Document 13505728
Mg
3
Al
2
Si
3
O
12
3
Mn
3
2
Al
2
3
Si
3
12
O
12
Cr
2
Si
3
O
12
Ca
3
3
Fe
2
2
Si
3
3
O
12
12
The structure of garnets consists of a continuous network of SiO
4 apices.
tetrahedra bonded to BO
6
octahedra at the
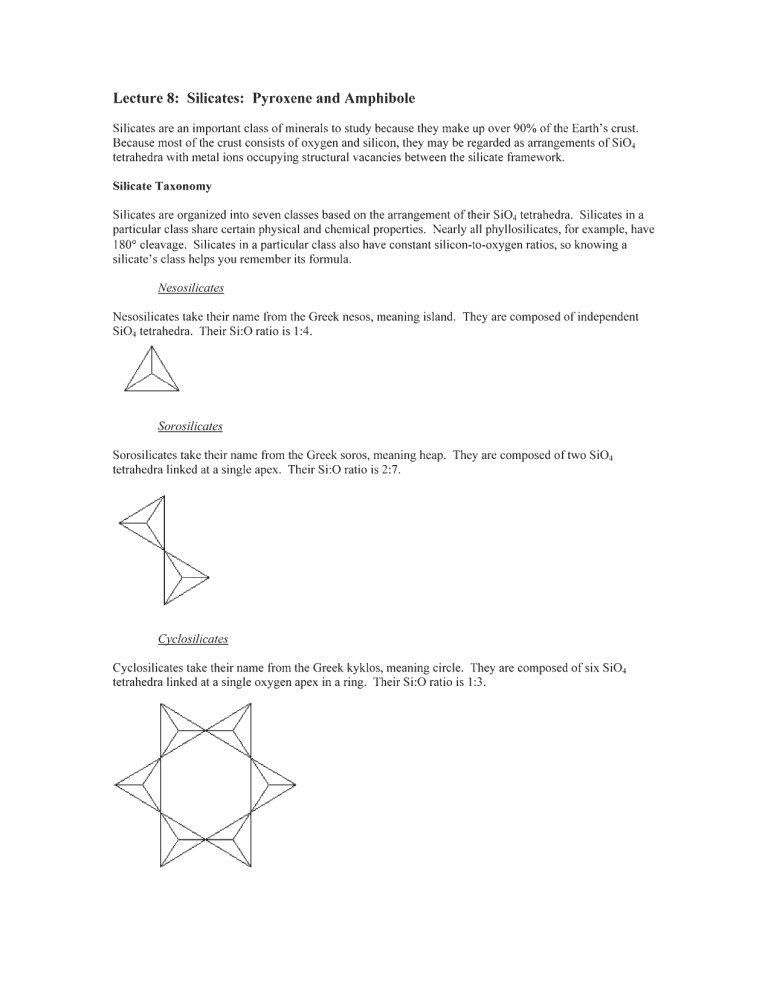
Sorosilicates
Sorosilicates are fairly rare. The most important group of minerals in this class is the epidote group.
(Fe 3+ , Al)Al
)(Si
2
)(Si
2
2
O(SiO
O
O
7
7
4
)(Si
2
O
7
)(OH)
)(OH)
)(OH)
Single Chain Inosilicates
Pyroxenes are an important group of minerals in the single chain inosilicate class. They have the general formula XYZ O
6 by Si 4+ or Al
2
3+
, where X and Y represent octahedral sites and Z represents the tetrahedral sites occupied
. The most common pyroxenes can be represented as part of the ternary system whose endmembers are wollastonite (CaSiO
3
), enstatite (MgSiO
3
), and ferrosilite (FeSiO
3
).
The structure of pyroxenes consists of chains of tetrahedra and octahedra that run parallel to the c axis.
One chain of octahedra is sandwiched between two chains of tetrahedra. This arrangement of chains can be visualized as I-beams.
Pyroxenes differ in the direction of their I-beams. When the I-beams all have the same direction, the pyroxene is a clinopyroxene. When the I-beams alternate directions, the pyroxene is a protopyroxene.
When two I-beams pointing in the same direction are followed by another two I beams pointing in the opposite direction—and so on—the pyroxene is an orthopyroxene.
Direction of I beams
+
+
+
-
+
+ -
+
+
+
+
-
+
-
+
+ + clino proto ortho-
The arrangement of I-beams in pyroxene accounts for the 90 ° cleavage.
Double Chain Inosilicates
Amphiboles are an important group of minerals in the double-chain inosilicate class. They have the general formula W
0-1
X
2
Y
5 represents 6- to 8-coordinated sites occupied by Ca 2+ coordinated sites occupied by Fe 2+ , Fe 3+ occupied by Al 3+ or Si 4+ ferroactinolite (Ca
2
Z
8
O
Fe
5
22
(OH, F)
Si
8
O
22
2
. W represents 10- to 12-coordinated sites occupied by Na
(OH)
2
, Mn 2+
7
Si
, Fe
, Al
8
O
), and grunerite (Fe
7
22
2+
3+
, Mg
, or Ti 4+
(OH)
2
2+ , Mn 2+ , Na + , or Li +
), tremolite (Ca
(OH)
2
).
2
Mg
5
Si
+ or K
8
O
22
(OH)
+ ; X
, Mg 2+ system whose endmembers are anthophylite (Mg
; Y represents 6-
; and Z represents tetrahedral sites
. The most common amphiboles can be represented as part of the quaternary
2
),
Si
8
O
22
The structure of amphibole is very similar to the structure of pyroxene. It is based on chains of tetrahedra and octahedra that run parallel to the c-axis and can be visualized as I-beams. Just as in the structure of pyroxene, the directionality of the I-beams distinguishes different types of amphibole. Their arrangement accounts for cleavage.






