Document 13540031
advertisement

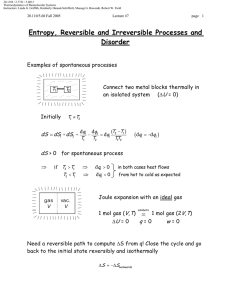
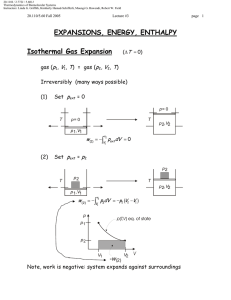
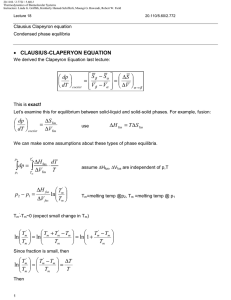
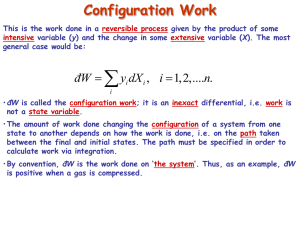
20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #6 page The Second Law ▲ First Law ∆U = q + w , showed the equivalence of work and heat ∫ dU = 0 for cyclic process ⇒ q = −w Suggests engine can run in a cycle and convert heat into useful work. ▲ Second Law • Puts restrictions on useful conversion of q to w • Follows from observation of a directionality to natural or spontaneous processes • Provides a set of principles for - determining the direction of spontaneous change - determining equilibrium state of system Need a definition: Heat reservoir Definition: A very large system of uniform T, which does not change regardless of the amount of heat added or withdrawn. Also called a heat bath. Real systems can come close to this idealization. Two classical statements of the Second Law: Kelvin Clausius and a Mathematical statement 1 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #6 page I. Kelvin: It is impossible for any system to operate in a cycle that takes heat from a hot reservoir and converts it to work in the surroundings without at the same time transferring some heat to a colder reservoir. q> 0 w< 0 -w= q T1 (hot) q T1 (hot) q1 -w q 1> 0 w< 0 q 2< 0 -w q 1= -w-q 2 -q 2 IMPOSSIBLE!! T2 (cold) OK!! II. Clausius: It is impossible for any system to operate in a cycle that takes heat from a cold reservoir and transfers it to a hot reservoir without at the same time converting some work into heat. q 2> 0 T1 (hot) q 1< 0 -q 1 -q 1= q 2 T1 (hot) q2 q2 IMPOSSIBLE!! T2 (c old) Alternative Clausius statement: -q 1 T2 (c old) q 2> 0 w> 0 q 1< 0 w -q 1= w+ q 2 OK!! All spontaneous processes are irreversible. (e.g. heat flows from hot to cold spontaneously and irreversibly) 2 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #6 ∫ đqrev T đ qrev = 0 ∫ Mathematical statement: page T is a state function = ∫ dS → and dS = ∫ đ qirrev < 0 T đqrev T S ≡ ENTROPY ∫ dS =0 → 2 ∆S = S2 − S1 = ∫ 1 2 qirrev qrev >∫ 1 T T irrev rev for cycle [1] ⎯⎯⎯ →[2] ⎯⎯⎯ →[1] 2 ∫1 2 ∫1 1 qrev q irrev q +∫ = ∫ irrev < 0 2 T T T 2 qirrev qirrev − ∆S < 0 ⇒ ∆S > ∫ 1 T T Kelvin and Clausius statements are specialized to heat engines. Mathematical statement is very abstract. Let’s Link them through analytical treatment of a heat engine. The Carnot Cycle - a typical heat engine All paths are reversible T1 (hot) p 1 q1 isotherm (T1) 2 adiabat w adiabat 4 isotherm (T2) 3 q2 T2 (cold) 3 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #6 page 1→2 isothermal expansion at T1 (hot) 2→3 adiabatic expansion (q = 0) 3→4 isothermal expansion at T2 (cold) ∆U = q2 + w 2 4→1 ⇒ ∴ Kelvin: ∆U = w1′ adiabatic compression (q = 0) Efficiency = 1st Law ∆U = q1 + w 1 ∆U = w 2′ work output to surroundings − (w 1 + w1′ + w 2 + w 2′ ) = heat in at T1 (hot) q v∫ dU = 0 ⇒ q1 + q2 = − (w1 + w1′ + w 2 + w 2′ ) Efficiency = q1 + q2 q =1+ 2 q1 q1 q2 < 0 → Efficiency ≡ ε < 1 (< 100%) -w = q1ε = work output Note: if the cycle were run in reverse, then q1 < 0, q2 > 0, w > 0. It’s a refrigerator! Carnot cycle for an ideal gas 1→2 2→3 ⎛V ⎞ 2 ∆U = 0; q1 = −w1 = ∫1 pdV = RT1 ln ⎜ 2 ⎟ V ⎝ q = 0; w1′ = CV (T2 −T1 ) Rev. adiabat ⎛T2 ⎞ ⎛V2 ⎞ ⎜ ⎟=⎜ ⎟ ⎝T1 ⎠ ⎝V3 ⎠ ⇒ ⎛V4 ⎞ ⎟ ⎝V3 ⎠ 4 ∆U = 0; q2 = −w2 = ∫3 pdV = RT2 ln ⎜ 4→1 q = 0; w2′ = CV (T1 −T2 ) ⇒ ⎠ γ −1 3→4 Rev. adiabat 1 ⎛T1 ⎞ ⎛V4 ⎞ ⎜ ⎟=⎜ ⎟ ⎝T2 ⎠ ⎝ V1 ⎠ γ −1 4 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #6 page q2 T2 ln (V4 V3 ) = q1 T1 ln (V2 V1 ) γ −1 γ −1 ⎛ V1 ⎞ ⎜ ⎟ ⎝V4 ⎠ ⎛T ⎞ ⎛V ⎞ =⎜ 2⎟=⎜ 2⎟ ⎝T1 ⎠ ⎝V3 ⎠ or q1 q2 + =0 ⇒ T1 T2 ⇒ v∫ ⎛V4 ⎞ ⎛V1 ⎞ ⎜ ⎟=⎜ ⎟ ⎝V3 ⎠ ⎝V2 ⎠ đqrev T ⇒ q2 T =− 2 q1 T1 =0 this illustrates the link between heat engines to the mathematical statement of the second law Efficiency ε =1+ q2 T =1− 2 q1 T1 For a heat engine (Kelvin): → 100% as T2 → 0 K q1 > 0, w < 0, T2 < T1 ⎛T −T2 ⎞ ⎟ q1 ⎠ Total work out = −w = ε q1 = ⎜ 1 ⎝ T1 ⇒ For a refrigerator (Clausius): q2 > 0, w > 0, T2 < T1 ( −w ) < q1 Note: In the limit T2 → 0 K, (-w) → q1, and ε → 100% conversion of heat into work. 3rd law will state that we can’t reach this limit! Total work in But q1 q =− 2 T1 T2 ⎛T −T ⎞ = w = ⎜ 2 1 ⎟ q1 ⎝ T1 ⎠ ⎛T −T ⎞ ⇒ w = ⎜ 1 2 ⎟ q2 ⎝ T2 ⎠ Note: In the limit T2 → 0 K, w → ∞. This means it takes an infinite amount of work to extract heat from a reservoir at 0 K ⇒ 0 K cannot be reached (3rd law). 5 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 • Lecture #6 page 6 The efficiency of any reversible engine has to be the same as the Carnot cycle, this can be shown by running the reversible engine as a refrigerator, using the work output of a Carnot engine to drive it so that the total work out is zero, and showing that, if the efficiency of the reversible engine is higher, then the second law is broken. Additionally: • We can approach arbitrarily closely to any cyclic process using a series of only adiabats and isotherms. ∴ So, for any reversible cycle • This is consistent with the mathematical statement of the second law, which defines Entropy, a function of state, with dS = Note: • đqrev T v∫ ∆S = S2 − S1 = ⇒ ∫ 2 1 đqrev T =0 đqrev T Entropy is a state function, but to calculate ∆S from q requires a reversible path. An irreversible Carnot (or any other) cycle is less efficient than a reversible one. p 1 irreversible isotherm with p ext = p 2 2 adiabat 4 adiabat isotherm (rev.) 3 1→2 ( −w )irrev < ( −w )rev ⇒ wirrev > w rev ∆U = qirrev + wirrev = qrev + w rev ∴ qirrev < qrev 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 ** Lecture #6 page An irreversible isothermal expansion requires less heat ** than a reversible one. ε irrev = 1 + also q2rev q2rev < 1 + = ε rev q1irrev q1rev đqirrev đqrev T < T ⇒ v∫ (q2 < 0) đqirrev <0 T • This leads to the Clausius inequality đq v∫ T ≤0 ⎧ đqrev ⎪⎪ v∫ T = 0 contains ⎨ ⎪ đqirrev < 0 ⎪⎩ v∫ T • Important corollary: The entropy of an isolated system never decreases (A): The system is isolated and irreversibly (spontaneously) changes from [1] to [2] (A) irreversible 1 2 (B) reversible (B): The system is brought into contact with a heat reservoir and reversibly brought back from [2] to [1] qirrev = 0 Path (A): Clausius đq v∫ T ≤0 ⇒ (isolated) ∫ 2 1 đqirrev T =0 ! +∫ 1 2 đqrev T ≤0 7 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field 20.110/5.60 Fall 2005 Lecture #6 ⇒ ∫ 1 2 đqrev T ∴ page 8 = S1 − S2 = −∆S ≤ 0 ∆S = S2 − S1 ≥ 0 This gives the direction of spontaneous change! For isolated systems 1 2 But! ∆Ssurroundings ∆S > 0 Spontaneous, irreversible process ∆S = 0 Reversible process ∆S < 0 Impossible ∆S = S2 − S1 independent of path depends on whether the process is reversible or irreversible (a) Irreversible: Consider the universe as an isolated system containing our initial system and its surroundings. ∆Suniverse = ∆Ssystem + ∆Ssurroundings > 0 ∴ (b) ∆Ssurr > −∆Ssys Reversible: ′ =0 ∆Suniv = ∆Ssys + ∆Ssurr ∴ ′ = −∆Ssys ∆Ssurr ∆Suniverse ≥ 0 for any change in state (> 0 if irreversible, = 0 if reversible)