Joint Research Office
advertisement

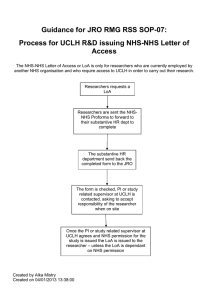
Joint Research Office Office Location: 1st Floor Maple House 149 Tottenham Court Road London W1T 7DN Postal Address: UCL, Gower Street London WC1E 6BT Tel No. 0203 447 9995 Fax No 0203 447 9937 Web-sites: www.ucl.ac.uk/jro; www.uclh.nhs.uk JRO RMG RSS SOP-02 Standard Operating Procedure (SOP) for Categorisation and Sponsorship of “Simple” Studies SOP ID Number Version Number JRO RMG RSS SOP-02 Version 1.0 Author: Name and Job Title Approved by Date: Dr Susan Kerrison Head of Risk and Regulation Target Trusts UCLH and Royal Free Target Audience SRA, DIO and RNCs Effective Date March 2012 Related SOPs SOP e-Document kept: G: \RM&G Team\NIHR RMG RSS SOPs\ Final; S:RMG\NIHR RSS SOPs JRO RMS SOP 02 SOP for Categorisation and Sponsorship for “Simple” studies 1 of 11 Review Date March 2014 Revision Chronology Previous SOP ID Number Effective Date Reasons for Change JRO RMS SOP 02 SOP for Categorisation and Sponsorship for “Simple” studies 2 of 11 Author Joint Research Office Standard Operating Procedure (SOP) for Categorisation and Sponsorship of “Simple” Studies 1. SUMMARY This SOP is part of the suite of SOPs to implement the NIHR Research Support Services Framework. It describes the processes for granting UCL, UCLH and RFH sponsorship to “simple” studies. 2. DEFINITIONS Box 1 “Simple” or “Complex” Any study where the following boxes are selected for screening question 2 on the NHS R and D and NHS REC form should be deemed a “simple” study. Any other studies should be treated as "complex". • • • • Study administering questionnaires/ interviews for quantitative analysis, or using mixed quantitative/qualitative methodology Study involving qualitative methods only Study limited to working with human tissues samples (or other biological samples) and data Study limited to working with data “External sponsored studies” - studies not sponsored by UCL, UCLH or RFH for which UCLH or RFH host approval is required. 3. PURPOSE and SCOPE The purpose of this SOP is to describe the process for classifying studies as “simple” or “ complex” outline steps required to process a “simple” study including deciding on sponsorship, establishing whether contract is required identify the responsibilities for processing “simple” studies The SOP does not cover the specific requirements for NHS permission which is dealt with in NIHR RSS RMG SOP 05 This SOP does not apply to UCL sponsored CTIMPs. A list of published UCL sponsored CTIMP SOPs are available at: http://www.ucl.ac.uk/jro/standingoperatingprocedures/document-library JRO RMS SOP 02 SOP for Categorisation and Sponsorship for “Simple” studies 3 of 11 4. RELATED SOPs JRO RMG RSS SOP 03 JRO RMG RSS SOP 05 JRO RMG RSS SOP 07 JRO RMG RSS SOP 06 Sponsorship and Approval of “Complex” studies NHS Permissions Honorary contracts and letters of access External Agreements: Set up and Control 5. JOINT RESEARCH OFFICE (JRO) POLICY All SOPs produced from the JRO must be used in conjunction with local NHS Trust and UCL policies and procedures. Where applicable there is also a requirement to include stakeholder engagement as appropriately identified. The JRO represents both UCL, UCLH or RF as the Sponsor and UCLH and RF as Participating Sites, depending on the type of research study. The JRO is responsible to ensure that appropriate research management and governance processes are in place. The RMG RSS SOPs will provide a quality assurance system for these requirements. 6. BACKGROUND This SOP complies with Research Governance Framework for Health and Social Care 2005 (2nd Edition), NIHR Research Support Services framework JRO RMS SOP 02 SOP for Categorisation and Sponsorship for “Simple” studies 4 of 11 7. RESPONSIBLE PERSONNEL AND THEIR DUTIES Responsible person 1 Database and Information Officer (DIO) Summary of duies 2 Senior Research Administrator (SRA) 3 RM & G Network Manager/ QA manager 4 Research Network Coordinator (students) 5 Research Network Coordinators (RNC) Assesses whether project is a research study. Assesses whether project is “simple” or “complex”. Sets REDA flag accordingly Refers “complex” studies to RNC. Assesses whether a sponsor is required Refers hosted studies to SRA Assesses eligibility for UCL sponsorship Refers study to contract/finance team is required If eligible processes study for UCL sponsorship If UCLH or RFH NHS permission is required, refers to SRAs Assesses whether project is research Assesses whether project is “simple” or “complex”. Sets REDA accordingly Refers “complex” studies to RNC. Assesses whether a sponsor is required Refers study to contract/finance team if required Processes sponsorship for UCLH and RFH if required Processes NHS permissions for UCLH and RFH in accordance with RMG RSS SOP 05 Assesses whether projects referred by DIO, RNC or SRA are research Quality control host approval and sponsorship processes as outlined in relevant SOP Advise and confirm sponsorship decision on a cases by case basis when required Reviews and processes student studies Assesses whether project is a research study Assesses whether project is “simple” or “complex Processes “complex” studies in accordance with RMG RSS SOP 03 Assesses whether projects referred by DIO, RNC or SRA are research Advises and confirms a decision whether contractual arrangements are required 6 Head of Risk and Regulation 7 Contracts Manager JRO RMS SOP 02 SOP for Categorisation and Sponsorship for “Simple” studies 5 of 11 8. PROCEDURE 8.1 The following people may receive studies from research teams Database Information Officer (DIO) Senior Research Administrators (SRA) Research Network Co-ordinators (RNC) On receipt of a study, the DIO, SRA, or RNC should assess whether the study is research. Information about this can found at http://www.nres.npsa.nhs.uk/applications/is-your-projectresearch. If the DIO, SRA or RNC is uncertain whether to classify the project as a research study then they should refer the study or the Senior RNC or Head of Risk and Regulation for an opinion. 8.2 If the study is deemed a research study then the DIO, SRA and RNC should assess whether the study is to be categorised as “simple” or “complex” as in Box 1 above. After categorisation the flag should be set in REDA. 8.3 All “complex” studies will be referred to the RNC for processing. DIO and SRAs will handle both the sponsorship and host approval processes for “simple” studies. 8.4 For simple studies, the DIO or SRA will decide whether the study requires sponsorship or has a sponsor outside UCL, UCLH or RF. If UCLH, RFH or UCL sponsorship is required the DIO or SRA will assess which model is applicable - see box 2. After contracts review see 8.5 below, studies with a sponsorship which is not UCLH, RFH, or UCL which require UCLH or RFH NHS permission will be then be approved in accordance with JRO RSS RMG SOP 05 Box 2 Rules for UCLH, RFH or UCL Sponsorship of “Simple” studies 1. UCLH and Royal Free will sponsor non CTIMPs where • The study is registered and managed within the JRO • The study is single site and recruiting only NHS patients at the site sponsoring the study. 2. UCL will sponsor other “simple” studies if Chief Investigator (CI) has a substantive employment contract or an honorary contract/passport with UCL. 3. UCL will sponsor student studies, if students are undertaking a recognised course with UCL 4. The JRO has flexibility in how it allocates sponsorship particularly for single site and student studies. In some circumstances, it might be advisable to take a pragmatic approach to allocation of sponsorship. The Research Network Manager will advise. 8.5 Studies will handled by the following staff UCLH or RFH hosted “simple” studies SRAs UCLH or RFH sponsored “simple” studies SRAs JRO RMS SOP 02 SOP for Categorisation and Sponsorship for “Simple” studies 6 of 11 UCL students studies Other UCL sponsor “simple” studies RNC (students) DIO Contracts 8.6 The RNC (students) will assess the need for agreement for student studies. Only in exceptional circumstances will formal agreements be required for student studies. In other circumstances, the requirements for studies will be written into a specifically tailored approvals letter. 8.7 At the time of finalising this SOP, a review of requirements for contracts is in progress which may change the following requirements. When this SOP was approved, studies in following categories needed to be referred to the contracts/finance team for a review studies where monies are transferred between institutions eg UCL and UCLH or UCL and North Midx involves a commercial organisation involves the transfer of identifiable data or human tissue between institutions including UCL. Identifiable data is data which contains person information in particular the names and addresses or dates of birth of patient. That is data which has not been anonymised or pseudonymised. Pseudonymised data is data which has identifiable information removed and replace with a unique key. Processing UCL, UCLH and RFH sponsored and hosted “simple” studies 8.8 For UCL sponsored “simple” studies, no UCL risk assessment form, or UCL insurance form is required to be completed. Investigators will be asked to complete a Data Protection Form but this is currently under review. The investigator will be advised on the appropriate wording for complaints and insurance for Subject Information Sheet and Protocol as in Appendix 1. 8.9 For studies hosted UCLH or RFH which meet the criteria for either UCLH or RFH sponsorship as in Box 2, no other sponsorship approval processes are required. 8.10 Studies which require NHS permissions will then be processed under JRO RMG RSS SOP 05. 8.11 The study documentation should then be entered onto REDA. JRO RMS SOP 02 SOP for Categorisation and Sponsorship for “Simple” studies 7 of 11 Flow diagram for a “simple” study Study sent to SRA, DIO or RNC SRA. DIO or RNC to register and assign R and D number SRA, DIO or RNC decides “simple” or “complex” according to definition below based on question A5 of ethics form - see Box 1. Flag set on REDA accordingly Simple Complex SRA or DIO to decide if sponsorship required – see Box 2 for sponsorship rules. Yes Trust eg RFH or UCLH sponsorship processed by SRA RNC. Then follow SOP03 No Yes UCL sponsorship DIO Other sponsor ie hosted study Does the study need a contract ? Yes Refer to contracts and finance team. Contracts to inform SRA or DIO if contract required and flag set No Is UCLH or RFH NHS permission required ? Yes S No Agree study after documentation entered onto database JRO RMS SOP 02 SOP for Categorisation and Sponsorship for “Simple” studies 8 of 11 9. IMPLEMENTATION & TRAINING This SOP will be implemented according the procedure agreed by the Approvals Development Group. 10. PUBLICATION & COMMUNICATION This SOP is published JRO website: http://www.ucl.ac.uk/jro. G: \RM&G Team\NIHR RMG RSS SOPs\ Final; S:RMG\NIHR RSS SOPs The fully approached and signed master copy is also stored in a designated binder within the JRO. 11. REVIEW SOPs will be reviewed every 2 years unless an earlier review is required. 12. REFERENCES JRO Website http://www.ucl.ac.uk/jro Research Governance Framework 2005 (2nd Edition) http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digita lasset/dh_4122427.pdf NIHR RSS Framework (including Appendix 1 – Glossary of Terms and Acronyms) http://www.nihr.ac.uk/systems/Pages/RSS_Documents.aspx 13. SIGNATURES Author: Name / Job Title Signature / Date: Reviewed by: Name / Job Title Signature / Date: JRO RMS SOP 02 SOP for Categorisation and Sponsorship for “Simple” studies 9 of 11 Authorised by: Name / Job Title Signature / Date: 16. ACRONYMS CI CTIMP DIO GCP ISF JRO MHRA NIHR RSS PI QA REC REDA RM&G RNC RFH SI SOP SRA TMF UCL UCLH Chief Investigator Clinical Trial of Investigational Medicinal Products Database & Information Officer Good Clinical Practice Investigator Site File Joint UCL/UCLH/Royal Free Research Office Medicines and Healthcare products Regulatory Agency National Institute for Health Research (NIHR) Research Support Services Principal Investigator Quality Assurance Research Ethics Committee Research Database Research Management & Governance Research Network Co-ordinator Royal Free Hospital NHS Trust Statutory Instrument Standard Operating Procedure Senior Research Administrator Trial Master File University College London University College London Hospitals NHS Foundation Trust JRO RMS SOP 02 SOP for Categorisation and Sponsorship for “Simple” studies 10 of 11 Appendix 1 Wording for patient information sheets and protocols where study is sponsored by UCL Wording for patient or subject information sheets “What if there is a problem “ or “What happens if something goes wrong ? “ If you wish to complain, or have any concerns about any aspect of the way you have been approached or treated by members of staff you may have experienced due to your participation in the research, National Health Service or UCL complaints mechanisms are available to you. Please ask your research doctor if you would like more information on this. In the unlikely event that you are harmed by taking part in this study, compensation may be available. If you suspect that the harm is the result of the Sponsor’s (University College London) or the hospital's negligence then you may be able to claim compensation. After discussing with your research doctor, please make the claim in writing to the [add name of Chief Investigator] who is the Chief Investigator for the research and is based at [add location name for Chief Investigator]. The Chief Investigator will then pass the claim to the Sponsor’s Insurers, via the Sponsor’s office. You may have to bear the costs of the legal action initially, and you should consult a lawyer about this. Wording for protocols University College London holds insurance against claims from participants for harm caused by their participation in this clinical study. Participants may be able to claim compensation if they can prove that UCL has been negligent. However, if this clinical study is being carried out in a hospital, the hospital continues to have a duty of care to the participant of the clinical study. University College London does not accept liability for any breach in the hospital’s duty of care, or any negligence on the part of hospital employees. This applies whether the hospital is an NHS Trust or otherwise.” JRO RMS SOP 02 SOP for Categorisation and Sponsorship for “Simple” studies 11 of 11