∫ EXPANSIONS, ENERGY, ENTHALPY Isothermal Gas Expansion
advertisement

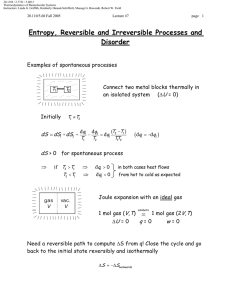
20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #3 page 1 EXPANSIONS, ENERGY, ENTHALPY Isothermal Gas Expansion ( ∆T = 0) gas (p1, V1, T) = gas (p2, V2, T) Irreversibly (many ways possible) (1) Set pext = 0 p= 0 T T p= 0 p 1,V1 p 2,V2 v2 w(1) = − ∫ pext dV = 0 V1 (2) Set pext = p2 p2 T p2 T p 2,V2 p 1,V1 v2 w(2) = −∫ p2dV = −p2 (V2 −V1 ) V1 p p1 p2 V1 -w(2) V2 Note, work is negative: system expands against surroundings 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 (3) Lecture #3 page 2 Carry out change in two steps gas (p1, V1, T) = gas (p3, V3, T) = gas (p2, V2, T) p1 > p3 > p2 p2 p3 p3 T T T p 2,V2 p 3,V3 p 1,V1 v3 v2 V1 V3 w(3) = − ∫ p3dV − ∫ p2dV = − p3 (V3 −V1 ) −p2 (V2 −V3 ) p p1 More work delivered to surroundings in this case. p3 p2 V1 V3 V2 -w(3) (4) Reversible change p = pext throughout V p wrev = − ∫ 2 pdV V1 p1 p2 V1 - V2 rev For ideal gas: V wrev = − ∫ 2 V1 Maximum work delivered to surroundings for isothermal gas expansion is obtained using a reversible path V p nRT dV = −nRT ln 2 = nRT ln 2 V V1 p1 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #3 page 3 The Internal Energy U dU = d-q + d-w (First Law) dU = C pathdT − pext dV And U (T ,V ) ⇒ ⎛ ∂U ⎞ ⎛ ∂U ⎞ dU = ⎜ ⎟ dT + ⎜ ⎟ dV ∂ T ∂ V ⎝ ⎠V ⎝ ⎠T Some frequent constraints: dU = d-qrev + d-wrev = d-qrev – pdV Reversible ⇒ • Isolated ⇒ d-q = d-w = 0 • Adiabatic ⇒ d-q = 0 • Constant V ⇒ • (p = pext ) ⇒ dU = d-w reversible = -pdV w = 0 ⇒ dU = d-qV Constant V ∂U ⎞ ⎛ ∂U ⎞ dU = ⎛⎜ ⎟ dT + ⎜ ⎟ dV ⎝ ∂T ⎠V ⎝ ∂V ⎠T ⎛ ∂U ⎞ ⇒ d- qV = ⎜ ⎟ dT ⎝ ∂T ⎠V But also d-qV = CV dT So ⇒ ⎛ ∂U ⎞ ⎟ = CV ⎜ ⎝ ∂T ⎠V very important result!! ⎛ ∂U ⎞ ⎟ ⎝ ∂V ⎠T dU = CV dT + ⎜ dV what is this? 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #3 ⎛ ∂U ⎞ ⎟ ) ⎝ ∂V ⎠T Joule Free Expansion of a Gas gas (to get ⎜ vac gas (p1, T1, V1) = gas (p2, T2, V2) Since q = w = 0 Recall ⇒ ⎛ ∂U ⎞ ⎜ ⎟ ⎝ ∂V ⎠T ⎛ ∂U ⎞ ⎟ ⎝ ∂V ⎠T • q=0 Expansion into Vac. (pext=0) w=0 Constant U dV = 0 dVU = −CV dTU ⎛ ∂U ⎞ ⎜ ⎟ = −CV ⎝ ∂V ⎠T Joule did this. Adiabatic dU or ∆U = 0 dU = CV dT + ⎜ page 4 ⎛ ∂T ⎞ ⎜ ⎟ ⎝ ∂V ⎠U measure in Joule exp't! ⎛ ∆T ⎞ ⎜ ⎟ ⎝ ∆V ⎠U ⎛ ∆T ⎞ ⎛ ∂T ⎞ ∴ dU = CV dT − CV ηJ dV ⎟ =⎜ ⎟ ≡ ηJ ⎝ ∆V ⎠U ⎝ ∂V ⎠U Joule coefficient lim ⎜ ∆V → 0 For Ideal gas ⇒ ηJ = 0 dU = CV dT U(T) exactly Always for ideal gas only depends on T The internal energy of an ideal gas depends only on temperature Consequences ⇒ ⇒ ∆U = 0 ∆U = ∫ CV dT For all isothermal expansions or compressions of ideal gases For any ideal gas change in state 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #3 H(T,p) Enthalpy page 5 H ≡ U + pV Chemical reactions and biological processes usually take place under constant pressure and with reversible pV work. Enthalpy turns out to be an especially useful function of state under those conditions. reversible = gas (p, T1, V1) gas (p, T2, V2) const . p U1 U2 ∆U = q + w = qp − p ∆V ∆U + p ∆V = qp define as H ∆U + ∆ ( pV ) = qp H ≡ U + pV Choose H (T , p ) ∂H • ∆H = q p ⇒ ⎞ What are ⎛⎜ ⎟ ⎝ ∂T ⎠ p ⎛ ∂H ⎞ ⎜ ⎟ ⎝ ∂T ⎠ p ∆ (U + pV ) = qp ⇒ for a reversible constant p process ⎛ ∂H ⎞ ⎛ ∂H ⎞ dH = ⎜ ⎟ dp ⎟ dT + ⎜ ⎝ ∂T ⎠ p ⎝ ∂p ⎠T ⎛ ∂H ⎞ and ⎜ ⎟ ? ⎝ ∂p ⎠T ⇒ for a reversible process at constant p (dp = 0) ⇒ dH = đq p ⇒ ⎛ ∂H ⎞ and dH = ⎜ ⎟ dT ⎝ ∂T ⎠ p ∂H ⎞ ⎟ dT ⎝ ∂T ⎠ p đq p = ⎛⎜ ∴ but ⎛ ∂H ⎞ ⎜ ⎟ = Cp ⎝ ∂T ⎠ p đqp = C p dT also 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 • ⎛ ∂H ⎞ ⎜ ⎟ ⎝ ∂p ⎠T Lecture #3 ⇒ page 6 Joule-Thomson expansion adiabatic, q = 0 porous partition (throttle) gas (p1, T1) w = pV 1 1 − pV 2 2 ⇒ ∴ gas (p2, T2) = ∆U = q + w = pV 1 1 − pV 2 2 = −∆ ( pV ∆U + ∆ ( pV ) = 0 ⇒ ∴ ) ∆ (U + pV ) = 0 ∆H = 0 Joule-Thomson is a constant Enthalpy process. ⎛ ∂H ⎞ ⎟ dp ⎝ ∂p ⎠T dH = C p dT + ⎜ ⇒ ⎛ ∂H ⎞ ⎛ ∂T ⎞ ⎜ ⎟ = −C p ⎜ ⎟ ⎝ ∂p ⎠T ⎝ ∂p ⎠H Define ∴ ⎛ ∂H ⎜⎜ ⎝ ∂p ⎛ ∂H ⎞ ⎟ dpH ⎝ ∂p ⎠T C p dT = − ⎜ ⇒ ← can measure this ⎛ ∆T ⎞ ⎜ ⎟ ⎝ ∆p ⎠ H ⎛ ∆T ⎞ ⎛ ∂T ⎞ lim ⎜ =⎜ ⎟ ⎟ ≡ µJT ← Joule-Thomson Coefficient ∆p → 0 ⎝ ∆p ⎠H ⎝ ∂p ⎠H ⎞ ⎟⎟ = −C p µJT ⎠T and dH = C p dT − C p µJT dp 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #3 U(T), For an ideal gas: page 7 pV=nRT H ≡ U (T ) + pV = U (T ) + nRT H (T ) only ⎛ ∂H ⎜ ⎝ ∂p ⇒ depends on T, no p dependence ⎞ ⎟ = µJT = 0 for an ideal gas ⎠T For an ideal gas C p = CV + R ⎛ ∂H ⎞ ⎟ , ⎝ ∂T ⎠ p ⎛ ∂U ⎞ ⎟ ⎝ ∂T ⎠V Cp = ⎜ CV = ⎜ H = U + pV , ↓ ⎛ ∂H ⎞ ⎛ ∂U ⎞ ⎜ ⎟ =⎜ ⎟ +R ∂ ∂ T T ⎝ ⎠p ⎝ ⎠p ↑ ↑ ⎛ ∂U ⎞ ⎛ ∂V ⎞ C p = CV + ⎜ ⎟ ⎜ ⎟ +R ⎝ ∂V ⎠T ⎝ ∂T ⎠ p = 0 for ideal gas ∴ C p = CV + R pV = RT