Signalling through GPCRs Kinetic effects in GPCR Signalling signal regulation
advertisement

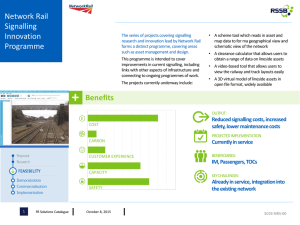
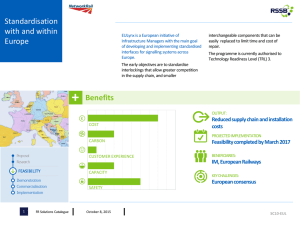
Signalling through GPCRs Benjamin Smith , Marcin Jurdzinski & Graham Ladds What happens if effector molecules are in abundance? Kinetic effects in signal regulation GPCR Signalling Activity Figure 5. Simulation of GTPase cycle in the presence and absence of RGS. Initial conditions were set such that effector molecules are always available to be activated. The curves begin to meet in the plateau region. Signalling under normal circumstances At Low Ligand Concentration: •Low levels of GPCR stimulation lead to a low rate of Gα activation. The RGS can hydrolyse Gα –GTP as fast it is produced so little signalling occurs. (GPCR) At High Ligand Concentration •High levels of GPCR stimulation lead to a high rate of guanine nucleotide exchange on Gα subunits. RGS cannot then overcome the large number of Gα –GTPs so effector molecules are activated and a response occurs. Figure1. Types of signalling in a mammalian cell. There are many different types of signalling system in mammalian cells. We study signalling through G Protein Coupled Receptors (GPCRs), highlighted in red. They are a ubiquitous family of 7span transmembrane receptors. -2 160.000 LacZ Activity 120.000 DeltaRgs1 100.000 Rgs1 (Wild Type) 80.000 •60% of all prescription drugs (with a value of >$200 billion per year) target GPCRs. 3 10 30 100 300 •Figure 3 shows a surprising result. RGS proteins can reduce signalling at low levels of ligand stimulation but increase it at high levels. •This has variously been observed in GPCR signalling (e.g. G protein gated K+ channels [Fujita et al. 2000, Ulens et al. 2000] and pheromone response pathway in the yeast Schizosaccharomyces pombe (Figure 3)) but no satisfactory explanation exists. Gα •Experiments to test this are very difficult to design. •By modelling the GTPase cycle as a dynamical system it should be possible to investigate this phenomenon more thoroughly. Figure 6. Simulation of GTPase cycle in the presence and absence of RGS. The rate constants involved in the 15000 hydrolysis, autohydrolysis and effector activation reactions 10000 were experimented with. The deltaRGS 5000 autohydrolysis rate constant RGS (R4) is ~100 fold lower than Log [Ligand] RGS catalysed hydrolysis -1 1 2 (R6&7). Activation of effector is fast. Binding of Gα-GTP to effector is slower when Gα-GTP is bound to RGS. Now the graphs begin to display the behaviour we expect. Activity Figure 7. Simulation of GTPase cycle in the presence and absence of RGS. By setting the rate constants such that binding of Gα-GTP to inactive effector (R8), happens faster than subsequent activation of effector (R9), GαGTP is sequestered if no RGS is available to recycle it. The curves display the behaviour seen in experiment. 7000 6000 5000 4000 3000 deltaRGS 2000 RGS 1000 Log [Ligand] -1 -0.5 0.5 1 1.5 2 •Through a process of questioning and simulation (Figures 4-7) a detailed understanding of the GTPase cycle was reached. •The simulation produces good qualitative agreement with experimental data indicating that in Sz. pombe downstream components have little effect on the extent of the signalling response. •The model now needs to be validated through further experiment. Experimental Validation The insight provided by working with the mathematical model prompted the design of some biologically relevant experiments: GTP GDP 2 1000 •The effect is suspected to be due to the kinetics of the system, with the components we have identified in the GTPase cycle being sufficient to produce it. GPCR 1.5 [Ligand] •They are implicated in most diseases. Premature births, cardiac disease, cancer, neurological β γ 1 Is there a sequestering effect? 1 Figure 3. Graph of β-galactosidase activity (response to stimulation) vs. concentration of ligand with which Schizosaccaromyces pombe cells were stimulated. Sz. pombe express only one GPCR pathway, with a GTPase cycle very similar to that shown in Figure 2. Sz. pombe RGS is called Rgs1. The Magenta curve shows the dose response of wild type cells. The Blue curve shows the dose response of cells lacking RGS protein (delta Rgs1). The two curves very clearly cross at high levels of stimulation (highlighted in red), with the RGS apparently increasing signalling in this region. This result seems counterintuitive, how can “switching off” Gα lead to increased signalling? disorders. β γ Gα 0.5 40.000 0 GPCR -0.5 60.000 0.000 ACTIVATION RGS 15000 20000 180.000 20.000 Ligand deltaRGS 17500 How do hydrolysis rates of Gα-GTP compared to rates of activation of effector affect signalling? 200.000 •They interact with most intracellular effector systems, including ion channels and transcription factors The GTPase cycle 20000 Activity 140.000 How is GPCR signalling regulated?... 22500 Log [Ligand] •Maximum levels of receptor stimulation lead to activation of all available Gα. At this point signalling plateaus. Further increase in ligand concentration fails to increase signalling. It is sometimes hard to reach this plateau stage in experiments as such a high response can be lethal to cells •Human cells express a complex network of GPCR signalling pathways. Some 400 non-sensory receptors exist (and >500 olfactory receptors) 25000 -1 At Very High Ligand Concentration •Cells live and die by signalling. A large proportion of a cells energy is devoted to processing information about its extracellular environment and translating this into an adaptive response. Signalling through GPCRs (highlighted in Figure 1) plays a vital role in this process [Ladds et al., 2005]. 27500 Overexpression of Gα GTP autohydrolysis •Effects of overexpression of Sz. pombe Gα on signalling can be compared with predictions made by simulation. GTP hydrolysis Fast and Slow Cycling Mutants RGS DEACTIVATION RESPONSE Figure 2. Schematic of a basic GPCR signalling system. A heterotrimeric G protein (Gαβγ) associates with a transmembrane GPCR to form a pre-activation complex (top left). When ligand binds to GPCR a conformational change occurs in the receptor allowing the Gα subunit to release GDP and bind GTP, thus causing activation (top right). The activated G protein can then bring about a response by interacting with downstream effector molecules (bottom right). Hydrolysis of Gα -GTP returns the Gα subunits to their inactive state, thereby terminating the signal. This hydrolysis reaction is catalysed by a class of proteins called the Regulators of G protein Signalling (RGS). •Figure 2 shows the basic unit of GPCR signalling, whereby binding of a ligand to a receptor brings about a response in cells through guanine nucleotide exchange on a Gα subunit [Ladds et al., 2005] . •In some systems the Gβγ subunit interacts with downstream effector molecules to produce a response. • The above reaction scheme describes all the reactions we believe occur in the GTPase cycle. It was turned into a set of ordinary differential equations. •These were implemented in Mathematica™ and solved by numerical integration. • The resulting time domain data was used to output a simulation of the dose response, resembling the biological system used in experiment. •In systems of equations such as this, the rate constants for each of the reactions as well as the initial concentrations of the chemical species determine the qualitative behaviour of the system. Initially these were set with our best guess for relative values and the simulation was run. Activity •Signalling is regulated by GTPase Activating Proteins known as Regulators of G protein Signalling (RGS) [Dohlman and Thorner, 1997]. 1.2 1 0.8 • In mammalian systems, where a number of different classes of G protein, GPCR and RGS exist and may interact, the picture quickly becomes complex. 0.6 deltaRGS 0.4 RGS 0.2 Log [Ligand] Is the basic GTPase cycle fully understood?... -1 -0.5 0.5 1 1.5 2 Figure 4. Simulation of GTPase cycle in the presence and absence of RGS. Parameters and initial conditions were set to values that seemed sensible. The resulting graphs do not produce the crossing over effect seen in experiment. •Sequence comparison with other G proteins indicates that specific single point mutations can produce fast and slow cycling versions of Sz. pombe Gα subunit which can be cloned into the yeast and assayed for effects on signalling. Results will be compared with identical in silico experiments. Future Work •Move into looking at rat heart cells and human myometrium. •Investigate the control, tolerance and exploitation of noise in the Sz. pombe system. Selected References 1.Didmon, M., K.Davis, P.Watson, G.Ladds, P.Broad, and J.Davey. 2002. Identifying regulators of pheromone signalling in the fission yeast Schizosaccharomyces pombe. Curr. Genet. 41:241-253. 2.Dohlman, H.G. and J.Thorner. 1997. RGS Proteins and Signaling by Heterotrimeric G Proteins. J. Biol. Chem. 272:3871-3874. 3.Fujita, S., A.Inanobe, M.Chachin, Y.Aizawa, and Y.Kurachi. 2000. A regulator of G protein signalling (RGS) protein confers agonist-dependent relaxation gating to a G protein-gated K+ channel. Journal of Physiology-London 526:341-347. 4.Ladds, G., A.Goddard, and J.Davey. 2005. Functional analysis of heterologous GPCR signalling pathways in yeast. Trends Biotechnol. 23:367-373. 5.Ulens, C., P.Daenens, and J.Tytgat. 2000. Changes in GIRK1/GIRK2 deactivation kinetics and basal activity in the presence and absence of RGS4. Life Sci. 67:2305-2317.