and storage. 9. Annal. Agriculturae Fennial 2:1-74. Bhatia, I. S., K. L. Bajaj and A. K. Verma. 1971. A general spray reagent for phenolic compounds on thin-layer plates. Chromatog. 62:471-472. J. 60 10. Boonyakiat, D. 1980. Factors affecting storage of Yellow Globe Danvers onion: abscisic acid during maturation and storage and phenolic substances inhibitory to Botrytis allii and Botrytis cinerea. 11. M.S. Thesis. Boswell, V. R. 1923. Oregon State University. Influence of the time of maturity of onions upon rest period, dormancy, and response to various stimuli designed to create the rest period. Proc. Amer. Soc. Hort. Sci. 20:225-233. 12. Boswell, V. R. 1923. Influence of the time of maturity of onions on the behavior during storage, and the effect of storage temperature on subsequent vegetative and reproductive development. Proc. Amer. Soc. Hort. Sci. 20:234-239. 13. Brewster, J. L. 1977. The physiology of oinion. Bureau of Horticultural and Plantation Crops Commonwealth Hort. Abstr. 47(1): 9-23. 14. Brewster, J. L. 1977. The physiology of onion. Commonwealth Bureau of Horticultural and Plantation Crops Hort. Abstr. 47(2): 103-112. 15. Bubl, C. E. 1978. The Chemical Curing of Oregon Danver Onions. M.S. Thesis, Oregon State University. 16. Byrde, R. J. W. rot. II. apple. 17. The varietal resistance of fruits to brown The nature of resistance in some varieties of cider J. Hort. Sci. 32:227-238. Cadmans, C. H. . tannins. 1956. 1960. Inhibition of plant virus infection by In J. B. Pridham (Ed.) Phenolics in Plants in Health and Disease. Pergamon Press, New York. 61 18. Catlin, P. B. and E. A. Olsson. 1968. Identification of inter- specific hybrids of Pyfus after paper chromatography of leaf extracts. 19. J. Amer. Soc. Hort.Sci. 93:88-109. Catlin, P. B. and E. A. Olsson. 1966. Identification of some Pyrus species after paper chromatography of leaf and bark extracts. J. Amer. Soc. Hort. Sci. 88:127-144. 20. Clark, C. A. and J. W. Lorbeer. vars to Botrytis brown stain. 21. 1973. Reaction of onions culti- Plant Dis. Rep. 57:210-214. Clark, C. A. and J. W. Lorbeer. 1973. Symptomatology, etiology, and histopathology of Botrytis brown stain of onion. Phyto- pathology 63:1231-1235. 22. Clark, C. A. and J. W. Lorbeer. Botrytis brown stain of onion. 23. 1975. The role of phenols in Phytopathology 65(3):338-341. Clark, C. A. and J. W. Lorbeer. 1976. Comparative histopathology of Botrytis squamosa and B^. cinerea on onion leaves. Phytopath. 66(11):1279-1289. 24. Clark, J. E. and 0. V. S. Heath. of onion plant. V. J. Exp. Bot. 13:227-249. Claypool, L. L. and R. M. Keefer. CO Studies in the physiology An investigation into the growth substances content of bulbing onions. 25. 1962. 1942. determination in respiration studies. A colorimetric method for J. Amer. Soc. Hort. Sci. 40:177-186. 26. Drinkwater, W. 0. and B. E. Jones. 1955. Effect of irrigation and soil moisture on maturity yield and storage of two onion hybrids. Proc. Amer. Soc. Hort. Sci. 66:267-278. 62 27. Ellerbrook, L. A. and J. W. Lorbeer. of onion flower blight. 1977. Etiology and control Phytopathology 67(2):155-159. B. cinerea, B.* allii conidi-. caused flower blighting and seed yield losses of 98, 421 respectively. 28. Farkas, G. L. and Z. Kiraly. 1962. Role of phenolic compounds in the physiology of plant diseases and disease resistance. Phyto- pathology 2(4A):105-150. 29. Foskett, R. L. and C. E. Peterson, 1950. The relation of dry matter content and storage quality in some onion varieties and hybrids. 30. J. Amer. Soc. Hort. Sci. 55:314-318. Goodman, R. N., Z. Kiraly and M. Zoitlin. 1967. and Physiology of Infectious Plant Diseases. Company, Inc. 31. Gull, D. D. The Biochemistry D. Van Nostrand 354 pp. 1960. Artificially curing Florida onions. Proc. Fla. Sta. Hort. Soc. 73:153-156. 32. Gunkel, W. W., J. W. Lorbeer, J. Kaufman'and H. A. Smith, Jr. 1971. Artificial drying - a method for control of Botrytis neck rot in bulk stored onions. Ann. Prog. Report, N. Y. Farm Electri- fication Council No. 28:71-80. 33. Gutzman, V. L. and N. C. Hayslip. 1962. Effect of time of seeding and varieties on onion production and quality when grown in two soil types. 34. Proc. Fla. State Hort. Soc. 75:156-162. Haard, F. N. and D. K. Salunkhe. Handling of Fruits and Vegetables. Publishing Company, Inc. 191 pp. 1925. Postharvest Biology and Westport Connecticut, AVI 63 35. Hancock, J. G. and J. W. Lorbeer. 1963. Pathogenesis of Botrytis cinerea, B^. squamosa and B^. allii on onion leaves. Phytopathology 53:669-673. 36. Harborne, J. B. 1967. Comparative Biochemistry of the Flavonoids. Academic Press, London and N. Y. 37. Hatfield, W. C. 1948. to disease resistance. 38. Hoyle, B. J. 1947. 383 pp. Antibiotic substances in onion in relation J. Agri. Res. 77:115-135. Storage breakdown of onions as affected by stage of maturity and length of topping. Proc. Amer. Soc. Hort. Sci. 50:353-359. 39. Hulme, A. C. and K. L. Edney. 1960. Phenolic substances in the peel of Cox's Orange Pippin apples with reference to infection by (j. perenhans. 40. In J. B. Pridham (Ed.) Phenolics in Plants in Health and Disease. Pergamon Press, New York. Irwin, R. D. The Etiology of Bacterial Soft Rot of Onions 1974. and Identification of the Causal Organism. 87-94 pp. Ph.D. Thesis. Oregon State University. 41. Isenberg, F. M. and J. K. Ang. on onions. 42. 1956. The use of maleic hydrazide J. Amer. Soc. Hort. Sci. 68:343-350. Isenberg, F. M. and J. K. Ang. 1963. Northern grown onions: curing, storing and inhibiting sprouting. Cornell Ext. Bull. No. 1012, 15 pp. 43. Isenberg, F. M. and J. K. Ang. 1964. Effects of maleic hydrazide field sprays on storage quality of onion bulbs. Hort. Sci. 84:378-385. Proc. Amer. Soc. 6A 44. Isenberg, F. M., T. H. Thomas and M. Pendergrass. 1974. Hormonal and histological differences between normal and maleic hydrazide treated onions stored over winter. Int. Soc. Hort. Sci. 1(38): 95-108. 45. Jones, H. A. 1921. Preliminary report on onion dormancy studies. Proc. Amer. Soc. of Hort. Sci. 44:479-484. 46. Jones, H. A. 1927. The influence of storage temperature on seed production in the Ebenezer onion. Proc. Amer. Soc. Hort. Sci. 24:61-63. 47. Jones, H. A. and L. K. Mann. 1963. Onions and Their Allies. Interscience Publishers, Inc., New York. 48. Jordan, R. W. Minnesota. 49. 1915. Onions. Webb Publishing Company, St. Paul, 95 pp. Kawamoto, S. 0. and J. W. Lorbeer. leaves by Pseudomonas cepacia. 50. Kato, T. 286 pp. 1966. 1974. Infection of onion Phytopathology 64:1440-1445. Physiological studies on bulb foirmation and dor- mancy in the onion plant. VIII. Relationship bewteen dormancy of the bulb and properties of its juice. J. Japan Soc Hort. Sci. 35:95-101. 51. Kaufman, J. and J. W. Lorbeer. 1967. Control of Botrytis neck rot of onions by fungicidal dusts and desiccant chemicals. Plant Dis. Rept. 51:696-699. 52. Knott, J. E. 1933. The relation of bulb size to the thickness of the outer scales of the onion. 562. J. Amer. Soc. Hort. Sci. 30:561- 65 53. Kunkel, R. 1947. The effect of various levels of nitrogen and potash on the yield and keeping quality of onions. Proc. Amer. Soc. Hort. Sci. 50:361-367. 54. Lee, S. and D. Le Tourneau. 1958. Chlorogenic acid content and Verticillium wilt resistance of potatoes. Phytopathology 48: 268-274. 55. Leopold, A. C. and P. E. Kriedemann. Development. 56. 2nd edition. Levy, D. and N. Kedar. initiation in onion. 57. 1975. McGraw-Hill Inc. 1970. Plant Growth and 545 pp. Effect of ethrel on growth and bulb HortSci. 5(2):80-82. Levy, D., N. Kedar and R. Karacingue. 1973. Effect of ethephon on bulbing of onion under noninductive photo-period. HortSci. 8(3):228-229. 58. Link, K. P., H. R. Angell and J. C. Walker. 1929. The isolation of protocatechuic acid from pigmented onion scales and its significance in relation to disease resistance in onions. J. Biol. Chem. 81:369-375. 59. Link, K. P. and J. C. Walker. 1933. The isolation of catechol from pigmented onion scales and its significance in relation to disease resistance in onions. 60. Lipe, W. N. 1976. J. Biol. Chem. 100:379-383. Effect of ethephon on rate of bulb enlarge- ment, maturity, and yield in onions. 61. Lorenz, 0. A. and B. J. Hoyle. HortSci. 11(4):424-425. 1946. Effect of curing and time of topping on weight loss and chemical composition of onion bulbs. Proc. Amer. Soc. Hort. Sci. 47:301-308. 66 62. Lutz, J. M. and R. E. Hardenburg. 1977. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks. Agriculture Handbook No. 66. 63. Mahotiere, S. 1972. USDA 94 pp. Onion dormancy in relation to temperature, applied growth substances, and an endogenous growth inhibitor. Ph.D. Thesis, Michigan State University. 64. Ill pp. Mahotiere, S., R. C. Herner, and F. G. Dennis. 1976. Effect of temperature on growth of shoot apices excised from onions in rest. HortSci. 11(2):154-155. 65. Mahotiere, S., R. C. Herner and F. G. Dennis. 1976. Effect of applied growth substances on growth of shoot apices excised from onions in rest. 66. J. Amer. Soc. Hort. Sci. 101(3):211-213. Matson, W. E., N. S. Mansour and D. G. Richardson. Storage: Guidelines for Commercial Growers. 948, Oregon State University Ext. Service. Q^')'>^y67. Maude, R. B., and A. H. Presley. Botrytis allii. \/68. 1. Extension Circular 15 pp. Infection of onions by 1917. 1977. Neck rot (Botrytis allii) Seed-borne infection and its relationship to the disease in the onion crop. Munn, M. T. Onion Ann. Appl. Biol. 85:165. Maude, R. B., and A. H. Presley. of bulb onions. 69. 1977. 1978. Ann. Appl. Biol. 86:163-180. Neck Rot Disease of Onions. N. Y. (Geneva) Exp. Sta. Bull. No. 437:365-402. 70. Neter, J. and W. Warrerman. Models. 1974. Richard D. Irwin, Inc. Applied Linear Statistical Homewood, Illinois. 841 pp. 67 71. Owen, J. H., J. C. Walker and M. A. Stahmann. 1950. Pungency, color and moisture supply in relation to disease resistance in the onion. 72. Phytopathology 40:292-297. Pappelis, A. J., K. V. Somasekhara, J. N. Bemiller and D. Wilner. 1976. Increased resistance to spread of B^. allii in onion bulbs and induced chlorogenic acid synthesis. (Abstract). Proc. Am. Phytopath. Soc. 3:232. 73. Paterson, D. G., and S. H. Wittwer. 1953. Further investigation on the use of maleic hydrazide as a sprout inhibitor for onions. Proc. Amer. Soc. Hort. Sci. 62:405-410. 74. Patil, S. S., M. Zucker and A. E. Dimond. 1966. Biosynthesis of chlorogenic acid in potato roots resistant and susceptible to Verticillium albo-atrum. 75. Phytopathology 56:971-974. Powell, L. E., and S. D. Seeley. 1974. The metabolism of absci- sic acid to a water soluble complex in apple. J. Amer. Soc. Hort. Sci. 99(5):439-441. 76. Pridham, J. B. 1960. Phenolics in Plants in Health and Disease. Symposium publications division Pergamon Press, N. Y. 77. Richardson, D. G., C. Bubl and N. S. Mansour. 1976. 131 pp. Effects of top desiccants on curing and subsequent storage quality of Danvers Yellow Globe onions. 78. Acta Hort. 62:317-325. Risueno, M. C, J. L. Diez, G. Gimenez-Martin and C. de la Torre. 1971. Ultra-structural study of the effect of abscisic acid on cell elongation in plant cells. 323-328. Onions. Protoplasma 73(3/4): 68 79. Robinson, J. E., K. M. Browne and W. G. Burton. 1975. characteristics of some vegetables and soft fruits. Biol. 80. Ryall, A. L. and W. J. Lipton. 1979. Handling, Transportation, Vol. 1, 2nd edition. Publishing Company, Inc. Westport, Connecticut. 82. 83. Ryall, A. L. and W. T. Pentzer. _ n 84. ^j, 1974. Vol. 2. Company, Inc. 545 pp. Westport, Connecticut. Steel, R. G. and J. H. Torrie. 1960. Statistics. McGraw-Hill Book Co. Inc. Stow, J. R. 1976. AVI 587 pp. Handling, Transportation, and Storage of Fruits and Vegetables. AVI Publishing Principles and Procedure of 481 pp. The effect of defoliation on storage potential of bulbs of the onion. S 5 s-5? Ann. Appl. 81:399-408. and Storage of Fruits and Vegetables. 81. Storage Ann. Appl. Biol. 84:21-79. Talieva, N. M. and L. V. Runkova. 1976. Changes in the movements of phenolic compounds in the onion leaf epidermis under the influence of conidal inoculation with 15. allii Munn. Summarized in: Rev. of Plant Path. 55:970. 85. Taylor, Roy. Valley. 1975. Observations on Onion Storage in the Treasure Extension Agricultural Engineer. University of Idaho Moscow, Idaho. 86. Thomas, T. H. and F. M. Isenberg. onion bulbs during dormancy. 87. 88. 1972. Hormone physiology of Exp. Hort. No. 23:48-51. Thomas, T. H. and F. M. Isenberg. 1972. The role of growth sub- stances in regulation of bulb dormancy. J. Exp. Bot. 20:124-132. Thompson, H. C. and W. C. Kelly. 1957. Vegetable Crops,. 5th ed. McGraw-Hill Book Co. Inc., N. Y. 611 pp. 69 89. Torre, C. de la, J. L. Diez, J. F. Lopez-Saez and G. GimenezMartin. 1970. of root growth. 90. Tucker, W. G. Effect of abscisic on the cytological components Onions. 1974. Cytologia 37(2):197-205. Onion respiration during long term storage. Acta Hort. 38:127-129. 91. Vaughan, E. K., M. G. Cropsey and E. N. Hoffman. 1964. Effects of field-curing practices, artificial drying and other factors in the control of neck rot in stored onions. Agri. Exp. Sta. OSU Tech. Bull. 77:22 pp. 92. Walker, J. C. N.Y. 93. 1969. Plant Pathology (3rd edition). McGraw-Hill 1925. Studies on disease resistance in the onion. 819 pp. Walker, J. C. Proc. Nat. Acad. Sci. 11:183-189. 94. Walker, J. C. and R. J. Larsen. control. 95. Ag. Hndbk. No. 208. 1961. Onion diseases and their USDA. Walker, J. C. and C. G. Lindgren. 1924. Further studies on the relation of onion scale pigmentation to disease resistance. J. Ag. Res. 29:507-514. 96. Walker, J. C. and R. B. Maude. 1975. The natural occurrence of growth of Gliocladium roseum on the mycelium and sclerotia of B^. allii Munn. Trans. Br. Mycol. Soc. 65:335-338. 97. Walker, J. C. and M. A. Stahman. resistance in plants. 98. Waltz, A. and J. Burr. Storage. 1955. Chemical nature of disease Annu. Rev. Plant Physiol. 6:351-366. 1977. Preparing Onions for Harvest and University of Idaho College of Agriculture. Extension Service Agri. Exp. Stat. Cooperative 70 99. Ward, C. M. 1976. The influence of temperature on weight loss from stored onion bulbs due to desiccation, respiration, and sprouting. 100. Ann. Appl. Biol. 83:149-155. Ward, C. M. and W. G. Tucker. 1976. Respiration of maleic hydrazide treated and untreated onion bulbs during storage. Ann. Appl. Biol. 82:135-142. 101. Wittwer, S. H., R. C. Shama, L. E. Weller and H. M. Sell. 1950. The effect of preharvest foliage sprays of certain growth regulators on sprout inhibition and storage quality of carrots and onions. 102. Plant Physiol. 25:539-549. Woodman, R. M. and H. R. Barnell. 1937. The connection between the keeping qualities of commercial varieties of onions and the rates of water loss during storage. 103. Wukasch, R. T. and G. Hofstra. Ann. Appl. Biol. 24:219-235. 1977. interaction in onion leaf dieback: Ozone and Botrytis spp field studies. J. of Amer. Soc. Hort. Sci. 102(5):543-546. 104. Yamaguchi, M., H. K. Pratt and L. L. Morris. 1957. Effect of storage temperatures on the keeping quality and composition of onion bulbs and on subsequent darkening of dehydrated flakes. Proc. Amer. Soc. Hort. Sci. 69:421-426. APPENDICES 71 Appendix 1. Flow diagram for free ABA extraction from onion tissue. equivalent of 5g ground tissue in CH OH 3 i Filtrate 10 DPM14C-ABA- -25 ml of Na HCO3 buffer at pH 8.0 CH OH evaporated off Partitioned against CH„C10 3 x 50 ml. 2 2^ 3, discard CH2C12 . aqueous fraction pH adjusted to 2.5 I Partitioned against CH Cl 3 x 50 ml J: discard aqueous fraction I CH Cl 2 fraction ^ Partitioned against KaOH buffer Ph 10 3 x 50 ml JT discard CH Cl VK aqueous fraction pH adjusted to 2.5 Partitioned against Ethyl acetate 3 x 50 ml. ^ discard aqueous fraction 14 count of C ABA for % recovery ^ Taken to dryness and methyl esterification by diazomethane Injection of esterified ABA into GLC 72 Appendix 2. Sampling date Free ABA concentration (ng/g dry weight) in onion tissues during maturation and storage. Approximately % top fall Foliage Neck Bulb Aug. 20, 79 0 0.37 0.15 . 0.04 Aug. 30, 79 40 1.81 0.27 0.17 Sept. 10, 79 60 0.53 0.33 0.31 Sept. 20, 79 100 0.28 0.27 0.21 0.01 0.15 0.20 Oct. 1, 79 Nov. 1, 79 0.25 Jan. 1, 80 0.15 Feb. 1, 80 0.53 Mar. 1, 80 0.45 Apr. 1, 80 0.28 Apr. 1, 80 sprout 0.24 73 Appendix 3. Comparison of respiration rate of eight onion varieties after 8 months storage at 0oC. Respiration rate root sprout mg CO /kg/hr (0-5) leave (cm) XP-H667 26.46 3.05 2.73 3.20 XP-H742 28.18 2.15 3.76 2.75 Yellow Globe 33.06 2.17 4.11 3.21 Autumn Beauty 27.68 1.79 4.19 3.33 XP-W207 20.26 1.41 2.85 3.15 XP-H348 22.05 2.01 2.39 3.24 Danvers 18.41 0.88 3.04 4.95 XP-H195 25.39 0.70 1.46 3.33 Varieties length of 1st no. of outer s> Sapparo Yellow Globe 74 Appendix 4. ABA concentration and respiration rate of eight onion varieties after 1 month storage at 0oC. 74a Appendix 4. (,_&>! ^JM 5UJ)ZOO O CD CM CD I CD ro (^^^^^^ l CD a ^^^^^^ s^^s^^^i^^^ CD > 00 K) I o 2 O o CD LU 2 O or m^^^^ < < CD < Kasss5S*ssss*s o>i 1 in i m CO UJ OZ r^- OJ i I CO LJ I ro (UJ6/6U) CD i a V8V I 75 Appendix 5. IR spectrum of unknown phenolic with Rf = 0.86 in BAW and RF = 0.74 in 2% acetic acid. A. Unknown B. Protocatechuic acid C. Catechol D. Catechol (Sample) ^fc , 1 No. 007-1493 M ■ <1S9"I""!! "•"■t 4& f rONCFNTRATION ■ SCAN MODE ACCYa ' HI ENERGV D PHA'iF RESOLUTION □ REMARKS OPERATOR 3.0 5 MICROMETERS (urn) 6 auF*4lO, '.EA lOHn 4^^ SPECTRUM NO SA/v\PLE +<.s' ----- •• ''"■ ORIGIN DATE " 2.5 5URVEYD CAL □ THirKNF<;<; J0*PMlCCJN13Ul^.JHCCn«Il0f. 7 1 12 13 14 16 18 20 _L 4000 - 3600 3200 2800 2400 2000 '1400 ^1 *v 0Bk No. 007-U9J CONCENTRATION SCAN MODE THirKNFSS uiC .^■.ti-.-.y SURVEV n ACC. a HI ENERGY C PHASE ^^r? CA, G y-iff-lC^h-IHCLj JHfOMAIlUN B ""-» ■" -1 SPFf.TRIJM NO l-.UPIF L'-.. ..''■ -. ' *• -;.,.. RESCLUTIOM D REMA?K5 ORIGIN .:ATE. OPERATOR-- MICROMET;-.; >m 1000 3600 3200 2800 2400 2000 1800 1600 ':■■ 1400 1200 100') 300 600 75C ****"*. >>--* ■xii Jv ^ 1 z' o s D C ■ 01 ^ n £ 0 2 S. o s z< « tl i i g ■ o UJ o z O .J-I in t!. .-'t. -!<;•»sn-.'''■'.;ii iMMH ^^ rONrFNTRATION THir.KNESS No. 007-1493 CnAPMlC CONIROIS COfPORAIlON •. ■? S&NMODE PHASE REMARKS SURVEYD ^^ HI ENERGY D CAL. □ B\lffJ>\.0 NE« TOUK ^^ SPFaRUM NO. SAMPIF C^. RESOLUTIOM □ nPFParnp 5 ORIGIN HATF MICROMETERS (un 6 _; : 7 JL O 76 Appendix 6. 1. Chemical structures of major phenolics constituents of Yellow Globe Danvers onion. Catechin HO 2. Catechol OH 3. H Chlorogenic acid COOH OK ^ ./\ OH OH HO \ 4. Caffeic acid HO CH.'CHCO.H -OH 5. Quercetin 77 6. 7. Arbutin H H OHH HOCHC-C-C-rf-CHOK' Protocatechuic acid CO OH OH OH N )-0H 78 Appendix 7. Comparison of respiration rate of eight onion varieties after 8 month storage at 0oC. Respiration rate (mg of C02/kg/hr) with dry outer scale without dry outer scale XP-H667 29.48 28.81 XP-H742 37.56 35.69 Sapparo Yellow Globe 26.35 40.27 Autumn Beauty 32.55 36.58 XP-W207 21.11 30.20 XP-H348 27.50 35.97 Yellow Globe Danvers 21.42 34.16 XP-H195 24.08 49.45 Varieties