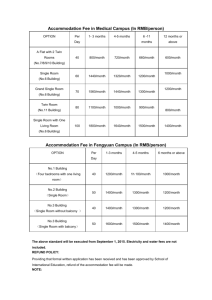
Multi-Wavelength, Depth Resolved, Scattering and Pathlength Corrected in-vivo Near-
advertisement

Biomedical Optics (BIOMED) 2010 paper: BTuB7 OSA Technical Digest (CD) Multi-Wavelength, Depth Resolved, Scattering and Pathlength Corrected in-vivo NearInfrared Spectroscopy of Brain Tissue. ILIAS TACHTSIDIS1, T.S. Leung1, A. Ghosh2, M. Smith2, C.E. Cooper3, C.E. Elwell1 1 Biomedical Optics Research Laboratory, Department of Medical Physics and Bioengineering, University College London, Gower Street, London WC1E 6BT, Email: iliastac@medphys.ucl.ac.uk 2 Neurocritical Care Unit, National Hospital for Neurology and Neurosurgery, University College London Hospital, Queen Square, London, UK 3 Department of Biological Sciences, University of Essex, Colchester Essex, UK Introduction isobestic Specific Extinction Coefficient (OD/M/cm) 5000 4500 4000 3500 λ1 λ2 Water Scaled HbO2 HHb ox-redCCO 3000 2500 2000 1500 1000 500 0 650 675 700 725 750 775 800 825 850 875 900 925 950 Wavelengths (nm) 1. With multi-spectral data, more chromophores can be resolved while improving spectroscopic resolution. Introduction 2. Multi-distance attenuation data reduces sensitivity to the optical coupling and to the attenuation changes in the superficial layers. Introduction Accurate absolute quantification of tissue chromophores requires knowledge of light scattering. I: Light Intensity 3. The tissue transport scattering (µs’), can be measured with frequency (or time) domain instrumentation. Instrumentation: Hybrid Optical Spectrometer [pHOS] Multi-Distance Frequency Domain Multi-Distance Broadband Spectrometer Instrument 1 Four wavelength (690, 750, 790, 850nm) multi-distance frequency domain (110MHz) spectrometer (MDFD). The instrument allows for the quantitative assessment of the absorption and scattering coefficients at two different source-detector distances (3 and 3.5cm)at each wavelength. [D.M. Hueber Phys. Med. Biol. 46, 41-62 (2001)] Instrument 2 White light source (50W halogen bulb) with a short pass filter to minimise temperature effects. Four distances (2.0, 2.5, 3.0 and 3.5cm) utilising four detector fibres with different diameters. Optical bandwidth 504 -1068nm and mean wavelength resolution 4nm. Detectors Emitter Detectors Distal End Instrumentation Operation Background Aim Here we report on the use of our novel multi-distance frequency and broadband domain system to measure brain oxygenation, haemodynamics and metabolism during changes in cerebral oxygenation secondary to carbon-dioxide induced changes in cerebral blood flow (CBF) in a healthy adult. Study Protocol and Systemic Measurements + CO2 Hypercapnia Hyperventilation Baseline Hypocapnia 0 5 10 15 20 25 (minutes) -Mean Velocity of Middle Cerebral Artery (Vmca) -Mean Blood Pressure (MBP) -Arterial Oxygen Saturation (SaO2) -Breathing Gases including End-Tidal CO2 (EtCO2) Results EtCO2 10 9 Hypercapnia Hypocapnia 70 (cm/sec) 8 7 (kPa) Vmca 80 Hypercapnia Hypocapnia 6 5 4 3 60 50 40 30 20 2 10 1 0 0 0 200 102 400 600 800 1000 1200 Time (seconds) Hypocapnia 1400 0 200 400 600 800 1000 1200 1400 Time (seconds) SaO2 MBP 180 Hypercapnia Hypercapnia Hypocapnia 160 140 (mmHg) (%) 100 98 96 94 120 100 80 60 40 92 20 0 90 0 200 400 600 800 1000 Time (seconds) 1200 1400 0 200 400 600 800 1000 1200 Time (seconds) 1400 Optical Algorithm 1 MDFD µa (MDFD) µs’ (MDFD) DPF( MDFD) = 3µs' ( MDFD) Pathlength Detector 1 L1(790nm)=DPF(790nm) × 3.5cm 2 µa ( MDFD) Specific Extinction Coefficient (OD/M/cm) (OD/M/cm Fitting from 740 to 900nm Pathlength Detector 2 L2(790nm)=DPF(790nm) × 3.0cm Detector 1 (3.5cm): ∆[ HbO2 ] 1 ∆[ HHb] = L1( 790 nm ) ∆[oxCCO] ( Det1) specific extinction coefficient multiplied by the correction factors for the wavelength dependence of the optical pathlength at each wavelength i: chromophores j: wavelengths ∆A(λn) (Det1) ∆A(λn) (Det2) ∆A(λn) (Det3) ∆A(λn) (Det4) Wavelengths (nm) ε: MDBBS Detector 2 (3.0cm): ∆[ HbO2 ] 1 ∆[ HHb] = L2( 790 nm ) ∆[oxCCO] ( Det 2) ∆A( λ1) ∆ A (λ 2) −1 ⋅ (ε i , j ) ⋅ ...... ∆A( λn ) ( Det1) ∆A( λ1) A ∆ ( λ 2) −1 ⋅ (ε i , j ) ⋅ ...... A ∆ ( λn ) ( Det 2 ) µ’s 12.0 11.5 11.0 10.5 10.0 9.5 9.0 8.5 8.0 7.5 7.0 6.5 6.0 Hypocapnia µa 0.15 Hypercapnia Hypocapnia 0.13 0.12 0.11 0.10 690nm 790nm 0.09 750nm 850nm 690nm 790nm 750nm 850nm 0.08 0 200 400 600 800 1000 1200 1400 0 200 400 Pathlength (Det 1 3.5cm) 34 Hypocapnia 600 800 1000 1200 1400 Time (s) Time (s) 30 Hypercapnia 32 28 30 26 (cm) (cm) Hypercapnia 0.14 (cm-1) (cm-1) MDFD Pathlength Measurement 28 26 Pathlength (Det 2 3.0cm) Hypocapnia Hypercapnia 24 22 24 690nm 790nm 750nm 850nm 22 20 690nm 750nm 790nm 850nm 18 0 200 400 600 800 Time (s) 1000 1200 1400 0 200 400 600 800 1000 Time (s) 1200 1400 pHOS Brain Tissue ∆[Concentrations] From the changes in light attenuation (MDBBS) and using the continuous measured Pathlength for 790nm (MDFD) the changes in concentrations of [HbO2], [HHb] and [oxCCO] were calculated by fitting from 740 to 900nm and correcting for the wavelength dependency of DPF. ∆[Concentrations] 3.5cm (Det 1) ∆[Concentrations] 3.0cm (Det 2) ∆[ ∆[ 1.5 Hypocapnia 1.5 Hypercapnia Hypercapnia 1.0 1.0 0.5 (µ µM) 0.5 (µ µM) Hypocapnia 0.0 -0.5 0.0 -0.5 -1.0 -1.0 HbO2 oxCCO HHb -1.5 -1.5 HbO2 oxCCO HHb -2.0 0 200 400 600 800 1000 Time (s) 1200 1400 0 200 400 600 800 1000 Time (s) 1200 1400 Hybrid Spatially Resolved Algorithm MDFD MDBBS Calculate Attenuation Slope: ∂Aλn/∂ρ µa (MDFD) Aλn (Det1) µs’ (MDFD) Aλn (Det2) µ aSRS ( λn ) Power Law: µs’ (λn) = bλ-a a, b: free parameters in the fit ∂A( λn ) ⋅ ln 10 ⋅ = ∂ρ 3 ⋅ µ 's( λn ) 1 Scale µaSRS(λn) using µa (MDFD) A 2 − ρ 2 Aλn (Det3) Aλn (Det4) Absolute µaHybrid(λn) Specific Extinction Coefficient (OD/M/cm) 4500 4000 3500 HbO2 HHb Water*10^6 Fitting from 680 to 860nm 3000 [ HbO2 ] [ HHb] [ H 2 O] 2500 2000 1500 1000 500 0 650 675 700 725 750 775 800 825 850 875 900 925 950 975 1000 Wavelengths (nm) µ aHybrid ( λ1) Hybrid −1 µ a (λ 2) = (ε i , j ) ⋅ ...... µ Hybrid ( λn ) a i: chromophores j: wavelengths pHOS Brain Optical Measurement µ’s 11.0 ∂A/∂ρ ρ 0.93 0.92 10.5 0.91 (OD/cm) (cm-1) 10.0 9.5 9.0 8.5 0.90 0.89 0.88 0.87 0.86 8.0 0.85 7.5 0.84 7.0 680 0.83 680 700 720 740 780 800 820 840 860 740 760 780 800 820 µaSRS µaHybrid 0.13 0.070 0.11 (cm-1) 0.12 0.065 0.09 0.055 0.08 0.050 680 0.07 680 740 760 780 800 Wavelengths (nm) 820 840 860 840 860 0.10 0.060 720 720 Wavelengths (nm) 0.075 700 700 Wavelengths (nm) 0.080 (cm-1) 760 700 720 740 760 780 800 820 Wavelengths (nm) 840 860 Results pHOS Brain Tissue Concentrations [Concentrations] 60 Hypercapnia Hypocapnia 72 50 70 (%) 40 (µ µM) [Tissue Saturation] 74 Hypercapnia Hypocapnia 30 68 66 20 64 HbO2 HHb HbT 10 0 62 60 0 200 400 600 800 1000 1200 1400 0 200 400 600 Hypocapnia 80 Hypercapnia 5 1200 1400 Hypercapnia Hypocapnia 70 4 3 60 2 50 1 (%) (µ µM) 1000 [Water] ∆[Concentrations] ∆[ 6 800 Time (s) Time (s) 0 40 -1 30 -2 20 -3 HbO2 -4 HHb -5 10 0 0 200 400 600 800 Time (s) 1000 1200 1400 0 200 400 600 800 Time (s) 1000 1200 1400 Results pHOS Brain Tissue Absorption Fitting Baseline 0.13 [HbO2] : 31µM [HHb] : 16µM [Water]: 45% 0.12 0.11 (cm-1) We estimate back µa from the calculated absolute concentrations and compare with the measured µaHybrid to assess the fitting (residuals). µaFitted 0.10 0.09 µaHybrid 0.08 0.07 680 700 720 740 760 780 800 820 840 860 Wavelengths (nm) Hypocapnia 0.12 µM [HbO2] : 29µ [HHb] : 18µ µM [Water] : 37% 0.11 µaFitted µM [HbO2] : 34µ [HHb] : 15µ µM [Water] : 45% 0.12 µaFitted 0.11 0.10 0.09 µaHybrid 0.08 Hypercapnia 0.13 (cm-1) (cm-1) 0.13 0.10 0.09 0.08 0.07 µaHybrid 0.07 680 700 720 740 760 780 800 820 Wavelengths (nm) 840 860 680 700 720 740 760 780 800 820 Wavelengths (nm) 840 860 Discussion • We demonstrated the use of our pHOS system to investigate oxygenation, haemodynamics and metabolic signals in a healthy adult head. • The combination of multi-distance frequency domain and broadband spectrometers allows: 1. to measure pathlength continuously and hence correctly scale the changes in concentrations; 2. to obtain multi-wavelength, depth resolved measurements of absorption. • The second approach is especially important for NIRS measurement of the adult brain where broad wavelength coverage and increased sensitivity to deeper layers are required. Discussion • These preliminary results show that during hypercapnia the changes in haemoglobin concentrations calculated with the conventional differential spectroscopy method are smaller than those calculated using the hybrid SRS method, possibly indicating different contributions from the intra- and extracerebral layers. • This technology is currently being used for studies in human adult volunteers and in critically ill brain-injured patients. The pHOS system in use on a traumatic brain injured patient at the National Hospital for Neurology and Neurosurgery in London, UK. Acknowledgments Many thanks to Prof. Matthias Kohl-Bareis and Dr Dennis Hueber for technical advice and support. The authors would like to thank the EPSRC (EP/D060982/1) for the financial support of this work. This work was undertaken at University College London Hospitals and partially funded by the Department of Health's National Institute for Health Research. Dr Ilias Tachtsidis is supported by The Wellcome Trust