Specimen characterization with the Electron Microprobe Massachusetts Institute of Technology
advertisement

Specimen characterization
with the
Electron Microprobe
Massachusetts Institute of Technology
Signals produced in the
Electron Microprobe
Cathodoluminescence
(CL)
Electron
beam
Back-scattered
electron (BSE)
Characteristic X-ray
Secondary
electron (SE)
Specimen
Electron-specimen interactions
Beam electron
Specimen atom
Scattered beam electron
Elastic Scattering
E1 = E0 , large e
Inelastic Scattering
E1<E0 , small i
( e>> i)
- Back-scattered
electron
- Characteristic X-rays
- Secondary electron
- Cathodoluminescence
Elastic scattering cross-section
Q(> e) =
1.62x10-20
(Z /E )cot2( e/2)
2
2
Q: cross section (events.cm2/e-.atom)
e: elastic scattering angle
E1 = E0 , large
e
Z: atomic number
E: beam energy
Electron interaction volume
•
•
Increases with voltage (electron
beam energy)
Decreases with sample atomic
number
Typical depths (15 kV, perpendicular beam):
Carbon (C, At# 6)
1.8 m
Iron (Fe, At# 26)
1.1 m
Uranium (U, At#92)
0.8 m
Electron Back-scattering
(High angle elastic scattering)
Backscattered electron image
Back-scattered electron
Polished surface
Function of
composition
Plane polarized transmitted light
Thin section
Function of optical
properties
Phase identification: EDS X-ray spectra
Mean Atomic Number
ilm
hbl
plg
Understanding X-rays:
Energy and Wavelength
E=h
h : Planck's constant
(6.626x10-34 Joule.sec
or, 6.626x10-34/1.6021x10-16 keV.sec)
: frequency (= c/ )
(c : speed of light in vacuum
= 2.99793x1017 nm/sec
: wavelength)
nm) = c/ = hc/E = 1.2398/E (keV)
Understanding X-rays:
The electromagnetic spectrum
UM
3.17 keV
0.39 nm
SiK
1.74 keV
0.71 nm
nm) = 1.2398/E (keV)
The X-ray spectrum
Characteristic X-rays
Continuum
X-rays
X-ray background
(maximum energy = electron beam
energy, E0)
Continuum X-rays: background in
X-ray spectra
Phase 1
Phase 2
Neither phase contains Cr
But background counts at Cr :
in
1
in
2
Characteristic X-ray generation
Inner-shell ionization
X-ray and electron transition
K : L to K-shell K M to K-shell
L : M to L-shell L N to L-shell
M N to M-shell
K-shell
Ti K
L-shell
Fe K
Flowchart for
X-ray generation
Ti K
Fe K
Overvoltage
U = E0/Ec
where, E0 is the electron beam energy (usually 10-25 keV)
Ec : critical excitation energy for inner shell ionization
Best analytical condition, U≈5
keV
K
L
M
Imaging with X-rays:
compositional mapping
Mg
Ca
Na
Ti
Beam-rastered image: electron beam rasters over the area to be imaged
Stage-rastered image: electron beam is stationary, stage moves
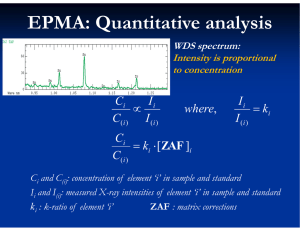
EPMA: Quantitative analysis
WDS spectrum:
Intensity is proportional
to concentration
Ci
C( i )
Ii
I (i )
Ci
C( i )
ki [ ZAF ]i
where,
Ii
I (i )
ki
Ci and C(i): concentration of element ‘i’ in sample and standard
Ii and I(i): measured X-ray intensities of element ‘i’ in sample and standard
ki : k-ratio of element ‘i’
ZAF : matrix corrections
Matrix (ZAF) corrections
Z : atomic number correction
A : absorption correction
F : fluorescence correction
Atomic number (Z) correction
R( i )
Zi
S (i )
Ri
Si
Ri = CjRij
R = #X-rays generated / #X-rays if
there were no electron backscattering
Si = CjSij
S = -(1/ )(dE/ds), stopping power
( ): standard
a function of E0 and composition
(Duncumb and Reed)
Z, a function of E0 and composition
Measuring Cu
in
Cu-Al alloy
ZCuKα
Pure Cu
standard
CuAl2
standard
X-ray absorption
I = I0 exp-(
/ )( x) =
I0 exp-(
/ )( z cosec )
I: Intensity emitted; I0: Intensity generated
/ : mass absorption coefficient
: density; z: depth; : take-off angle
Mass absorption
coefficient, (
)
energy
absorber
ZnK is highly
absorbed in Ni
Energy
Ec(K-shell)
(
)
energy
Ni
__________(keV)_____(keV)____(cm2/g)______
CoK
6.925
53
NiK
7.472
8.331
60
CuK
8.041
49
ZnK
8.632
311
Absorption (A) correction
Absorption function,
Ai
f(
(i )
f ( i)
)
f( i) =
Ii(emitted)/Ii(generated)
( ): standard
a function of E0, and composition
(Philibert)
A, a function of E0,
composition
and
ANiK in Fe-Ni alloy
1.7
1.6
1.6
CFe
0.1
0.3
0.5
0.5
0.7
1.4
0.1
1.5
0.3
ANiK at 40o
ANiK at 15.5o
1.5
0.9
1.3
CFe
1.4
0.3
0.7
0.9
1.3
0.1
1.5
ANiK at 52.5o
1.6
CFe
1.2
0.5
1.4
0.7
0.9
1.3
1.2
1.2
1.1
1.1
1
1.1
1
10
15
20
E0 (keV)
25
30
1
10
15
20
E0 (keV)
25
30
10
15
20
E0 (keV)
25
30
X-ray fluorescence
A consequence of X-ray absorption
when
Eabsorbed X-ray > Ec(absorber shell)
Absorption-Fluorescence in Fe-Ni alloy
NiK is absorbed in Fe, and Fe is fluoresced
K-shell excitation energy of Fe = 7.111 keV; NiK energy 7.478 keV
NiKα =379.6 cm2/g
( )
Fe
Characteristic fluorescence (F)
correction
1
Fi
1
{
I
{
f
( ij )
I (i )
I ijf I i
}
}
If : fluoresced intensity
I : e-beam generated intensity
( ): standard
Fluorescence correction for an element includes the
summation of fluoresced intensities by other
elements in the compound
a function of E0 and composition
(Castaing-Reed)
F, a function of E0 and composition
FFeK in Fe-Ni alloy
1
1
1
0.9
0.9
CFe
0.1
0.3
0.8
0.1
0.3
0.7
0.9
0.9
15
20
25
30
10
0.9
15
20
25
30
0.3
0.5
0.5
ANiK at 40o
1.4
0.9
1
1
1
25
30
30
1.2
1.1
20
0.9
1.3
1.1
E0 (keV)
25
0.7
1.1
15
0.5
1.4
1.2
1.2
30
0.3
0.7
1.3
1.3
25
0.1
1.5
ANiK at 52.5o
0.3
0.9
20
CFe
0.1
1.5
0.7
10
15
1.6
CFe
0.1
ANiK at 15.5o
10
E0 (keV)
1.6
CFe
1.4
0.5
E0 (keV)
1.7
1.5
0.3
0.7
E0 (keV)
1.6
0.1
0.7
0.7
10
CFe
0.8
0.5
0.7
0.7
0.9
CFe
0.8
0.5
FFeK at 52.5o
1.1
FFeK at 40o
1.1
FFeK at 15.5o
1.1
10
15
20
25
30
E0 (keV)
ANiK in Fe-Ni alloy
10
15
20
E0 (keV)
MIT OpenCourseWare
http://ocw.mit.edu
12.119 Analytical Techniques for Studying Environmental and Geologic Samples
Spring 2011
For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.