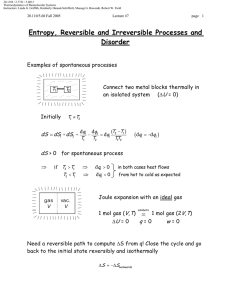
Equilibrium in Solution
advertisement

20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #11 page 1 Equilibrium in Solution The chemical potential for molecules in solution is given by a formula that is very similar to that for ideal gases: µA (T , p , cA ) = µAo (T , p ) + RT ln cA = µAo (T , p ) + RT ln [A ] The precise definition of the standard chemical potential µAo (T , p ) is now more complicated; it is defined at a given pH, salt concentration, etc…, all solution properties that need to be defined in advance. We will not go through those and take it as a given that the standard state is appropriately defined. Given a standard chemical potential µAo (T , p ) , then the analysis that we did for the ideal gas follows straight through and we find for a solution process νA A(g, T, p) + νB B(g, T, p) = νC C(g, T, p) + νD D(g, T, p) that following the ideal gas analysis in our previous lecture ⎛ [C ]νC [D ]νD ∆G (ε ) = ε ⎡⎣ν C µC (T ) + ν D µD (T ) ⎤⎦ − ⎡⎣ν A µA (T ) + ν B µB (T ) ⎤⎦ + RT ln ⎜ ⎜ [A ]ν A [B ]ν B ⎝ o o o o ⎞ ⎟ ⎟ ⎠ and the equilibrium constant K comes out through o ∆Grxn = −RT ln K , Where K = Qeq K = e −∆G o RT [C ] [D ] at equilibrium as before, and where the = ν [A ]ν [B ] νC νD A B concentrations Q are equilibrium concentrations. 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #11 page 2 Temperature dependence of K (or Kp) ln K (T ) = − ∆G o RT ⇒ d ln K d ⎛ ∆G o ⎞ ∆G o 1 d ∆G o = − = − dT dT ⎜⎝ RT ⎟⎠ RT 2 RT dT But at fixed pressure and/or solutions properties (p = 1 bar, pH constant, etc..) d ∆G o ⎛ ∂∆G o ⎞ =⎜ ⎟ dT ⎝ ∂T ⎠1 bar,pH constant, etc... and from fundamental equation ⎛ ∂G ⎞ dG = −SdT +Vdp ⇒ ⎜ ⎟ = −S ⎝ ∂T ⎠ p ∴ o o 1 d ln K ∆H (T ) −T ∆S (T ) = + ∆S o (T 2 dT RT RT d ln K (T ) ∆H o (T = dT RT 2 ⎛ ∂∆G o ⎞ o ⎜ ⎟ = −∆S (T T ∂ ⎝ ⎠p ⇒ ) ) ) T2 ∆H o (T )dT Integrating: ln K (T2 ) = ln K (T1 ) + ∫ At constant p: ∆H o (T ) = ∆H o (T1 ) + ∆C p (T −T1 ) T1 T2 ln K (T2 ) = ln K (T1 ) + ∫ T1 RT 2 ∆H o (T1 ) + ∆C p (T −T1 ) RT 2 dT Over small T ranges, ∆C p (T −T1 ) can be assumed small and ∆H o independent of T. ⇒ ∆H o ⎛ 1 1 ⎞ ∆H o ⎛T2 −T1 ⎞ ln K (T2 ) ≈ ln K (T1 ) + ⎜ − ⎟ = ln K (T1 ) + ⎜ ⎟ R ⎝T1 T2 ⎠ R ⎝ TT 1 2 ⎠ 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 If Lecture #11 ∆H o (T ) < 0 (Exothermic) page 3 T2 >T1 means K p (T2 ) < K p (T1 ) The equilibrium shifts toward reactants If ∆H o (T ) > 0 (Endothermic) T2 >T1 means K p (T2 ) > K p (T1 ) The equilibrium shifts toward products This is Le Chatelier’s principle for Temperature • Example: The Haber process ½ N2(g, T, p) + 3/2 H2(g, T, p) = NH3(g, T, p) o ∆Hrxn (298 K ) = −46.21 kJ/mol o ∆Grxn (298 K ) = −16.74 kJ/mol Kp = pNH 3 pH3 2 pN1 2 2 =p 2 −1 XNH 3 XH3 2XN1 2 2 =e 16,740 J/mol (8.314 J/K-mol)(298 K ) = 860 2 For p = 1 bar this is pretty good, lots of product. However, the reaction at room T is slow (this is kinetics, not thermodynamics). Raising T to 800 K can speed it up. But since ∆H o (T ) < 0 (exothermic), Le Chatelier tells us that the equilibrium will shift toward the reactants. Indeed: What to do? K p ( 800 K ) = 0.007 ⇒ Note above KX = p K p 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #11 page 4 Again use Le Chatelier, but with pressure! If we increase p, Eq. shifts toward products. ⇒ Run reaction at high T and high p For p = 1 bar, T = 800 K, Kp = 0.007 KX = XNH 3 XH3 2XN1 2 2 But at p = 100 bar, • = (1 ) K p = 0.007 2 much better! K X = (100 ) K p = 0.7 Heterogeneous Equilibria If a product or reactant is a solid or liquid, it will not appear in the ratio of partial p’s for Kp or in the concentrations if the equilibrium is in solution. However, it must be used in ∆G. Why? Take νA A(s) + νB B(g) = νC C(l) + νD D(g) The solid and liquid are not mixed – they are pure states. ∆G = ⎡⎣ν C µC ( s, pure, p ) + ν D µD ( g, mix, p ) ⎤⎦ − ⎡⎣ν A µA ( l , pure, p ) + ν B µB ( g, mix, p )⎤⎦ And for (l) or (s) ⇒ µC ( pure, p ) ≈ µ o ( pure ) ∆G = ν C µCo + ν D µDo − ν A µAo − ν B µBo + RT ln (no p-dependence) pDνD = ∆G o + RT lnQ νB pB 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 ∴ Lecture #11 ⎡ pDνD ⎤ νB ⎥ ⎣ pB ⎦Eq . No A or C involved. Kp = ⎢ But we still have and page 5 o ∆Grxn = ν C µCo + ν D µDo − ν A µAo − ν B µBo ln K p = − o ∆Grxn RT e.g. the decomposition of limestone CaCO3 (s) = CaO (s) + CO2 (g) T = 25ºC Calculate equilibrium vapor pressure at room T and elevated T. Data at 25ºC: Substance CaCO3 (s) CaO (s) CO2 (g) µ o (kJ/mol) -1128.8 -604.0 -394.36 ∆Hfo (kJ/mol) -1206.9 -635.09 -393.51 At equilibrium, ∆G = µ ( CaO, s ) + µ ( CO2 , g) − µ ( CaCO3 , s ) = µ o ( CaO, s ) + µ o ( CO2 , g) + RT ln pCO2 − µ o ( CaCO3 , s ) = ∆G o + RT ln K p where K p = pCO2 (at eq.) The equilibrium constant includes only the gas, but ∆G o includes the solids too. ∆G o (kJ/mol) = -604.0 – 394.4 – (-1128.8) = 130.4 kJ/mol ∆H o (kJ/mol) = -635.1 – 393.5 – (-1206.9) = 178.3 kJ/mol 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #11 page 6 Equilibrium pressure: ln K p = − ∆G o RT =− 130, 400 J/mol = −52.50 (8.314 J/K-mol) (298.15 K ) K p = 1.43x 10 −23 bar Nothing there at room T ! Try 1100 K: ∆H o ⎛ 1 1 ⎞ − ⎜ R ⎝ 1100 K 298 K ⎟⎠ 178, 300 J/mol ⎛ 1 1 ⎞ = −52.50 − − ⎜ ⎟ = 0.17 8.314 J/K-mol ⎝ 1100 K 298 K ⎠ (1100 K ) ≈ 0.84 bar ln pCO2 (1100 K ) ≈ ln pCO2 (298 K ) + pCO 2 There’s probably some change in ∆Hfo over such a wide T range, but clearly the equilibrium shifts dramatically.