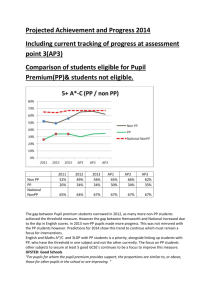
1-[2,4,6-Trimethyl-3,5-bis(4-oxopiper- idin-1-ylmethyl)benzyl]piperidin-4-one
![1-[2,4,6-Trimethyl-3,5-bis(4-oxopiper- idin-1-ylmethyl)benzyl]piperidin-4-one](http://s2.studylib.net/store/data/013791243_1-80a6806a5ba942cfb3eeae6f2d8378a0-768x994.png)
organic compounds
Acta Crystallographica Section E
Structure Reports
Online
ISSN 1600-5368
1-[2,4,6-Trimethyl-3,5-bis(4-oxopiperidin-1-ylmethyl)benzyl]piperidin-4-one
K. Rajesh, a
V. Vijayakumar,
Narasimhamurthy b a
‡ S. Sarveswari, a and Edward R. T. Tiekink c
T.
* a
Organic Chemistry Division, School of Advanced Sciences, VIT University, Vellore
632 014, India, b
Materials Research Centre, Indian Institute of Science, Bengaluru
560 012, India, and c
Department of Chemistry, University of Malaya, 50603 Kuala
Lumpur, Malaysia
Correspondence e-mail: edward.tiekink@gmail.com
Received 4 May 2010; accepted 6 May 2010
Key indicators: single-crystal X-ray study; T = 293 K; mean (C–C) = 0.004 A
R factor = 0.062; wR factor = 0.183; data-to-parameter ratio = 14.9.
In the structure of the title compound, C
27
H
39
N
3
O
3
, each of the (4-oxopiperidin-1-yl)methyl residues adopts a flattened chair conformation (with the N and carbonyl groups being oriented to either side of the central C
4 plane) and they occupy positions approximately orthogonal to the central benzene ring [C benzene
—C—C methylene
—N torsion angles
103.4 (2), 104.4 (3) and 71.9 (3) ]; further, two of these residues are oriented to one side of the central benzene ring with the third to the other side. In the crystal packing, supramolecular layers in the ab plane are sustained by C—
H O interactions.
Experimental
Crystal data
C
27
H
39
N
3
O
3
M r
= 453.61
Triclinic, P 1 a = 7.9315 (16) A b = 12.449 (3) A c = 14.618 (3) A
= 67.641 (3)
= 87.749 (4)
Data collection
Bruker SMART APEX CCD diffractometer
Absorption correction: multi-scan
( SADABS ; Sheldrick, 1998)
T min
= 0.981, T max
= 0.987
Refinement
R [ F
2
> 2 ( F
2
)] = 0.062
wR ( F
2
) = 0.183
S = 1.02
4490 reflections
= 73.630 (3)
V = 1277.0 (5) A
3
Z = 2
Mo K radiation
= 0.08 mm
1
T = 293 K
0.28
0.21
0.17 mm
12284 measured reflections
4490 independent reflections
3008 reflections with I > 2 ( I )
R int
= 0.026
301 parameters
H-atom parameters constrained max
= 0.26 e A
3 min
= 0.14 e A
3
Related literature
For background to the biological significance of piperidin-4one and analogous pyran and thiopyran species, see: El-
Subbagh et al.
(2000); Ganellin et al.
(1965); Hagenbach &
Gysin (1952); Ileana et al.
(1985); Mokio et al.
(1989); Pathak et al.
(2007). For a related structure, see: Vijayakumar et al.
(2010).
Table 1
).
D —H A D —H H A
C20—H20a N3
C9—H9a O2 i
C21—H21b O3 ii
0.96
0.97
0.97
2.46
2.60
2.48
Symmetry codes: (i) x þ 1 ; y 1 ; z ; (ii) x 1 ; y ; z .
D A
3.184 (4)
3.412 (5)
3.252 (4)
D —H A
132
142
136
Data collection: SMART (Bruker, 2001); cell refinement: SAINT
(Bruker, 2001); data reduction: SAINT ; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008); molecular graphics:
ORTEP-3 (Farrugia, 1997) and DIAMOND (Brandenburg, 2006); software used to prepare material for publication: publCIF (Westrip,
2010).
VV is grateful to DST India for funding through the Young
Scientist Scheme (Fast Track Proposal). TN acknowledges the establishment of the CCD facility under the IRHPA-DST programme at the Indian Institute of Science, Bangalore.
‡ Additional correspondence author, e-mail: kvpsvijayakumar@gmail.com.
o1306
Rajesh et al.
doi:10.1107/S1600536810016570 Acta Cryst.
(2010). E 66 , o1306–o1307
organic compounds
Supplementary data and figures for this paper are available from the
IUCr electronic archives (Reference: HG2682).
References
Brandenburg, K. (2006).
DIAMOND . Crystal Impact GbR, Bonn, Germany.
Bruker (2001).
SMART and SAINT . Bruker AXS Inc., Madison, Wisconsin,
USA.
El-Subbagh, H. I., Abu-Zaid, S. M., Mahran, M. A., Badria, F. A. & Alofaid, A.
M. (2000).
J. Med. Chem .
43 , 2915–2921.
Farrugia, L. J. (1997).
J. Appl. Cryst.
30 , 565.
Ganellin, C. R. & Spickett, R. G. (1965).
J. Med. Chem .
8 , 619–625.
Hagenbach, R. E. & Gysin, H. (1952).
Experimentia , 8 , 184–187.
Ileana, B., Dobre, V. & Nicluescu-Duvaz, I. (1985).
J. Prakt. Chem .
327 , 667–
674.
Mokio, I. G., Soldatenkov, A. T., Federov, V. O., Ageev, E. A., Sergeeva, N. D.,
Lin, S., Stashenku, E. E., Prostakov, N. S. & Andreeva, E. L. (1989).
Khim.
Farm. Zh .
23 , 421–427.
Pathak, C., Karthikeyan, S., More, K. & Vijayakumar, V. (2007).
Indian J.
Heterocycl. Chem .
16 , 295–296.
Sheldrick, G. M. (1998).
SADABS . University of Go¨ttingen, Germany.
Sheldrick, G. M. (2008).
Acta Cryst.
A 64 , 112–122.
Vijayakumar, V., Rajesh, K., Suresh, J., Narasimhamurthy, T. & Lakshman, P.
L. N. (2010).
Acta Cryst.
E 66 , o170.
Westrip, S. P. (2010).
J. Appl. Cryst.
43 . Submitted.
Acta Cryst.
(2010). E 66 , o1306–o1307 Rajesh et al.
C
27
H
39
N
3
O
3 o1307
supporting information
supporting information
Acta Cryst.
(2010). E
66
, o1306–o1307 [doi:10.1107/S1600536810016570]
1-[2,4,6-Trimethyl-3,5-bis(4-oxopiperidin-1-ylmethyl)benzyl]piperidin-4-one
K. Rajesh, V. Vijayakumar, S. Sarveswari, T. Narasimhamurthy and Edward R. T. Tiekink
S1. Comment
Piperidin-4-one and their analogous pyran and thiopyran species attract interest owing to their biological properties, viz. anti-viral, anti-tumour (El-Subbagh et al.
, 2000), central nervous system (Ganellin et al.
, 1965), local anesthetic
(Hagenbach et al.
, 1952), anti-cancer (Ileana et al.
, 1985), and anti-microbial (Mokio et al.
, 1989; Pathak et al.
, 2007) activities. As a continuation of structural studies of piperidine-4-ones (Vijayakumar et al.
, 2010), the title compound, (I), was synthesised and characterised by X-ray crystallography.
In compound (I), Fig. 1, the (4-oxopiperidin-1-yl)methyl residues containing the N1 and N2 atoms lie to one side of the central benzene ring and that with the N3 atom to the other. Owing to the presence of methyl substituents on either side of each 4-oxopiperidin-1-yl)methyl residue, the piperidin-4-one rings adopt side-on conformations to minimise steric interactions so that the N atoms occupy positions approximately normal to the plane through the benzene rings. This is quantified by the C2–C1–C7–N1 [103.4 (2) °], C2–C3–C14–N2 [-104.4 (3) °], and C4–C5–C21–N3 [71.9 (3) °] torsion angles. Each of the six-membered piperidin-4-one rings adopts a slightly flattened chair conformation with the N and carbonyl groups lying to either side of the central C
4
plane in each case. Only the amine-N3 atom forms a significant intra- or inter-molecular interaction, i.e. an intramolecular C–H···N contact, Table 1. In the crystal packing, molecules are sustained into layers by C–H···O interactions; Table 1. Layers are formed in the ab plane and stack along the c axis, Fig.
2.
S2. Experimental
To a suspension of 1.5 equiv. of 4-piperidone hydrochloride monohydrate in benzene (20 ml), 3.0 equiv of K
2
CO
3
was added. After stirring well for 30 min, 2,4,6-tris(bromomethyl)mesitylene (0.5 equiv) in benzene (10 ml) was added, followed by refluxing for 10 h. The completion of reaction was monitored by TLC. The reaction mixture was then allowed to cool to room temperature, filtered to remove the insoluble solids and then the filter cake was washed with dichloromethane. Excess solvents were removed under reduced pressure and the obtained crude product was purified by crystallization using 1:1 ratio of chloroform and methanol; m.pt. 483 K.
S3. Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.96 to 0.97 Å) and were included in the refinement in the riding model approximation, with U iso
(H) set to 1.2–1.5
U equiv
(C).
Acta Cryst.
(2010). E 66 , o1306–o1307 sup-1
supporting information
Figure 1
The molecular structure of (I) showing the atom-labelling scheme and displacement ellipsoids at the 35% probability level.
Acta Cryst.
(2010). E 66 , o1306–o1307 sup-2
supporting information
Figure 2
A view in projection down the a axis of the unit cell contents in (I) highlighting the stacking of layers (mediated by C–
H···O contacts, shown as orange dashed lines) along the c axis.
1-[2,4,6-Trimethyl-3,5-bis(4-oxopiperidin-1-ylmethyl)benzyl]piperidin-4-one
Crystal data
C
27
H
39
N
3
O
3
M r
= 453.61
Triclinic, P 1
Hall symbol: -P 1 a = 7.9315 (16) Å b = 12.449 (3) Å c = 14.618 (3) Å
α = 67.641 (3)°
β = 87.749 (4)°
γ
Z
= 73.630 (3)°
V = 1277.0 (5) Å
= 2
F (000) = 492
θ = 2.9–21.9°
µ = 0.08 mm −1
3
D x
= 1.180 Mg m −3
Mo Kα radiation, λ = 0.71073 Å
Cell parameters from 969 reflections
Acta Cryst.
(2010). E 66 , o1306–o1307 sup-3
supporting information
T = 293 K
Block, colourless
Data collection
Bruker SMART APEX CCD diffractometer
Radiation source: fine-focus sealed tube
Graphite monochromator
ω scans
Absorption correction: multi-scan
( SADABS ; Sheldrick, 1998)
T min
= 0.981, T max
= 0.987
0.28 × 0.21 × 0.17 mm
12284 measured reflections
4490 independent reflections
3008 reflections with I > 2 σ ( I )
R int
= 0.026
θ max
= 25.0°, θ min
= 1.5° h = −9→9 k = −14→14 l = −17→17
Refinement
Refinement on F 2
Least-squares matrix: full
R [ F 2 > 2 σ ( F 2 )] = 0.062
wR ( F 2 ) = 0.183
S = 1.02
4490 reflections
301 parameters
0 restraints
Primary atom site location: structure-invariant direct methods
Secondary atom site location: difference Fourier map
Hydrogen site location: inferred from neighbouring sites
H-atom parameters constrained w = 1/[ σ 2 ( F o
2 ) + (0.0929
P ) 2 + 0.3308
P ] where P = ( F o
2 + 2 F c
2 )/3
(Δ/ σ ) max
= 0.007
Δ ρ max
= 0.26 e Å −3
Δ ρ min
= −0.14 e Å −3
Special details
Geometry
. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate
(isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes.
Refinement
. Refinement of F 2 against ALL reflections. The weighted R -factor wR and goodness of fit S are based on F 2 , conventional R -factors R are based on F , with F set to zero for negative F 2 . The threshold expression of F 2 > 2 σ ( F 2 ) is used only for calculating R -factors(gt) etc. and is not relevant to the choice of reflections for refinement. R -factors based on F 2 are statistically about twice as large as those based on F , and R - factors based on ALL data will be even larger.
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å 2 )
C1
C2
C3
C4
C5
C6
C7
H7A
O1
O2
O3
N1
N2
N3 x
−0.1090 (3)
−0.5097 (3)
1.1430 (3)
0.2978 (2)
0.0198 (2)
0.6691 (2)
0.4104 (3)
0.3290 (3)
0.3003 (3)
0.3618 (3)
0.4516 (3)
0.4721 (3)
0.4264 (3)
0.5444
y
−0.3541 (2)
0.4895 (2)
0.1907 (3)
−0.20717 (17)
0.35113 (19)
0.18892 (18)
−0.0297 (2)
0.0681 (2)
0.1875 (2)
0.2095 (2)
0.1122 (2)
−0.0076 (2)
−0.1596 (2)
−0.2101
z
0.4155 (2)
0.2615 (2)
−0.0499 (2)
0.36948 (14)
0.26602 (16)
0.05220 (14)
0.33087 (17)
0.35901 (17)
0.29004 (19)
0.19514 (18)
0.16894 (17)
0.23580 (18)
0.39930 (19)
0.3991
U iso
*/ U eq
0.1134 (9)
0.1089 (8)
0.1334 (11)
0.0539 (5)
0.0603 (6)
0.0558 (5)
0.0502 (6)
0.0511 (6)
0.0532 (6)
0.0545 (6)
0.0520 (6)
0.0511 (6)
0.0592 (7)
0.071*
Acta Cryst.
(2010). E 66 , o1306–o1307 sup-4
0.1498
0.2698
0.3448
0.2003 (3)
0.1946
0.2641
−0.0916 (3)
−0.1012
−0.0373
−0.2748 (3)
−0.2675
−0.3491
−0.3573 (4)
−0.2391 (4)
−0.2927
−0.2249
−0.0583 (4)
0.4088
0.3258 (3)
0.3095
0.4462
0.1999 (4)
0.2340
0.2100
0.0127 (4)
−0.0149 (3)
−0.1333
−0.0036
0.1175 (3)
0.1019
0.0960
0.2677 (3)
0.0189
−0.0698
0.3263 (3)
0.4242
0.3107
0.2214
0.5171 (3)
0.5507
0.4215
0.6971 (4)
0.5887
0.7295
0.8419 (4)
0.8620
0.8064
1.0056 (4)
C16
H16A
H16B
C17
C18
H18A
H18B
C19
H13A
H13B
H13C
C14
H14A
H14B
C15
H15A
H15B
C10
C11
H11A
H11B
C12
H12A
H12B
C13
H7B
C8
H8A
H8B
C9
H9A
H9B
H21A
H21B
C22
H22A
H22B
C23
H23A
H23B
C24
H19A
H19B
C20
H20A
H20B
H20C
C21
0.0375
0.1097
−0.0303
0.2953 (2)
0.2693
0.3556
0.2725 (2)
0.2573
0.1953
0.3271 (3)
0.3275
0.2767
0.4534 (3)
0.5317 (3)
0.6024
0.5597
0.4656 (3)
−0.1636
−0.3350 (2)
−0.3439
−0.3799
−0.3884 (3)
−0.3963
−0.4689
−0.3128 (3)
−0.1805 (2)
−0.1373
−0.1616
−0.1385 (2)
−0.0531
−0.1479
0.0440 (3)
0.5170
0.4493
0.3379 (3)
0.3442
0.3926
0.3586
0.1390 (3)
0.0648
0.1966
0.2351 (3)
0.2925
0.1686
0.2969 (4)
0.3253
0.3665
0.2109 (3)
Acta Cryst.
(2010). E 66 , o1306–o1307
0.4643
0.4819
0.5091
0.3141 (2)
0.3854
0.2929
0.3062 (2)
0.3761
0.3004
0.2531 (2)
0.1866
0.2881
0.2471 (2)
0.2223 (3)
0.2380
0.1516
0.2789 (3)
0.4664
0.4315 (2)
0.4999
0.4284
0.3990 (3)
0.3369
0.4483
0.3857 (2)
0.3333 (2)
0.3415
0.2630
0.37301 (19)
0.3340
0.4410
0.46323 (19)
0.2551
0.3488
0.1196 (2)
0.0792
0.1532
0.0784
0.06593 (18)
0.0536
0.0174
−0.0532 (2)
−0.0905
−0.0758
−0.0716 (3)
−0.1416
−0.0530
−0.0130 (2) supporting information
0.102*
0.102*
0.102*
0.0655 (7)
0.079*
0.079*
0.0587 (6)
0.070*
0.070*
0.0770 (8)
0.092*
0.092*
0.0750 (8)
0.1045 (12)
0.125*
0.125*
0.0860 (10)
0.071*
0.0705 (8)
0.085*
0.085*
0.0792 (9)
0.095*
0.095*
0.0704 (8)
0.0684 (7)
0.082*
0.082*
0.0580 (6)
0.070*
0.070*
0.0677 (7)
0.103*
0.103*
0.0757 (8)
0.114*
0.114*
0.114*
0.0628 (7)
0.075*
0.075*
0.0842 (10)
0.101*
0.101*
0.1011 (12)
0.121*
0.121*
0.0797 (9) sup-5
supporting information
C25
H25A
H25B
C26
H26A
H26B
C27
H27A
H27B
H27C
0.9854 (3)
0.9698
1.0913
0.8270 (3)
0.8527
0.8071
0.5526 (3)
0.4736
0.5726
0.6625
C19
C20
C21
C22
C23
C24
C25
C26
C27
C11
C12
C13
C14
C15
C16
C17
C18
C2
C3
C4
C5
C6
C7
C8
C9
C10
O1
O2
O3
N1
N2
N3
C1
Atomic displacement parameters (Å 2 )
U 11
0.0518 (15)
0.0443 (13)
0.0547 (15)
0.0443 (14)
0.0414 (13)
0.0466 (15)
0.0468 (15)
0.0654 (19)
0.0603 (17)
0.0501 (15)
0.0425 (13)
0.0580 (16)
0.079 (2)
0.0575 (17)
0.0408 (14)
0.0408 (13)
0.0569 (15)
0.0747 (14)
0.0508 (12)
0.0656 (14)
0.0428 (10)
0.0401 (10)
0.0351 (10)
0.0308 (11)
0.0303 (11)
0.0307 (11)
0.0301 (11)
0.0289 (11)
0.0305 (11)
0.0401 (12)
0.0538 (15)
0.078 (2)
0.0621 (17)
U 22
0.0663 (17)
0.0559 (15)
0.0854 (19)
0.0750 (18)
0.0625 (16)
0.087 (2)
0.080 (2)
0.064 (2)
0.0647 (19)
0.0744 (19)
0.0917 (19)
0.116 (3)
0.122 (3)
0.123 (3)
0.105 (2)
0.0757 (18)
0.0853 (19)
0.0842 (16)
0.1078 (18)
0.245 (3)
0.0519 (12)
0.0613 (13)
0.0699 (13)
0.0641 (15)
0.0717 (17)
0.0663 (16)
0.0641 (16)
0.0755 (17)
0.0688 (16)
0.0672 (17)
0.0587 (17)
0.0552 (17)
0.0688 (18)
0.1473 (3)
0.2031
0.0811
0.0975 (2)
0.0309
0.0659
−0.1134 (3)
−0.1128
−0.1879
−0.1067
U 33
0.0783 (18)
0.0659 (16)
0.0636 (17)
0.0871 (19)
0.0712 (16)
0.098 (2)
0.085 (2)
0.153 (3)
0.136 (3)
0.086 (2)
0.0569 (15)
0.0561 (17)
0.086 (2)
0.092 (2)
0.084 (2)
0.0666 (16)
0.0825 (19)
0.159 (2)
0.160 (2)
0.131 (2)
0.0582 (12)
0.0751 (14)
0.0532 (12)
0.0553 (14)
0.0548 (14)
0.0676 (16)
0.0664 (16)
0.0561 (14)
0.0632 (15)
0.0615 (15)
0.0804 (19)
0.100 (2)
0.0790 (19)
0.0935 (2)
0.1269
0.1235
0.1068 (2)
0.0845
0.1767
0.2056 (2)
0.1572
0.2628
0.1777
U 13
0.0024 (13)
0.0056 (11)
0.0091 (12)
0.0092 (13)
0.0062 (11)
0.0010 (14)
0.0067 (13)
0.018 (2)
0.0241 (18)
0.0093 (14)
0.0075 (11)
0.0056 (13)
0.0246 (18)
0.0352 (16)
0.0092 (13)
0.0054 (11)
0.0234 (14)
0.0274 (15)
0.0228 (13)
0.0524 (14)
0.0099 (9)
0.0124 (9)
0.0068 (8)
0.0028 (10)
0.0042 (10)
0.0049 (10)
0.0025 (10)
0.0062 (10)
0.0105 (10)
0.0024 (11)
0.0117 (13)
0.0208 (17)
0.0131 (14)
U 12
−0.0207 (13)
−0.0132 (11)
−0.0163 (14)
−0.0201 (13)
−0.0131 (11)
−0.0117 (14)
−0.0035 (14)
−0.0025 (16)
−0.0224 (15)
−0.0166 (13)
−0.0282 (13)
−0.0340 (17)
−0.052 (2)
−0.0536 (18)
−0.0235 (14)
−0.0169 (12)
−0.0324 (14)
−0.0394 (13)
−0.0044 (12)
−0.0694 (18)
−0.0119 (9)
−0.0127 (9)
−0.0180 (9)
−0.0161 (10)
−0.0155 (11)
−0.0172 (11)
−0.0170 (11)
−0.0225 (11)
−0.0209 (11)
−0.0099 (11)
−0.0076 (13)
−0.0240 (15)
−0.0278 (14)
0.0755 (8)
0.091*
0.091*
0.0644 (7)
0.077*
0.077*
0.0691 (8)
0.104*
0.104*
0.104*
U 23
−0.0157 (14)
−0.0161 (12)
−0.0327 (15)
−0.0403 (15)
−0.0262 (13)
−0.0419 (18)
−0.0281 (16)
−0.022 (2)
−0.0402 (19)
−0.0149 (16)
−0.0257 (14)
−0.0031 (16)
−0.008 (2)
−0.060 (2)
−0.0388 (18)
−0.0166 (14)
−0.0440 (16)
−0.0133 (15)
−0.0575 (16)
−0.105 (2)
−0.0137 (10)
−0.0238 (11)
−0.0122 (10)
−0.0210 (12)
−0.0276 (13)
−0.0288 (13)
−0.0193 (13)
−0.0251 (13)
−0.0312 (13)
−0.0191 (13)
−0.0137 (14)
−0.0225 (16)
−0.0213 (15)
Acta Cryst.
(2010). E 66 , o1306–o1307 sup-6
Geometric parameters (Å, º)
C1—C7
C2—C3
C2—C13
C3—C4
C3—C14
C4—C5
C4—C20
C5—C6
C5—C21
C6—C27
C7—H7A
C7—H7B
C8—C9
C8—H8A
C8—H8B
C9—C10
C9—H9A
O1—C10
O2—C17
O3—C24
N1—C12
N1—C8
N1—C7
N2—C15
N2—C19
N2—C14
N3—C26
N3—C22
N3—C21
C1—C6
C1—C2
C9—H9B
C10—C11
C11—C12
C11—H11A
C11—H11B
C12—H12A
C12—H12B
C12—N1—C8
C12—N1—C7
C8—N1—C7
C15—N2—C19
C15—N2—C14
C19—N2—C14
C26—N3—C22
1.515 (3)
1.402 (3)
1.524 (3)
1.404 (3)
1.513 (3)
1.402 (3)
1.511 (4)
1.405 (3)
1.517 (3)
1.511 (3)
0.9700
0.9700
1.522 (4)
0.9700
0.9700
1.491 (4)
0.9700
1.209 (3)
1.205 (3)
1.202 (3)
1.455 (3)
1.457 (3)
1.468 (3)
1.447 (3)
1.465 (3)
1.474 (3)
1.444 (3)
1.457 (3)
1.480 (3)
1.407 (3)
1.409 (3)
0.9700
1.482 (4)
1.517 (3)
0.9700
0.9700
0.9700
0.9700
109.97 (18)
111.77 (19)
110.82 (19)
109.09 (19)
112.1 (2)
109.6 (2)
109.5 (2)
C18—H18B
C19—H19A
C19—H19B
C20—H20A
C20—H20B
C20—H20C
C21—H21A
C21—H21B
C22—C23
C22—H22A
C22—H22B
C23—C24
C23—H23A
C23—H23B
C24—C25
C25—C26
C25—H25A
C13—H13A
C13—H13B
C13—H13C
C14—H14A
C14—H14B
C15—C16
C15—H15A
C15—H15B
C16—C17
C16—H16A
C16—H16B
C17—C18
C18—C19
C18—H18A
C25—H25B
C26—H26A
C26—H26B
C27—H27A
C27—H27B
C27—H27C
N2—C15—C16
N2—C15—H15A
C16—C15—H15A
N2—C15—H15B
C16—C15—H15B
H15A—C15—H15B
C17—C16—C15 supporting information
0.9700
0.9700
0.9700
0.9600
0.9600
0.9600
0.9700
0.9700
1.516 (4)
0.9700
0.9700
1.474 (5)
0.9700
0.9700
1.480 (4)
1.525 (3)
0.9700
0.9600
0.9600
0.9600
0.9700
0.9700
1.518 (3)
0.9700
0.9700
1.493 (4)
0.9700
0.9700
1.477 (4)
1.526 (4)
0.9700
0.9700
0.9700
0.9700
0.9600
0.9600
0.9600
112.2 (2)
109.2
109.2
109.2
109.2
107.9
112.2 (2)
Acta Cryst.
(2010). E 66 , o1306–o1307 sup-7
C26—N3—C21
C22—N3—C21
C6—C1—C2
C6—C1—C7
C2—C1—C7
C3—C2—C1
C3—C2—C13
C1—C2—C13
C2—C3—C4
C2—C3—C14
C4—C3—C14
C5—C4—C3
C5—C4—C20
C3—C4—C20
C4—C5—C6
C4—C5—C21
C6—C5—C21
C5—C6—C1
C5—C6—C27
C1—C6—C27
N1—C7—C1
N1—C7—H7A
C1—C7—H7A
N1—C7—H7B
C1—C7—H7B
H7A—C7—H7B
N1—C8—C9
N1—C8—H8A
C9—C8—H8A
N1—C8—H8B
C9—C8—H8B
H8A—C8—H8B
C10—C9—C8
C10—C9—H9A
C8—C9—H9A
C10—C9—H9B
C8—C9—H9B
H9A—C9—H9B
O1—C10—C11
O1—C10—C9
C11—C10—C9
C10—C11—C12
C10—C11—H11A
C12—C11—H11A
C10—C11—H11B
C12—C11—H11B
H11A—C11—H11B
N1—C12—C11
Acta Cryst.
(2010). E 66 , o1306–o1307
118.9 (2)
121.0 (2)
119.8 (2)
120.9 (2)
119.2 (2)
112.09 (18)
109.2
109.2
109.2
109.2
107.9
112.0 (2)
109.2
109.2
109.2
109.2
107.9
111.39 (19)
108.5 (2)
120.0 (2)
118.4 (2)
121.5 (2)
119.8 (2)
120.1 (2)
120.0 (2)
120.0 (2)
121.7 (2)
118.3 (2)
120.2 (2)
119.6 (2)
120.2 (2)
120.0 (2)
112.8 (2)
109.0
109.0
109.0
109.0
107.8
121.5 (3)
123.7 (3)
114.8 (2)
111.3 (2)
109.4
109.4
109.4
109.4
108.0
111.6 (2)
C17—C16—H16A
C15—C16—H16A
C17—C16—H16B
C15—C16—H16B
H16A—C16—H16B
O2—C17—C18
O2—C17—C16
C18—C17—C16
C17—C18—C19
C17—C18—H18A
C19—C18—H18A
C17—C18—H18B
C19—C18—H18B
H18A—C18—H18B
N2—C19—C18
N2—C19—H19A
C18—C19—H19A
N2—C19—H19B
C18—C19—H19B
H19A—C19—H19B
C4—C20—H20A
C4—C20—H20B
H20A—C20—H20B
C4—C20—H20C
H20A—C20—H20C
H20B—C20—H20C
N3—C21—C5
N3—C21—H21A
C5—C21—H21A
N3—C21—H21B
C5—C21—H21B
H21A—C21—H21B
N3—C22—C23
N3—C22—H22A
C23—C22—H22A
N3—C22—H22B
C23—C22—H22B
H22A—C22—H22B
C24—C23—C22
C24—C23—H23A
C22—C23—H23A
C24—C23—H23B
C22—C23—H23B
H23A—C23—H23B
O3—C24—C23
O3—C24—C25
C23—C24—C25
C24—C25—C26 supporting information
109.4
109.4
109.4
109.4
108.0
109.5
109.5
109.5
109.5
109.5
109.5
113.1 (2)
108.9
108.9
108.9
108.9
107.8
109.2
109.2
109.2
109.2
107.9
122.0 (3)
122.6 (3)
115.4 (2)
112.1 (3)
109.2
109.2
109.2
109.2
107.9
111.2 (3)
110.2 (3)
109.6
109.6
109.6
109.6
108.1
109.8 (3)
109.7
109.7
109.7
109.7
108.2
122.3 (3)
122.9 (3)
114.8 (2)
110.8 (2) sup-8
N1—C12—H12A
C11—C12—H12A
N1—C12—H12B
C11—C12—H12B
H12A—C12—H12B
C2—C13—H13A
C2—C13—H13B
H13A—C13—H13B
C2—C13—H13C
H13A—C13—H13C
H13B—C13—H13C
N2—C14—C3
N2—C14—H14A
C3—C14—H14A
N2—C14—H14B
C3—C14—H14B
H14A—C14—H14B
C6—C1—C2—C3
C7—C1—C2—C3
C6—C1—C2—C13
C7—C1—C2—C13
C1—C2—C3—C4
C13—C2—C3—C4
C1—C2—C3—C14
C13—C2—C3—C14
C2—C3—C4—C5
C14—C3—C4—C5
C2—C3—C4—C20
C14—C3—C4—C20
C3—C4—C5—C6
C20—C4—C5—C6
C3—C4—C5—C21
C20—C4—C5—C21
C4—C5—C6—C1
C21—C5—C6—C1
C4—C5—C6—C27
C21—C5—C6—C27
C2—C1—C6—C5
C7—C1—C6—C5
C2—C1—C6—C27
C7—C1—C6—C27
C12—N1—C7—C1
C8—N1—C7—C1
C6—C1—C7—N1
C2—C1—C7—N1
C12—N1—C8—C9
C7—N1—C8—C9
Acta Cryst.
(2010). E 66 , o1306–o1307
−179.76 (19)
2.2 (3)
−3.3 (3)
179.92 (19)
174.0 (2)
−2.8 (3)
−0.3 (3)
177.04 (19)
−177.6 (2)
−0.3 (3)
−61.7 (3)
175.3 (2)
−73.9 (2)
103.4 (2)
58.7 (3)
−177.2 (2)
3.8 (3)
−173.44 (19)
−177.93 (19)
4.8 (3)
−3.7 (3)
178.0 (2)
174.9 (2)
−3.3 (3)
0.1 (3)
−178.57 (19)
178.1 (2)
−0.6 (3)
3.4 (3)
−174.6 (2)
109.3
109.3
109.3
109.3
108.0
109.5
109.5
109.5
109.5
109.5
109.5
112.7 (2)
109.1
109.1
109.1
109.1
107.8
supporting information
C24—C25—H25A
C26—C25—H25A
C24—C25—H25B
C26—C25—H25B
H25A—C25—H25B
N3—C26—C25
N3—C26—H26A
C25—C26—H26A
N3—C26—H26B
C25—C26—H26B
H26A—C26—H26B
C6—C27—H27A
C6—C27—H27B
H27A—C27—H27B
C6—C27—H27C
H27A—C27—H27C
H27B—C27—H27C
O1—C10—C11—C12
C9—C10—C11—C12
C8—N1—C12—C11
C7—N1—C12—C11
C10—C11—C12—N1
C15—N2—C14—C3
C19—N2—C14—C3
C2—C3—C14—N2
C4—C3—C14—N2
C19—N2—C15—C16
C14—N2—C15—C16
N2—C15—C16—C17
C15—C16—C17—O2
C15—C16—C17—C18
O2—C17—C18—C19
C16—C17—C18—C19
C15—N2—C19—C18
C14—N2—C19—C18
C17—C18—C19—N2
C26—N3—C21—C5
C22—N3—C21—C5
C4—C5—C21—N3
C6—C5—C21—N3
C26—N3—C22—C23
C21—N3—C22—C23
N3—C22—C23—C24
C22—C23—C24—O3
C22—C23—C24—C25
O3—C24—C25—C26
C23—C24—C25—C26
136.7 (3)
−43.1 (4)
−61.9 (3)
175.0 (2)
52.8 (4)
71.4 (3)
−168.0 (2)
71.9 (3)
−111.3 (2)
−63.1 (3)
175.1 (3)
57.7 (4)
126.4 (3)
−50.7 (4)
−129.9 (3)
47.2 (4)
133.7 (3)
−45.6 (4)
−61.6 (3)
174.8 (2)
54.7 (3)
68.0 (3)
−170.7 (2)
−104.4 (3)
74.3 (3)
61.4 (3)
−177.0 (2)
−51.2 (3)
−137.8 (3)
42.1 (4)
109.2
109.2
109.2
107.9
109.5
109.5
109.5
109.5
109.5
109.5
109.5
109.5
109.5
109.5
108.1
112.1 (2)
109.2
sup-9
N1—C8—C9—C10
C8—C9—C10—O1
C8—C9—C10—C11
Hydrogen-bond geometry (Å, º)
D —H··· A
C20—H20a···N3
C9—H9a···O2 i
C21—H21b···O3 ii
Symmetry codes: (i) x +1, y −1, z ; (ii) x −1, y , z .
−49.5 (3)
−135.9 (3)
43.5 (4)
D —H
0.96
0.97
0.97
supporting information
C22—N3—C26—C25
C21—N3—C26—C25
C24—C25—C26—N3
60.0 (3)
180.0 (2)
−51.3 (3)
H··· A
2.46
2.60
2.48
D ··· A
3.184 (4)
3.412 (5)
3.252 (4)
D —H··· A
132
142
136
Acta Cryst.
(2010). E 66 , o1306–o1307 sup-10