Avoiding Bias in Longitudinal Image Processing © Martin Reuter
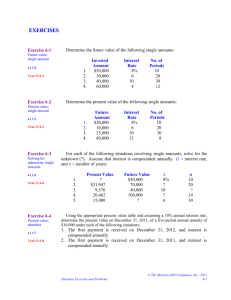
advertisement

Avoiding Bias in
Longitudinal Image
Processing
© Martin Reuter
mreuter@nmr.mgh.harvard.edu
http://reuter.mit.edu
Quantifying Imaging Biomarkers
Here we focus on www.FreeSurfer.net
• measure volume of cortical or subcortical structures
• compute thickness (locally) of the cortical sheet
• study differences of populations (diseased, control)
Neurodegenerative disorder:
14 time points, 6 years, Huntington’s Disease
We'd like to:
• exploit longitudinal information
(same subject, different time points))
Why longitudinal processing?
• to reduce variability on intra-individual morph. estimates
• to detect small changes, or use less subjects (power)
• for marker of disease progression (atrophy)
• to better estimate time to onset of symptoms
• to study effects of drug treatment
...
[Reuter et al, NeuroImage 2012]
Example (cross vs. long)
Example (subject as own control)
Challenges in Longitudinal Designs
1. Over-Regularization:
• Temporal smoothing
• Non-linear warps (regularization)
Ø Potentially underestimate change
2. Bias [Reuter and Fischl, NeuroImage 2011] , [Reuter et al. NeuroImage 2012]
• Interpolation Asymmetries [Yushkevich et al., NeuroImage 2010],
[Thompson, Holland, NeuroImage 2011], [Fox, Ridgway, Schott, NeuroIm. 2011]
• Asymmetric Information Transfer
Ø Often overestimate change
Interpolation Asymmetries (Bias)
Mapping follow-up to baseline:
• Keeps baseline image fixed (crisp)
• Causes interpolation artifacts in follow-up (smoothing)
• Often leads to overestimating change
Interpolation Artifacts
• Replicate by mapping an image somewhere and then
back (will interpolate twice).
• For example register A->B to get some transform T, then
map A via T and use the inverse transform to map it back.
mri_robust_register --mov A.nii --dst B.nii --lta T.lta --satit
mri_convert -at T.lta A.nii AatB.nii
mri_convert -ait T.lta AatB.nii AatA.nii
freeview -v A.nii AatA.nii
• Now analyze AatA as second time point with respect to A
Interpolation Asymmetries (Bias)
Left Subcortical Structures
Right Subcortical Structures
10
Sym. Pct. Volume Change
Sym. Pct. Volume Change
10
5
0
−5
−10
5
0
−5
−10
Thal
Caud
Put
Pal
Hippo
Amyg CortGM
WM
Thal
Caud
Put
Pal
Hippo
Amyg CortGM
WM
MIRIAD dataset: 65 subjects
First session first scan compared to twice interpolated image.
http://miriad.drc.ion.ucl.ac.uk
Interpolation Asymmetries (Bias)
Left Cortical ROIs
Left White Matter ROIs
15
−5
Sym. Pct. Volume Change
Sym. Pct. Volume Change
0
−10
−15
10
5
−20
−25
Cuneus PreCun CaMidFr SupFron PreCen SupTmp ParaHip InfPar
0
Cuneus PreCun CaMidFr SupFron PreCen SupTmp ParaHip InfPar
MIRIAD dataset: 65 subjects
First session first scan compared to twice interpolated image.
http://miriad.drc.ion.ucl.ac.uk
Tri-Linear vs. Cubic B-Spline
Interpolation (Bias)
Left Cortical ROIs
Left Subcortical Structures
[Tri−Linear] (65)
[Cubic] (65)
0
Sym. Pct. Volume Change
Sym. Pct. Volume Change
10
5
0
−5
−5
−10
−15
−20
−10
[Tri−Linear] (65)
[Cubic] (65)
Thal
Caud
Put
Pal
Hippo
Amyg CortGM
WM
−25
Cuneus PreCun CaMidFr SupFron PreCen SupTmp ParaHip InfPar
Regional!
Not finding it does not mean it is not there!
We need to treat all time points the same!
Robust Registration
[Reuter et al., NeuroImage, 2010]
Inverse consistency:
• a symmetric displacement model:
1 ⎞ S⎛ 1 ⎞
T⎛
r ( p) = I ⎜ x − d ( p) ⎟ − I ⎜ x + d ( p) ⎟
2
2
⎝
⎠
⎝
⎠
• resample both source and target to an unbiased
half-way space in intermediate steps (matrix
square root)
M
Source
Half-Way
Target
M
−1
Robust Registration
[Reuter et al., NeuroImage, 2010]
Tumor data courtesy of Dr. Greg Sorensen
Tumor data with significant intensity differences in the
brain, registered to first time point (left).
Robust Registration [Reuter et al 2010]
Target
Target
Robust Registration [Reuter et al 2010]
Registered Src FSL FLIRT
Registered Src Robust
mri_robust_register
• mri_robust_register is part of FreeSurfer
• can be used for pair-wise registration (optimally within
subject, within modality)
• can output results in half-way space
• can output ‘outlier-weights’
• see also Reuter et al. “Highly Accurate Inverse
Consistent Registration: A Robust Approach”,
NeuroImage 2010. http://reuter.mit.edu/publications/
• for more than 2 images: mri_robust_template
Asymmetric Information Transfer
Example:
1. Process baseline
2. Transfer results from
baseline to follow-up
3. Let procedures
evolve in follow-up
(or construct skullstrip
in baseline, or Talairach
transform …)
Can introduce bias!
[Reuter 2011, 2012]
Robust Unbiased Subject Template
1. Create subject
template (iterative
registration to median)
2. Process template
3. Transfer to time points
4. Let it evolve there
- All time points are
treated the same
- Minimize overregularization by letting
tps evolve freely
[Reuter et al., NeuroImage, 2012]
Robust Template Estimation
• Minimization problem for N images:
ˆ 'ˆi } := argmin
{I,
I,'i
N
X
E(Ii 'i , I) + D('i )2
i=1
• Image Dissimilarity:
Z
E(I1 , I2 ) =
|I1 (x)
I2 (x)| dx
⌦
• Metric of Transformations:
D(~t, r)2 =k ~t k2 + k R
1 k2F
Biased Information Transfer
MIRIAD Data (within session at baseline)
Biased Information Transfer
MIRIAD Data (within session at baseline)
Bias in Subcortical Volumes (MIRIAD Session)
6
6
[BASE1] (65)
[BASE2] (65)
[FS−LONG] (65)
[FS−LONG−rev] (65)
2
0
−2
2
0
−2
−4
−4
−6
−6
LPallidum
LHippoc LAmygdala RPallidum
RHippoc RAmygdala
PreCen
0
−2
−4
LatOcc
Cuneus
PreCun
[BASE1] (65)
[BASE2] (65)
[FS−LONG] (65)
[FS−LONG−rev] (65)
4
Sym. Pct. Volume Change
Sym. Pct. Volume Change
2
ParaHip
6
[BASE1] (65)
[BASE2] (65)
[FS−LONG] (65)
[FS−LONG−rev] (65)
4
CACing
Bias in Left Cortical GM Volumes (MIRIAD Session)
Bias in Left Cortical GM Volumes (MIRIAD Session)
6
[BASE1] (65)
[BASE2] (65)
[FS−LONG] (65)
[FS−LONG−rev] (65)
4
Sym. Pct. Volume Change
4
Sym. Pct. Volume Change
Bias in Left Cortical GM Volumes (MIRIAD Session)
2
0
−2
−4
−6
−6
CaMidFr
SupTmp
InfPar
Lingual
MedOrbFr MidTemp
ParaCen
Perical
PostCen
SupPari
SupMarg TransTemp
How and Why:
How to minimize over regularization:
ü Only initialize processing, evolve freely
How to avoid processing bias:
ü Treat all time points the same
Why not simply do independent processing then?
Ø Increase reliability, statistical power
Ø See for yourself …
Test-Retest Reliability
[Reuter et al., NeuroImage, 2012]
Subcortical
Cortical
Left Subcortical Structures TT−115
Left Cortical Gray Matter Parcellation TT−115
8
[CROSS] (115)
[LONG] (115)
7
Abs. Sym. Pct. Volume Change
Abs. Sym. Pct. Volume Change
7
8
6
5
4
3
2
1
0
[CROSS] (115)
[LONG] (115)
6
5
4
3
2
1
Thalamus
Caudate
Putamen
Pallidum Hippocamp Amygdala
0
Cuneus PreCun CaMidFr SupFron PreCen SupTmp ParaHip InfPar
[LONG] significantly improves reliability
115 subjects, ME MPRAGE, 2 scans, same session
Test-Retest Reliability
[Reuter et al., NeuroImage, 2012]
Diff. ([CROSS]-[LONG])
of Abs. Thick. Change:
Significance Map
[LONG] significantly improves reliability
115 subjects, ME MPRAGE, 2 scans, same session
Increased Power
[Reuter et al., NeuroImage, 2012]
Left Hemisphere:
Right Hemisphere
100
90
80
70
60
50
40
30
20
10
0
Sample Size Reduction (Right Hemisphere)
Percent Subjects needed (LONG vs. CROSS)
Percent Subjects needed (LONG vs. CROSS)
Sample Size Reduction (Left Hemisphere)
Thalamus Caudate
Putamen
Pallidum Hippocamp Amygdala
100
90
80
70
60
50
40
30
20
10
0
Thalamus Caudate
Putamen
Pallidum Hippocamp Amygdala
Sample Size Reduction when using [LONG]
Huntington’s Disease (3 visits)
[Reuter et al., NeuroImage, 2012]
Independent Processing
Longitudinal Processing
Atrophy in Huntington’s Disease [CROSS]
Atrophy in Huntington’s Disease [LONG]
2
[CN] (10)
[PHDfar] (16)
[PHDnear] (19)
[HD] (9)
1
0
−1
−2
−3
−4
LThalamus LCaudate LPutamen RThalamus RCaudate RPutamen
3
Pct. Volume Change (per year w.r.t. baseline)
Pct. Volume Change (per year w.r.t. baseline)
3
2
[CN] (10)
[PHDfar] (16)
[PHDnear] (19)
[HD] (9)
1
0
−1
−2
−3
−4
LThalamus LCaudate LPutamen RThalamus RCaudate RPutamen
[LONG] shows higher precision and better discrimination
power between groups (specificity and sensitivity).
Huntington’s Disease (3 visits)
[Reuter et al., NeuroImage, 2012]
Rate of Atrophy
Baseline Vol. (normalized)
Atrophy in Huntington’s Disease [LONG]
−3
2
[CN] (10)
[PHDfar] (16)
[PHDnear] (19)
[HD] (9)
1
0
−1
−2
−3
6
Stucture Volume / Intracranial Volume
Pct. Volume Change (per year w.r.t. baseline)
3
5
x 10
Volume at Baseline in Huntington’s Disease
CN (10)
PHDfar (16)
PHDnear (19)
HD (9)
4
3
2
1
−4
LThalamus LCaudate LPutamen RThalamus RCaudate RPutamen
0
LThalamus LCaudate LPutamen RThalamus RCaudate RPutamen
Putamen atrophy rate is significant between CN and PHD
far, but baseline volume is not.
Sources of Bias during Acquisition
BAD: these influence the images directly and cannot
be easily removed!
• Different Scanner Hardware (Headcoil, Pillow?)
• Different Scanner Software (Shimming Algorithm)
• Scanner Drift and Calibration
• Different Motion Levels Across Groups
• Different Hydration Levels (season, time of day)
Hydration Levels
14 subjects, 12h dehydration, rehydration 1L/h
[with A. Bartsch et al. – submitted]
Links:
1. Software and Wiki: http://freesurfer.net
•
http://freesurfer.net/fswiki/LongitudinalProcessing
2. Facebook: http://facebook.com/FreeSurferMRI
3. Data (MIRIAD): http://miriad.drc.ion.ucl.ac.uk
4. Publications:
•
•
Reuter, Rosas, Fischl: Highly Accurate Inverse Consistent
Registration: A Robust Approach. NeuroImage 53(4):
1181-1196, 2010.
http://reuter.mit.edu/papers/reuter-robreg10.pdf
Reuter et al.: Within-Subject Template Estimation for
Unbiased Longitudinal Image Analysis. NeuroImage 61(4):
1402-1418, 2012.
http://reuter.mit.edu/papers/reuter-long12.pdf