Document 13683868
advertisement

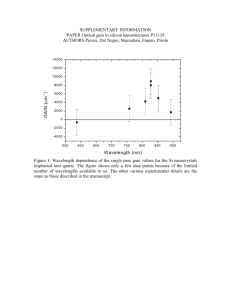
Copyright © 2005 American Scientific Publishers All rights reserved Printed in the United States of America Journal of Nanoscience and Nanotechnology Vol. 5, 1745–1748, 2005 Fabrication of a One-Dimensional Array of Nanopores Horizontally Aligned on a Si Substrate Hongguo Zhang,1 Zhi Chen,1 ∗ Tianxiang Li,2 and Kozo Saito2 1 Department of Electrical and Computer Engineering and Center for Nanoscale Science and Engineering, University of Kentucky, Lexington, KY 40506, USA 2 Department of Mechanical Engineering, University of Kentucky, Lexington, KY 40506, USA Delivered by Ingenta to University of Kentucky Libraries (cid 57004677), University of Kentucky (cid 70025377), University of A one-dimensional array of nanopores horizontally aligned on a silicon substrate was successfully Kentucky (cid 292596), University Kentucky Libraries (cidSEM 1058) fabricated by anodic aluminum oxidation (AAO) using aof modified two-step procedure. pictures IP : 127.0.0.1 show clear nanostructures of well-aligned one-dimensional nanopore arrays without cracks at the Thu, 01processes Sep 2005 interfaces of the sandwiched structures. The are13:17:18 compatible with the planar silicon integrated circuit processing technology, promising for applications in nanoelectronics. The formation mechanism of a single nanopore array on Si substrates was also discussed. Keywords: Nanopore Arrays, Anodization, AAO, 1-D Array, Nanotemplate. ∗ Author to whom correspondence should be addressed. J. Nanosci. Nanotech. 2005, Vol. 5, No. 10 fabrication of 1-D AAO templates using a sandwiched structure on a glass substrate (Al2 O3 /Al/Glass). However, they have only achieved very limited success, because no follow-up research has ever been carried out and the pore structures are hardly distinguishable in their scanning electron microscope (SEM) pictures.10 In addition, in their process, the Al2 O3 film and glass substrates were used, which are not compatible with the silicon integrated circuit processes. We attempted to improve their process so that it may be compatible with the Si integrated circuit processes. We found that the major difficulty for realization of the 1-D AAO nanopore array using conventional anodization was the cracks occurring at the interface between the SiO2 and the AAO layer. The presence of cracks suggests that this approach is unreliable and substantial improvement is needed. This might be why it is hard to view Masuda’s SEM images. In this communication, we will present successful fabrication of horizontal 1-D AAO templates without any cracks at the two interfaces using a modified two-step process, based on two types of structures, SiO2 /Al/SiO2 /Si and SiO2 /Al/Si. The fabrication processes are completely compatible with the Si integrated circuit processes. The fabrication processes of the two structures, SiO2 /Al/ SiO2 /Si and SiO2 /Al/Si, are illustrated schematically in Figure 1. First, For the SiO2 /Al/SiO2 /Si structure, a 100-nm layer of SiO2 was thermally grown on an undoped 1533-4880/2005/5/1745/004 doi:10.1166/jnn.2005.157 1745 COMMUNICATION In the past decade, as an effective self-assembly template, nanoscale anodic aluminum oxide (AAO) has attracted much interest for fundamental scientific research as well as industrial applications due to the high aspect ratio (∼1000), high pore density (∼1011 pores/cm2 , high level of ordering and uniformity.1–6 It is a very simple and facile approach because AAO templates are well-defined and their lengths and diameters are easily controlled. If the AAO template fabrication can be integrated into the mature Si integrated circuit processing technologies, it will be more useful for fabrication of nanoscale electronic devices. Up to now, it is still very difficult to utilize these AAO templates for fabrication of nanoelectronic devices, because all the AAO templates were vertically grown in two-dimensions (2-D) on substrates, not compatible with the mainstream Si planar processing technology. If a onedimensional (1-D) AAO array with nanopores horizontally aligned on a Si substrate can be formed, it will be much more promising for fabrication of nanoelectronic devices and nano-electromechanical systems (NEMS) using the planar processing technology. Although another material, mesoporous silica with a sub-10 nm pore structure, shows promising as a self-organized template,7–9 it is still very difficult to fabricate a single array of nanopore channels using mesoporous silica. Masuda et al.10 attempted Fabrication of a One-Dimensional Array of Nanopores Horizontally Aligned on a Si Substrate SiO2 SiO2 Al Zhang et al. (a) (b) (c) (d) Al SiO2 Si substrate Si substrate Hexagonal Cells Hexagonal Cells SiO2 Pores Al Pores SiO2 Al SiO2 Si substrate (a) Si substrate (b) Fig. 1. Fabrication procedures of one-dimensional horizontal nanopore arrays based on two types of structures: (a) SiO2 /Al/SiO2 /Si and (b) SiO2 /Al/Si. COMMUNICATION Si substrate. For the SiO2 /Al/Si structure, a non-oxidized Si substrate was used. Then, an aluminum layer with a Delivered by Ingenta to thickness ranging from ∼140 to 600 nm and a silicon University of Kentucky Libraries (cid 57004677), University of Kentucky (cid 70025377), University of oxide layer of ∼2 m were deposited on the oxidized Kentucky (cid 292596), University of Kentucky Libraries (cid 1058) and non-oxidized Si substrates using an e-beam evaporaIP : 127.0.0.1 tion apparatus from an aluminum source of 99.99% and Thu, respectively. 01 Sep 2005 13:17:18 a sintered silicon oxide source of 99.95% After formation of the lead contact on the aluminum layer with conductive epoxy, the subsequent sealing of the specFig. 2. Scanning electron microscope (SEM) images of AAO arrays imen except for the fractured surface with positive photo based on SiO2 /Al/SiO2 /Si structure with perfect interfaces for 600-nm thick aluminum film (a) (b) and 140 nm thick aluminum film (c) (d): resist was carried out. The clean specimen was anodized (a) 4–5 arrays of nanopores at a lower magnification and (b) their details in an oxalic acid solution of 0.20 M at 5–25 C at 40 V at a higher magnification; (c) One array of nanopores at a lower magnififor 3–7 minutes (the first step). Then the anodized oxide cation and (d) its details at a higher magnification. The modified two-step layer was removed by dipping the specimen into a mixture procedure is as follows: 5 min for the first step, 20 min for the second of phosphoric acid (6 wt%) and chromic acid (1.8 wt%) step, and 20 min for pore widening. at 55 C for 2–4 minutes. The reanodization (the second cross section is near the surface. It is well known that the step) was performed for 10–20 minutes under the same pores should be better organized deep inside the structure.1 conditions as the first step. Finally, the specimens were When the thickness of the Al film is about 140 nm, we dipped into a 5 wt% phosphoric solution at 20 C for obtained a well-aligned 1-D nanopore array (See Figs. 2c 20 minutes for pore widening. Field-emission scanning and 2d). The pore diameter is about 50 nm. The pore depth electron microscope (FE-SEM, JEOL JSM-6100) was used is about 500 nm according to the general anodization rate to examine all the specimens. at the same voltage and the same electrolyte solution. The basic assumption is that if the thickness of the aluminum film in the sandwiched structure is equivalent to the Cracks at interfaces are a great challenge during fabrisize of 4 cells, there should be 4 arrays of nanopores, and cation of the multi-layer structure, especially for our samif the thickness of the aluminum film in the sandwiched ples, which were dipped and etched in the acid solutions structure is equivalent to the size of a single cell, one array for a long time. For the conventional two-step anodizaof nanpores should be expected. Table I shows the cell tion method, the first step lasts for a very long time (up sizes of AAO anodized in various parameters. The porous to 10 hrs.) and the second step lasts for a short time (less structures of an Al film with a thickness of 600 nm were than 30 min.)11 Figures 3a and 3c show clearly cracks shown in Figures 2a and 2b. There are about 4–5 arrays occur at the SiO2 /AAO interface using the conventional of cells. The pores were not well ordered because the two-step anodization method, where the first step lasts Table I. Pore diameter, wall thickness, and cell size of AAO formed in various parameters. Electrolyte 0.2 0.3 0.3 0.3 1746 M M M M Oxalic acid, 32 F Sulfuric acid, 75 F Sulfuric acid, 75 F Sulfuric acid, 75 F Voltage V (V) Pore diameter P (nm) Wall thickness T (Å/V) Cell size W (Å) 40 20 10 8 50 30 15 10 120 100 90 80 1460 700 330 228 J. Nanosci. Nanotech. 5, 1745–1748, 2005 Zhang et al. Fabrication of a One-Dimensional Array of Nanopores Horizontally Aligned on a Si Substrate based on the SiO2 /Al/SiO2 /Si structure using the conventional anodization procedure showing cracks at the AAO/SiO2 interface (a)(c) and those using the modified two-step procedure showing no cracks at the AAO/SiO2 interface (b)(d). The conventional two-step method:8 >10 min for the first step, 20 min for the second step, and 20 min for pore widening. The modified two-step procedure: 5 min for the first step, 20 min for the second step, and 20 min for pore widening. J. Nanosci. Nanotech. 5, 1745–1748, 2005 Acknowledgments: This work is supported by National Science Foundation (ECS 0304129), Department of Energy (DE-FG02-00ER4582 and DE-FG26-04NT42171), Army Research Laboratory (W911NF-04-2-0023), and Kentucky Science & Engineering Foundation (KSEF-148502-03-78). 1747 of COMMUNICATION longer than 10 min. In order to solve this problem, optimal anodization parameters for 1-D nanopore templates were explored based on references.1 11–14 We have developed a modified two-step anodization procedure, i.e., a properly short first anodization followed with a relatively long Si second anodization. For our samples, the anodization was carried out in 0.20 M oxalic acid solution with a DC voltage of 40 V at 5 C (rather than room temperature) for 5 minutes for the first step and 20 minutes for the second step respectively. Using this modified two-step procedure, we succeeded in solving the crack problem based the SiO2 /Al/SiO2 /Si structure, seen in Figures 3b and 3d. Meanwhile, using the same procedure, a well-aligned 1-D (a) (b) nanopore template without cracks at interfaces from the Fig. 4. (a) Scanning electron microscope (SEM) images of 1-D horiSiO2 /Al/Si structure was also fabricated (See Fig. 4). zontal nanopore array based on the SiO2 /Al/Si sandwiched structure and The mechanisms for formation of only a single array (b) a typical cross-sectional image of the structure. The modified two-step of nanopores instead of two arrays of half-pores may be procedure is as follows: 5 min for the first step, 20 min for the second understood based on the self-adjusting effect.15 Initially step, and 20 min for pore widening. Delivered by Ingenta to barrier layer (oxide) is formed. In a corrosive solution, University of Kentucky Libraries (cidbegin 57004677), University of Kentucky right after the barrier layer formation, pores to be Al/barrier-layer interface).15(cid The70025377), growth rate University of oxide of Kentucky Libraries (cid 1058) initiated. The hexagonalKentucky oxide cells(cid are 292596), formed firstUniversity and depends on the local electric field at the Al/barrier interIP : 127.0.0.1 then pores are initiated at the centers of the cells. The face and the rate of dissolution depends on the local elec01 Sep tric 2005 13:17:18 pore and cell diameters and the barrier layer Thu, thickness field at the solution/barrier interface. This local field increase and a number of major pores eventually propais inversely proportional to the curvature of the pore base. gate and certain of incipient pores cease to function.15 16 There exists an equilibrium curvature of pore base in In the steady state, the field-assisted dissolution rate of the steady state. If the curvature is less than its equilibthe pore base (the solution/barrier-layer interface) equals rium value, the local field at the solution/barrier interface the field-assisted growth rate of oxide at cell base (the will increase, leading to stronger dissolution of the barrier layer. This tends to increase the curvature of the pore (b) (a) base. If the curvature is large than its equilibrium, the local field will decrease, leading to less dissolution of the barrier layer. This will reduce the curvature of the pore base. The equilibrium value will be maintained in the steady state due to the self adjustment. The same argument can be applied to the cell base. If for some reason, two cells are cramped into the space for only a single cell in equilibrium, the curvature of both cells is much less than its equilibrium value. This may result in strong dissolution of No Crack Crack the barrier layer due to much stronger local electric field. Thus two cells may spread toward each other until eventually a single cell emerges. (c) (d) In summary, the well-ordered 1-D horizontal nanopore arrays with pore diameters of ∼50 nm in sandwiched Crack No Crack structures on Si substrates were fabricated using the modified two-step anodization procedure without any cracks at interfaces. SEM pictures show clear nanostructures of the well-aligned 1-D nanopore arrays. The processes are compatible with the planar silicon integrated circuit processing technology, promising for applications in nanoelectronics. Fig. 3. Scanning electron microscope (SEM) images of AAO arrays Fabrication of a One-Dimensional Array of Nanopores Horizontally Aligned on a Si Substrate References and Notes 1. H. Masuda and K. Fukuda, Science 268, 1466 (1995). 2. O. Jessensky, F. Muller, and U. Gosele, Appl. Phys. Lett. 72, 1173 (1998). 3. W. B. Choi, E. J. Bae, J. W. Lee, and J. O. Lee, Appl. Phys. Lett. 79, 22 (2001). 4. S.-H. Jeong, H.-Y. Hwang, and K.-H. Lee, Appl. Phys. Lett. 78, 14 (2001). 5. D. Crouse, L. Yu-Hwa, E. Miller, and M. Crouse, Appl. Phys. Lett. 76, 49 (2000). 6. A. Cai, H. Zhang, H. Hua, and Z. Zhang, Nanotechnology 13, 627 (2002). 7. H. Miyata and K. Kuroda, Chem. Mater. 12, 49 (2000). 8. A. Okabe, T. Fukushima, K. Ariga, and T. Aida, Angew. Chem. Int. 41, 3414 (2002). Zhang et al. 9. V. R. Koganti and S. E. Rankin, J. Phys. Chem. B 109, 3279 (2005). 10. H. Masuda, K. Nishio, and N. Baba, Appl. Phys. Lett. 63, 3155 (1993). 11. H. Masuda and M. Satoh, Jpn. J. Appl. Phys. Part 2. 35, L126 (1996). 12. W. C. Hu, D. W. Gong, Z. Chen, L. M. Yuan, K. Saito, C. A. Grimes, and P. Kichambare, Appl. Phys. Lett. 79, 3083 (2001). 13. J. W. Diggle, T. G. Downie, and C. W. Goulding, Chem. Rev. 69, 365 (1969). 14. J. W. Diggle, T. G. Downie, and C. W. Goulding, in Modern Aspects of Electrochemistry, edited by J. O. M. Bokris, R. E. White, B. E. Conway, Plenum Press, New York (1989) Vol. 23, p. 401. 15. J. P. O’Sullivan and G. C. Wood, Proc. Royal Soc. of London 317, 511 (1970). 16. F. Keller, M. S. Hunter, and D. L. Robinson, J. Electrochem. Soc. 100, 411 (1953). Received: 16 February 2005. Accepted: 1 March 2005. COMMUNICATION Delivered by Ingenta to University of Kentucky Libraries (cid 57004677), University of Kentucky (cid 70025377), University of Kentucky (cid 292596), University of Kentucky Libraries (cid 1058) IP : 127.0.0.1 Thu, 01 Sep 2005 13:17:18 1748 J. Nanosci. Nanotech. 5, 1745–1748, 2005